Publication
Research Article
International Journal of MS Care
Using Body-Worn Sensors to Detect Changes in Balance and Mobility After Acute Aerobic Exercise in Adults with Multiple Sclerosis
Author(s):
Abstract
Background:
Current mobility and functional assessments do not capture the subtle changes in balance and gait that may predispose people with multiple sclerosis (MS) to falling. The purpose of this study was to use clinical and instrumented measures to examine the effects of an acute bout of aerobic exercise on balance and gait in individuals with MS.
Methods:
Ten adults with MS performed 15 minutes of moderate-intensity recumbent cycling or 15 minutes of rest. Exercise and rest visit order was randomized and separated by 1 week. Balance and mobility were assessed before, immediately after, and 2 hours after each test condition.
Results:
There were no significant differences across measurement periods for Timed 25-Foot Walk test times or Brief Balance Evaluation Systems Test scores. Significant improvements in mean sway radius and sway velocity when standing on foam and in percentage of stance stride time variability were found immediately after exercise compared with immediately after rest.
Conclusions:
This study lends further evidence that individuals with MS can safely engage in single bouts of aerobic exercise without detrimental short-term effects on function and may actually receive some short-term benefit regarding standing postural sway and gait variability. Future research should examine the dose-dependent relationship of varying types, intensities, or timing of exercise necessary to elicit short-term functional benefit and long-term health outcomes.
Individuals with multiple sclerosis (MS) experience a range of symptoms with functional consequences that significantly affect daily living and quality of life.1 Although there is increasing evidence of the efficacy of regular physical activity for improving symptoms and function,2 individuals with MS are more sedentary and insufficiently physically active compared with the general population.3 Because curtailment of physical activity may, in part, be attributable to perception of worsening symptoms and function after exercise,4–6 there is a need to offer continued evidence of there being no deleterious effect after acute exercise. In doing so, practitioners may be better positioned to facilitate the adoption and adherence of health-promoting levels of sustained exercise in this group.
In response to this need, researchers have aimed to understand the acute effects of a single bout of exercise in people with MS. Several studies have shown that although some negative effects of acute exercise occur, these neither persist nor affect overall functioning.7–9 It has been found that, in most participants, any worsening in the number or intensity of sensory symptoms is temporary, with levels returning to preexercise status within 30 minutes to 1 hour.7 10 Research has also confirmed that ratings of general physical functioning are not significantly affected by a single exercise session8 9 and that changes in balance performance between rest and exercise conditions do not significantly relate to changes in fatigue across the sessions.11 There is also evidence showing that in one session of bicycle ergometry, in which perceived decrements in balance and gait were reported, walking endurance actually improved.10
Although such findings are promising in terms of the utility of exercise as a prescriptive health treatment modality, the findings are limited by considering symptoms as a composite measure rather than separate symptom domains or by examining only changes in the perception of these balance and mobility changes rather than objectively measured changes. For those with MS, instrumented measures of standing balance and gait have been shown to be more sensitive in detecting subtle gait deficits in the absence of clinical manifestation.12 13 Most significant is that instrumented assessments can offer greater objectivity and reliability14 and that fall risk can be more readily identified from instrumented balance and walking evaluation than from routine clinical measures.15
Because current mobility and functional assessments do not capture the subtle changes in balance and gait that may predispose an individual with MS to falling, there is a critical need to objectively and more sensitively assess fall risk after exercise in this population. If shown to be the case, these findings may have clinical relevance for clinicians who require responsive measures of balance and mobility decline after exercise so that prescriptive changes to exercise recommendations can be made. Thus, the purpose of this study was to compare short-term changes in balance and gait, measured using objective and sensitive assessment techniques, after an acute bout of aerobic exercise in individuals with MS. We hypothesized that instrumented balance and gait measures, unlike clinically measured parameters, would evidence change from before to after 15 minutes of moderate leg cycling and that these changes would persist for up to 2 hours after the exercise bout.
Methods
Design and Participants
Using a repeated-measures design of two sessions, each with three time points of measurement, participants in the study served as their own controls (Figure 1).
Type and timing of assessments throughout testing protocol
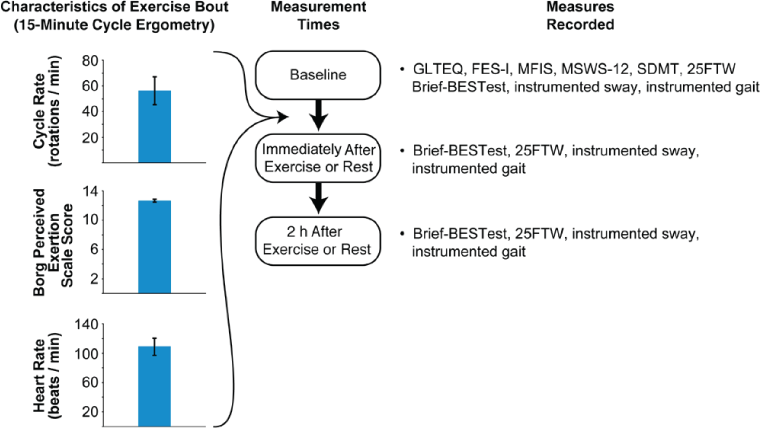
A convenience sample of ten individuals with MS was recruited. Participants were recruited through advertisements at the Multiple Sclerosis Center of the university medical center and in the local community. Individuals were included if they had physician-diagnosed MS, were able to exercise continuously for longer than 10 minutes, had a Patient-Determined Disease Steps score of 1 to 5,16 had not experienced a severe exacerbation of symptoms that required medical intervention in the previous month, and did not have an unrelated medical condition that prohibited participation in exercise. All the participants confirmed that they met the criteria for inclusion through self-report and gave written informed consent to participate in the institutional review board's approved protocol.
Measures
To assess the possibility of confounding influences on the exercise and rest session assessments, we measured physical activity levels (Godin Leisure-Time Exercise Questionnaire [GLTEQ]),17 concerns about falling (Falls Efficacy Scale-International [FES-I]),18 perceived fatigue (Modified Fatigue Impact Scale [MFIS]),19 and perceived mobility disability (12-item Multiple Sclerosis Walking Scale–version 2 [MSWS-12])20 at the start of each session.
Clinical Measures of Balance and Mobility
The eight-item Brief Balance Evaluation Systems Test (Brief-BESTest)21 was used as a clinical measure to identify underlying contexts of postural control that may contribute to poor functional balance. This test has been shown to be reliable and valid in the MS population.21 The Timed 25-Foot Walk test (T25FW) was also conducted because it represents a common measure of functional gait in people with MS.22
Instrumented Measures of Balance and Mobility
Balance and gait were also assessed during two instrumented tasks. Wireless inertial motion sensors (OPAL; APDM Inc, Portland, OR) were placed on the participants' wrists, feet, waist, and chest by Velcro elastic bands. Participants performed one instrumented trial of iSWAY while standing on a tiled floor and one trial of iSWAY while standing on 4 inches of foam, both with eyes closed. For the iSWAY, participants stood for 30 seconds with their arms crossed in front of their chest and with their feet close together but not touching. In addition, participants were asked to complete the iWalk, in which they were asked to walk as quickly and as safely as possible back and forth along a 25-foot tiled floor for 2 minutes.
Data from the OPAL sensors were collected wirelessly at 128 Hz onto a laptop computer. The outcome measures were then automatically generated by algorithms in the APDM Mobility Lab software. The instrumented walk generates measures of dynamic mobility, including mean and coefficients of variation (CVs) of stride length, percentage of time in stance, and the phase coordination index; these measures were selected to represent the spatial, temporal, and symmetry constructs of gait, respectively. For the iSWAY test, measures of standing body sway related to jerk, velocity, and sway radius were chosen because they represent the smoothness, control of sway speed, and amount of postural sway, respectively.
Procedures
Participants visited the laboratory two times separated by 1 week. The order of the experimental (Exercise) and control (Rest) visits was randomized across participants. Although the order of the clinical and instrumented measures was kept consistent within participants for the two sessions, these assessments were randomized across participants. Each participant performed either 15 minutes of moderate-intensity recumbent cycling or 15 minutes of rest. Exercise time and intensity was standardized across participants. Each participant bicycled at a rating of perceived exertion of 12 to 13 on the Borg scale, a reliable and valid mechanism to standardize exercise intensity in individuals with MS.23 Heart rate was also monitored during the exercise bout using a Polar Vantage heart rate monitor (Polar Electro, Kempele, Finland) to ensure that moderate-intensity exercise had been achieved and maintained throughout the 15-minute exercise bout. After the exercise bout, participants were asked to rest by sitting quietly for 5 minutes. They were then assessed on both the clinical and instrumented measures at this time and again 2 hours later. For the Rest session, the timing of all assessments was the same as for the Exercise session.
Statistical Analysis
Nonparametric analysis was required because Shapiro-Wilks tests identified violations of normality. To ensure that participants did not exhibit significant differences in baseline clinical status between the Exercise and Rest sessions, Wilcoxon tests were performed on scores from the GLTEQ, FES-I, MSWS-12, and MFIS. To determine whether the exercise bout affected clinical balance (Brief-BESTest) and mobility (T25FW) or instrumented measures of standing sway (sway jerk, velocity, and radius) and gait (stride length and its CV, percentage of time in stance and its CV, and the phase coordination index), Friedman tests were performed against each outcome measure's value across all six combinations of session and measurement time. If the Friedman test identified significant differences in outcome measures across the measurement times, then a priori paired comparisons between the Exercise and Rest sessions were performed at each measurement time by Wilcoxon tests.
Results
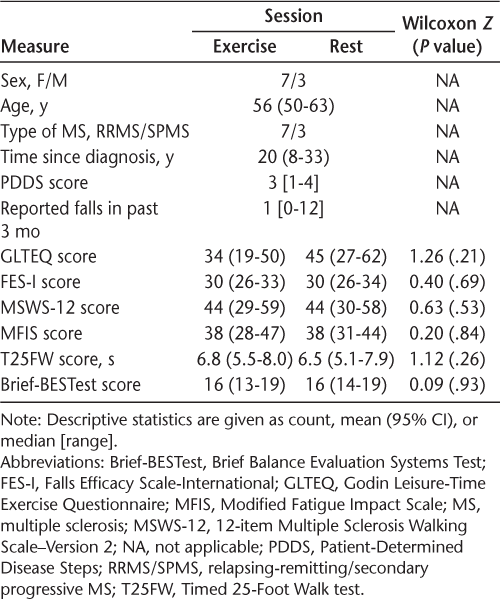
Ten people with MS participated in the study. The participants' baseline clinical status was not significantly different between the Exercise and Rest sessions (Table 1). Table 2 identifies the results of the statistical comparison tests for all the measures. Neither the T25FW nor the Brief-BESTest results significantly differed across combined sessions and measurement times; because the omnibus statistic was not significant, trends in the pairwise evaluations were not considered significant. For the Brief-BESTest, mean scores during the Rest session were 16.2, 15.7, and 16.7 at baseline, immediately after rest, and 2 hours after rest, respectively; scores during the Exercise session were 15.7, 16.2, and 17.2, respectively. For the T25FW, mean times during the Rest session were 6.53, 6.30, and 6.53 seconds at baseline, immediately after rest, and 2 hours after rest, respectively; times during the Exercise session were 6.79, 6.68, and 6.34 seconds, respectively.
Demographic characteristics and baseline clinical status at start of exercise and rest sessions
Statistical results for differences across measurement times with pairwise session differences at each time point
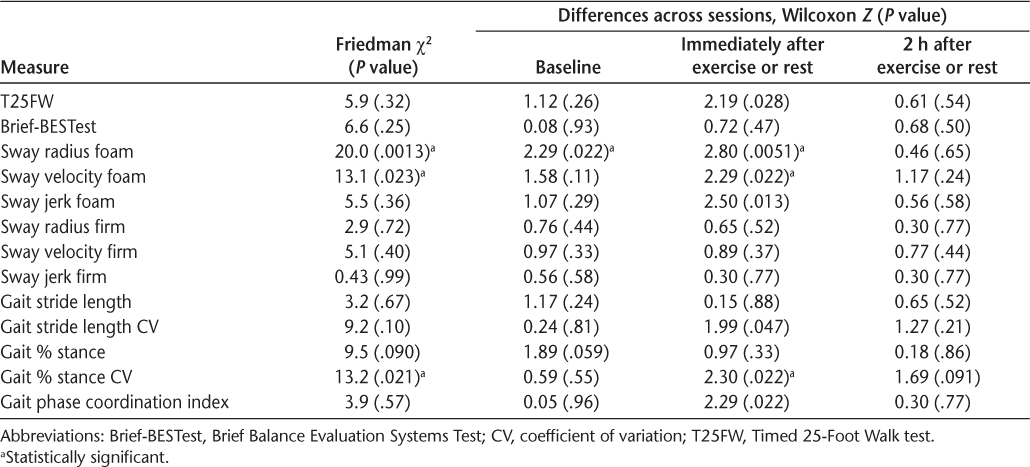
When standing on a foam surface, significant differences across measurement times were evident for mean sway and mean sway velocity (Table 2 and Figure S1 [published in the online version of this article at ijmsc.org]). Differences in mean sway radius and velocity were due to lower values immediately after exercise in the Exercise session compared with the same time point in the Rest session. Although not reaching significance with the Friedman test, similar trends were evident for sway jerk. No significant differences across measurement times were evident in any measure of sway when standing on a firm surface.
Significant differences across measurement times were not evident among instrumented measures of gait except for the CV of the percentage of stride time spent in stance, which was significantly lower immediately after exercise compared with the same time point during the Rest session (Table 2 and Figure S2). Although not reaching significance with the Friedman test, similar trends were evident for the phase coordination index.
Discussion
Determining the acute effects of exercise on standing and walking has important implications for individuals with MS who may fear a worsening of balance and increased risk of falling after exercising. Instrumented assessments have been found more effective in detecting subtle differences in balance and gait parameters than the more commonly used clinical tests12 13 and make it possible to objectively and accurately track changes in movement dynamics after exercise. The results of the present study suggest that a 15-minute bout of moderate-intensity recumbent cycling caused no detrimental effects in performance and, more importantly, may actually provide short-term improvement of standing postural sway and of gait variability when measured with body-worn inertial sensors.
The present findings that the clinical, uninstrumented measures did not change with exercise are commensurate with other MS research on short-duration exercise.8 9 Although these results are important for recommendations to those who may be hesitant to engage in exercise out of fear of deteriorating symptoms and performance, functional outcomes evaluated by clinical measures cannot granulate exercise-induced changes in postural control and gait in a way that more sensitive and objective measures can. Due to the unique but overlapping neural control systems required for standing and walking,24 the body-worn inertial sensors used in this study, similar to other studies,25 26 offered a means by which to detect subtle changes that were missed with the conventional clinical measures. Furthermore, these measurements revealed and characterized changes in distinct aspects of standing and mobility beneficially affected by the acute exercise. Not only did they support the preservation of function after acute exercise, they also revealed a subtle benefit of recumbent cycling in terms of gait variability and of postural sway when standing on foam. Given that increased gait variability is associated with a greater risk of falling in people with MS,27 these results offer additional evidence, and perhaps more confidence, for those with the disease when deciding whether to engage in moderate-intensity exercise.
Previous research has also demonstrated improved mobility after an acute bout of exercise. Moumdjian et al10 found improved walking endurance in participants with MS after bicycle ergometry regardless of perceived worsening of balance and gait symptoms. These researchers suggested that the exercise may have promoted changes in gait quality even with perceived decrements in gait pattern. We too speculate that this improvement in functioning may have a variety of neurophysiologic underpinnings. For one, previous research has demonstrated that both maintaining postural control while standing as well as bilaterally coordinating movements, such as walking, use attentional resources.28–30 Improved standing sway and gait variability in the present study may have occurred as a function of the related benefit to cognition obtained through the short bout of aerobic cycling. Indeed, there is increasing evidence to suggest that participation in single bouts of moderate-intensity aerobic exercise has an overall positive effect on aspects of cognition, including executive function and attention.31 The enhanced arousal and increased availability of processing resources derived from the exercise are believed to enable attention and the selection of task-relevant stimuli in a subsequent task, thereby, improving performance.31 32 This may be relevant as there exists an increased attentional demand with standing balance control as well as mobility in individuals with MS.33–35
In addition, there may have been some degree of transfer from the short bout of cycling to the subsequent task of walking in study participants. Although kinematically the movements are quite different,36 there is evidence of shared neural circuitry between cycling and walking. Research has elucidated phase-dependent modulation of background muscle activation and reflex amplitudes across rhythmic motor tasks, such as walking and cycling, that support common neural control.37 38
Notwithstanding these potential benefits, this study carries with it some methodological considerations. Given the size of the study sample, there is concern regarding adequate statistical power and the likelihood of detecting significant clinical effects. However, between sessions and at each time point, differences in the group means of the clinical measures did not exceed a clinically meaningful change or minimal detectable change of 2.7 seconds on the T25FW39 or of 4.3 points on the Brief-BESTest.40 Because participants did not exhibit clinically meaningful or statistically identifiable levels of change for clinical measures of gait speed or balance, it is likely that our interpretation of observing no exercise effects on physical function remains at least clinically accurate. Regarding generalizability, the sample represented those with primarily relapsing-remitting MS and mild-to-moderate disease severity (as indicated by Patient-Determined Disease Steps scores),16 and, thus, the results may not generalize to individuals with other characteristics of MS. Furthermore, this study evaluated recumbent cycling for 15 minutes at moderate intensity, taking place early in the day. Future research should examine the dose-dependent relationship between acute aerobic exercise and these outcomes to determine effects of different types, intensities, or timing of exercise.
Despite these empirical considerations, this study reinforces the lack of sensitivity in some clinical assessments to identify subtle changes in balance and gait after exercise and, although preliminary, provides evidence for potential benefits of short bouts of cycling in people with MS. Such insight is important when addressing patient concerns regarding impaired function and helping to increase confidence to exercise.
PRACTICE POINTS
Individuals with MS can safely engage in single bouts of exercise without detrimental short-term effects on physical functioning.
Short bouts of moderate-intensity recumbent cycling may provide some short-term benefit for standing postural sway and gait variability.
Financial Disclosures
The authors declare no conflicts of interest.
References
Patti F, Vila C. Symptoms, prevalence and impact of multiple sclerosis in younger patients: a multinational survey. Neuroepidemiology. 2014;42:211–218.
Giesser B. Exercise in the management of persons with multiple sclerosis. Ther Adv Neurol Dis. 2015;8:123–130.
Motl RW, McAuley E, Sandroff BM, Hubbard EA. Descriptive epidemiology of physical activity rates in multiple sclerosis. Acta Neurol Scand. 2015;131:422–425.
Ezeugwu V, Klaren RE, Hubbard E, Manns PT, Motl RW. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Prev Med Rep. 2015;2:241–246.
Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–463.
Halabchi1 F, Alizadeh1 ZI, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017;17:185.
Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:723–727.
Skjerbaek AG, Moller AB, Jensen E, et al. Heat sensitive persons with multiple sclerosis are more tolerant to resistance exercise than to endurance exercise. Mult Scler. 2013;19:932–940.
Learmonth YC, Paul L, McFayden AK, et al. Short-term effect of aerobic exercise on symptoms in multiple sclerosis and chronic fatigue syndrome: a pilot study. Int J MS Care. 2014;16:76–82.
Moumdjian L, Gervasoni E, Van Halewyck F, et al. Walking endurance and perceived symptom severity after a single maximal exercise test in persons with mild disability because of multiple sclerosis. Int J Rehabil Res. 2018;41:316–322.
Jackson K, Bigelow KE. Measures of balance performance are affected by a rested versus fatigued testing condition in people with multiple sclerosis. Phys Med Rehabil. 2013;5:949–956.
Spain RI, Mancini M, Horak FB, Bourdette D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture. 2014;39:958–964.
Spain RI, St. George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012;35:573–578.
Craig JJ, Bruetsch AP, Lynch SG, Horak FB, Huisinga JM. Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J NeuroEng Rehabil. 2017,14:43.
Sun R, McGinnis R, Sosnoff JJ. Novel technology for mobility and balance tracking in patients with multiple sclerosis: a systematic review. J Expert Rev Neurother. 2018;18:887–898.
Learnmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13:37.
Godin GS, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146.
van Vliet R, Hoang P, Lord S, Gandevia S, Delbaere K. Falls Efficacy Scale-International: a cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:883–889.
Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty D, LaRocca NG. Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society; 1997.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003;60:31–36.
Padgett P, Jacobs JV, Kasser SL. Is the BESTest at its best? a suggested brief version based on interrater reliability, validity, internal consistency, and theoretical construct. Phys Ther. 2012;92:1197–1207.
Bethoux F, Bennett S. Evaluating walking in patients with multiple sclerosis. Int J MS Care. 2011;13:4–14.
Morrison EH, Cooper DM, White LJ, et al. Ratings of perceived exertion during aerobic exercise in multiple sclerosis. Arch Phys Med Rehabil. 2008;89:1570–1574.
Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(suppl 2):ii7–ii11.
Pau M, Caggiari S, Mura A, et al. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: comparison with patient-based measure. Mult Scler Relat Disord. 2016;10:187–191.
Dandu SR, Engelhard MM, Qureshi A, et al. Understanding the physiological significance of four inertial gait features in multiple sclerosis. IEEE J Biomed Health Info. 2018;22:40–46.
Kalron A. Association between gait variability, falls and mobility in people with multiple sclerosis: a specific observation on the EDSS 4.0±4.5 level. NeuroRehabilitation. 2017;40:579–585.
Woollocott M, Shumway-Cook A. Attention and the control of posture and gait. Gait Posture. 2002;16:1–14.
Roerdink M, Hlavackova P, Vuillerme N. Center-of pressure regularity as a marker for attentional investment in postural control: a comparison between sitting and standing postures. Hum Mov Sci. 2011;30:203–212.
Stins JF, Michielsen ME, Roerdink M, Beek PJ. Sway regularity reflects attentional involvement in postural control: effects of expertise, vision, and cognition. Gait Posture. 2009;30:106–109.
Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101.
Chang YK, Pesce C, Chiang YT, Kuo CY, Fong DY. Antecedent acute cycling exercise affects attention control: an ERP study using attention network test. Front Hum Neurosci. 2015;9:156.
D'Orio VL, Foley FW, Armentano F, Picone MA, Kim S, Holtzer R. Cognitive and motor functioning in patients with multiple sclerosis: neuropsychological predictors of walking speed and falls. J Neuro Sci. 2012;316:42–46.
Hsieh KL, Sun R, Sosnoff JJ. Cognition is associated with gait variability in individuals with multiple sclerosis. J Neural Transm. 2017;124:1503–1508.
Negahban H, Mofateh R, Arastoo AA, et al. The effects of cognitive loading on balance control in patients with multiple sclerosis. Gait Posture. 2011;34:479–484.
Damiano DL, Norman T, Stanley CJ, Park HS. Comparison of elliptical training, stationary cycling, treadmill walking and overground walking. Gait Posture. 2011;34:260–264.
Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev. 2005;33:54–60.
Zehr EP, Balter JE, Ferris DP, Hundza SR, Loadman PM, Stoloff RH. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J Physiol. 2007;582(pt 1):209–227.
Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013;19:1784–1791.
Bravini E, Nardone A, Godi M, Guglielmetti S, Franchignoni F, Giordano A. Does the Brief-BESTest meet classical test theory and Rasch analysis requirements for balance assessment in people with neurological disorders? Phys Ther. 2016;96:1610–1619.







