Publication
Research Article
International Journal of MS Care
Brain Function Assessment of Patients with Multiple Sclerosis in the Expanded Disability Status Scale
Author(s):
Abstract
Background:
There is no consensus regarding assessment of the brain function functional system (FS) of the Expanded Disability Status Scale (EDSS) in patients with multiple sclerosis (MS). We sought to describe brain function FS assessment criteria used by Argentinian neurologists and, based on the results, propose redefined brain function FS criteria.
Methods:
A structured survey was conducted of 113 Argentinian neurologists. Considering the survey results, we decided to redefine the brain function FS scoring using the Brief International Cognitive Assessment for MS (BICAMS) battery. For 120 adult patients with MS we calculated the EDSS score without brain function FS (basal EDSS) and compared it with the EDSS score after adding the modified brain function FS (modified EDSS).
Results:
Of the 93 neurologists analyzed, 14% reported that they did not assess brain function FS, 35% reported that they assessed it through a nonstructured interview, and the remainder used other tools. Significant differences were found in EDSS scores before and after the inclusion of BICAMS (P < .001). Redefining the brain function FS, 15% of patients modified their basal EDSS score, as did 20% of those with a score of 4.0 or less.
Conclusions:
The survey results show the importance of unifying the brain function FS scoring criteria in calculating the EDSS score. While allowing more consistent brain function FS scoring, including the modified brain function FS led to a change in EDSS score in many patients, particularly in the lower range of EDSS scores. Considering the relevance of the EDSS for monitoring patients with MS and for decision making, it is imperative to further validate the modified brain function FS scoring.
The Expanded Disability Status Scale (EDSS)1 is the most popular tool for assessing disability in patients with multiple sclerosis (MS). Despite its limitations, the EDSS prevails as the main tool for assessing disability in clinical practice.2 Its final score, which can range from 0 (normal neurologic examination) to 10 (death by MS), is determined by a combination of intermediate scores obtained by assessing different functional systems (FSs) and mobility. Within FSs, the brain function FS is assessed and provides a score that ranges from 0 (normal) to 5 (dementia). The disadvantage of the brain function FS lies in the fact that it does not specify the tools used for defining the intermediate points. With the aim of improving reliability, Kappos et al3 developed Neurostatus by modifying gait assessment criteria and including new definitions for each FS. Although this approach to brain function FS is clearer, it does not propose a single test or homogeneous assessment criteria.
Considering the usefulness of the EDSS for monitoring patients and its important role in decision making,4 5 there is a need to provide clearer definitions of the different FSs to avoid intraobserver and interobserver variability between measurements and to improve quality. To standardize the brain function FS assessment, we aimed first to understand and describe the assessment criteria used by Argentinian neurologists for the brain function FS, and, considering these results, we decided to use an objective, easy-to-administer method for measuring brain function FS and evaluating its usefulness.
Methods
Overview
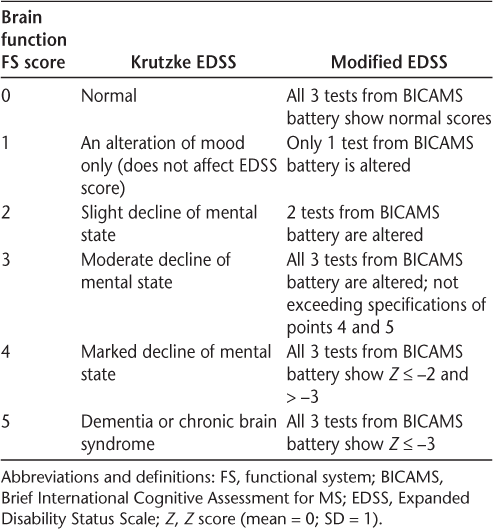
The study was performed in two stages. First we conducted a survey to learn about brain function FS assessment criteria used by Argentinian neurologists in the EDSS. Then, taking into account the results obtained from the survey, we redefined the brain function FS criteria in the EDSS using the Brief International Cognitive Assessment for MS (BICAMS) (Table 1). This study was exempted from informed consent by the ethics committee of Hospital J.M. Ramos Mejía.
Definitions of brain function FS scores
Survey of Argentinian Neurologists
Neurologists from Argentina were invited to participate in a structured survey specifically designed by the authors for this study. The survey was distributed online through Google Drive using the e-mail database of the Argentine Society of Neurology. The data were collected anonymously and previously approved by the ethics committee of the centers involved in this investigation. The results were directly captured in a database. The information surveyed included the professionals' sex, their years of practice as neurologists, whether they considered themselves MS specialists, the frequency with which they implemented the EDSS in their patients, and the brain function FS assessment criteria they used in the EDSS.
Assessment of Brain Function FS
A retrospective study was designed for two renowned MS centers in Buenos Aires City, Argentina, duly approved by the ethics committees of Hospital J.M. Ramos Mejía and Buenos Aires Institute of Neurosciences. One hundred twenty patients with MS were recruited. The inclusion criteria were a diagnosis of MS (clinically defined using the McDonald criteria6) and age 18 to 60 years. The exclusion criteria were a concomitant neurologic disorder other than MS, other systemic diseases that can cause cognitive impairment, a history of alcoholism or drug abuse, a diagnosis of psychiatric disorder, being in clinical relapse, or treatment with corticosteroids for the past 4 weeks.
The brain function FS was redefined in the EDSS by a new scoring system based on a change in assessment proposed by Saccà et al.7 The “basal EDSS” score (which excludes brain function) and the “modified EDSS” score, proposed for this work, were calculated. To calculate the modified EDSS score, the scores obtained in the tests of the BICAMS battery8 were used (Table 1). The basal and modified EDSS scores were then compared.
Measuring Instruments
The BICAMS9 is a neuropsychological battery for MS developed by a team of experts with the aim of designing a tool with a shorter administration time, yet still sensitive to cognitive impairment, for patients with MS. This battery has been validated in Argentina. It takes approximately 15 minutes to administer the BICAMS, which includes three tests: 1) The California Verbal Learning Test, Second Edition, measures learning and verbal memory. Through this test, the patient's ability to learn a list of 16 words and recall them over five trials is assessed. From the scores of the five recall trials, a total score is obtained. 2) The Brief Visuospatial Memory Test–Revised measures learning and visual memory. The patient is presented a matrix of six simple, abstract drawings over three trials lasting 10 seconds, each followed by a recall instance. The total score is obtained by summing the scores of the three learning trials. 3) The Symbol Digit Modalities Test (SDMT) displays a sheet of paper containing nine symbols, each one paired with a number. Below, there is a random sequence of these symbols, and the patient must verbally produce the number that corresponds to each of the symbols as quickly as possible. The test measures attention and information processing speed, and the final score is the total number of correct answers obtained during 90 seconds.
Statistical Analysis
The data were analyzed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp, Armonk, NY). A descriptive analysis was performed on all the variables included. The basal and modified EDSS scores were compared through a t test for two related samples. Because the addition of neuropsychological tests to the total calculation of the EDSS score affected only patients with basal EDSS scores of 4.0 or less, the analysis was repeated taking into account this subgroup of patients. The tests from the BICAMS battery were considered to be impaired if the score obtained was lower than 1.5 SDs below the population mean. This score is obtained by subtracting the population mean from the raw score and dividing it by the SD. Scores were adjusted according to educational level and age following the procedure established by previous studies.8 9 The scores considered in this work are shown in Table 1.
Results
Survey Responses
The survey was sent to 352 neurologists around the country, and 113 (32%) responded. Of the 113 neurologists who were surveyed, 20 did not implement the EDSS in their clinical practice and, therefore, were excluded from the analysis. Of the 93 neurologists who were finally considered for analysis, 54% were women and 33% considered themselves to be specialists in demyelinating diseases. Regarding brain function FS assessment in the EDSS, 35% of the neurologists reported that they conducted nonstructured interviews, 16% reported that they administered a Mini-Mental State Examination (MMSE),10 14% said that they did not include it in the final score of the EDSS, 9% reported using the Montreal Cognitive Assessment, 3% reported using BICAMS, and the remainder answered that they used other tests.
Assessment of Modified EDSS Scores
The patient group comprised 79 women (65.8%) and 41 men (34.2%). The mean ± SD patient age was 39.88 ± 12.27 years, schooling was 13.82 ± 3.42 years, EDSS score was 2.94 ± 2.05, and disease duration was 11.66 ± 9.47 years. Regarding MS type, 107 (89%) of the patients had relapsing-remitting, 8 (7%) had secondary progressive, and 5 (4%) had primary progressive.
A total of 52.5% of the patients showed cognitive impairment in at least one of the tests of the administered battery. After analyzing patient results in each one of the tests, it was found that 35.8% showed alterations in verbal memory (California Verbal Learning Test, Second Edition), 30.8% in visual memory (Brief Visuospatial Memory Test–Revised), and 36.7% in attention (SDMT). In relation to the number of tests that were impaired, the results indicated that 16.7% of the patients showed alterations in one test only, 20.8% in two tests, and 15.8% in all three tests. Of those patients who had alterations in all three tests, 26.3% had 2 to 3 SDs below the population mean in the three tests and 15.8% had more than 3 SDs below the population mean.
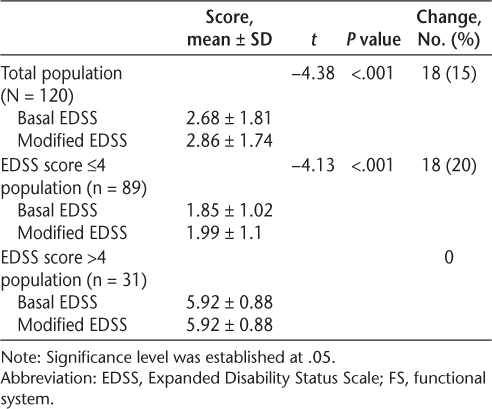
Comparing the basal EDSS scores with the modified EDSS scores, the latter were found to be higher, demonstrating that patients showed greater global disability when cognition was taken into account (P < .001). Eighteen patients (15%) showed a change in their EDSS score, all of whom had a basal EDSS score of 4.0 or less. After repeating the analysis in the subgroup of patients with a basal EDSS score of 4.0 or less, we discovered that the scores obtained in the modified EDSS were higher (P < .001) and that 20.22% of the patients showed different scores on the EDSS after correction through objective measures. Results are shown in Table 2.
Comparison of EDSS scores before and after modification of brain function FS criteria
Discussion
This study demonstrated the potential benefits of implementing an objective tool for measuring cognition, such as the BICAMS battery, in the calculation of brain function FS. According to the present results, one in five patients with an EDSS score of 4.0 or less without the brain function FS may have a higher EDSS score after inclusion of the modified brain function FS based on performance on the BICAMS. Although more than 50% of the patients showed alterations in one of the BICAMS tests, the benefits of incorporating the battery into the brain function FS assessment were evident only in patients who had an EDSS score of 4.0 or less. This phenomenon resembles what occurs in other FSs, which are underestimated in patients who have an EDSS score higher than 5.0 and where gait is the main modifying variable of the scale. The brain function FS assessment used in this study was significant in relation to the variation of the EDSS final score compared with the basal EDSS score. Those results are consistent with those reported by Saccà et al.7
The assessment of neurologic impairment in patients with MS is particularly relevant given that the disease can affect different FSs of the central nervous system and can have a variable course of evolution.11 In the past few years, a variety of scales have been developed to assess the impact of disability in patients who have MS, but none has been as widely accepted as the EDSS in clinical practice.2 The scale continues to be popular in the scientific community, despite its disadvantages, because it is not a linear scale, it has moderate interrater reliability, and its score is strongly oriented toward gait.12 However, despite the high prevalence of cognitive impairment in MS, the assessment of cognition in the EDSS is somewhat basic, ambiguous, and lacking sensitivity.1 Kurtzke13 reports that compromise of the brain function FS, leaving aside mood alterations, accounts for only 4.8%. In addition, brain function is considered to be the FS least involved in the final EDSS score.
Although cognitive impairment as a manifestation of MS was described by Charcot early in 1877,14 psychometric methods began to be firmly implemented a century later.15 Within the framework of an international consensus, a group of experts developed the BICAMS neuropsychological battery, a psychometric measure highly recommended for assessing cognition in patients with MS.9 Some studies show that there is a correlation between one of the BICAMS tests and parameters of magnetic resonance imaging (MRI), such as brain atrophy, burden of injury, and disease activity (gadolinium-positive injuries).16 Other studies show important associations with activities of daily living and employment.17
In the present study, we emphasize the need for unifying the brain function FS assessment criteria. From the survey data we were able to learn that 35% of Argentinian neurologists used a nonstructured interview to assess cognition, 16% used the MMSE, and the remainder either did not assess it or used a different tool. Previous studies by different authors showed a low correlation between neuropsychological performance in general and the information reported by patients18; therefore, the nonstructured interview should not be considered an objective tool for assessing cognitive impairment. In addition, the MMSE is considered to lack sensitivity for MS, and experts have recommended against its use since the 1990s.19
The results of this study show that by using a simple, easy-to-administer test to assess brain function FS, the modification of the EDSS score was significant. Another advantage of using the BICAMS battery for assessing brain function FS is its administration time: it requires less than 15 minutes. Similar to the study by Saccà et al,7 inclusion of the modified brain function FS did not affect the final EDSS score when scores were higher than 4.0. As previously stated, this is the result of the effect that gait alterations have on scores equal to or higher than 5. Nevertheless, it is always necessary to perform a correct estimation of all the FSs, given that a sharp increase in any of them could be the consequence of a relapse or a clinical progression regardless of the final EDSS score. In this regard, one of the BICAMS tests, the SDMT, proved to be sensitive to objectifying cognitive effects associated with relapses.20 21 Pardini et al22 found a correlation between patients with cognitive decline measured by the SDMT and enhancement with gadolinium on brain MRI, suggesting a possibility of cognitive relapse in those patients, even without compromise of other FSs in the EDSS. On the other hand, an adequate brain function FS assessment should be considered in the context of new therapeutic goals in MS oriented to evaluating no evidence of disease activity (NEDA) as a measure of optimal response to drugs.23 24 NEDA is the absence of clinical relapses, of EDSS score progression, and of MRI activity. Many authors suggest that the absence of cognitive impairment progression should be included in the definition of NEDA as a marker of activity.25 Underestimating the assessment of cognitive functions could, therefore, lead to an inadequate perception of NEDA.
Limitations of this study include that the traditional brain function FS was not included in the calculation of the baseline EDSS score. In addition, only 32% of the neurologists responded to the survey. Likewise, this investigation is retrospective and studied a limited number of patients. Future prospective evaluations are needed to compare the traditional brain function FS score with the modified score proposal of the brain function FS and to assess whether adding the BICAMS significantly increases the time needed to rate the EDSS.
Beyond the previously mentioned limitations, the findings of the present study allow us to conclude that standardizing the brain function FS in the EDSS could result in an improved integration of cognitive assessment in the routine physical examination of patients with MS. Other authors have found that clinical interview and standard neurologic examination are not sufficiently sensitive to detect cognitive impairment in MS and suggest the need for an accurate cognitive screen to complement routine neurologic evaluation.7 When there are no unified criteria for cognitive assessment, incorrect interpretations are a risk and the assessment of disability in patients with MS may be limited to physical aspects only, underestimating cognition and its effect on patient disability and quality of life.
Use of the BICAMS battery to assess cognition in daily practice could improve accuracy in the assessment of disability as measured by the EDSS. The BICAMS battery is highly recommended, and although it omits certain cognitive areas such as executive function and long-term storage of memory tests, it is currently the most widely used battery for assessing cognitive alterations in MS. Likewise, the BICAMS battery is a brief cognitive assessment for MS that can be used internationally, in small centers, with perhaps one or a few staff members who may or may not have formal neuropsychological training. It is proposed by a consensus of MS experts and is validated in multiple languages.
PRACTICE POINTS
The Expanded Disability Status Scale (EDSS) is the most popular tool for assessing disability in patients with MS. Despite the high prevalence of cognitive impairment in MS, the assessment of cognition in the EDSS is somewhat basic, ambiguous, and lacking sensitivity.
In our survey, Argentinian neurologists reported using different tools to assess cognition or rate the brain function functional system (FS) as part of the EDSS.
The brain function FS was redefined through a new assessment system using the Brief International Cognitive Assessment for MS battery. The present results show that by using a simple, easy-to-administer battery to rate the brain function FS, the final EDSS score changed in approximately 20% of patients.
Financial Disclosures
The authors declare no conflicts of interest.
References
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33:1444–1452.
Izquierdo G, Ruiz-Peña JL. Clinical evaluation of multiple sclerosis: quantification by use of scales [in Spanish]. Rev Neurol. 2003;36:145–152.
Kappos L, D'Souza M, Lechner-Scott J, Lienert C. On the origin of Neurostatus. Mult Scler Relat Disord. 2015;4:182–185.
Mark MS, Freedman S, Selchen D, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci. 2013;40:307–323.
Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263:1053–1065.
Polman C, Reingold S, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Saccà F, Costabile T, Carotenuto A, et al. The EDSS integration with the Brief International Cognitive Assessment for Multiple Sclerosis and orientation tests. Mult Scler. 2017;23:1289–1296.
Vanotti S, Smerbeck A, Benedict RH, Caceres F. A new assessment tool for patients with multiple sclerosis from Spanish-speaking countries: validation of the Brief International Cognitive Assessment for MS (BICAMS) in Argentina. Clin Neuropsychol. 2016;7:1023–1031.
Benedict RH, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol. 2012;12:55.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a longitudinal study comparing Disease Steps and EDSS to evaluate disease progression. Mult Scler. 1999;5:349–354.
Basil Sharrack R, Hughes AC. Clinical scales for multiple sclerosis. J Neurol Sci. 1996;135:1–9.
Kurtzke JF. Disability rating scales in multiple sclerosis. Ann N Y Acad Sci. 1984;436:347–360.
Charcot JM. Lectures on the Diseases of the Nervous System. New Sydenham Society; 1877.
Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis, I: frequency, patterns, and prediction. Neurology. 1991;41:685–691.
Rao SM, Martin AL, Huelin R, et al. Correlations between MRI and information processing speed in MS: a meta-analysis. Mult Scler Int. 2014;2014:975803.
Honan CA, Brown RF, Batchelor J. Perceived cognitive difficulties and cognitive test performance as predictors of employment outcomes in people with multiple sclerosis. J Int Neuropsychol Soc. 2015;21:156–168.
Benedict RH, Munschauer F, Linn R, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler. 2003;9:95–101.
Beatty WW, Goodkin DE. Screening for cognitive impairment in multiple sclerosis: an evaluation of the Mini-Mental State Examination. Arch Neurol. 1990;47:297–301.
Benedict RH, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:721–733.
Morrow SA, Jurgensen S, Forrestal F, et al. Effects of acute relapses on neuropsychological status in multiple sclerosis patients. J Neurol. 2011;258:1603–1608.
Pardini M, Uccelli A, Grafman J, et al. Isolated cognitive relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:1035–1037.
Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72:152–158.
Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4:329–333.
Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler. 2016;22:64–72.







