Publication
Research Article
International Journal of MS Care
Restless Legs Syndrome Severity and Cognitive Function in Adults With Multiple Sclerosis: An Exploratory Pilot Study
Author(s):
ABSTRACT
BACKGROUND
Restless legs syndrome (RLS) is a sleep disorder present in as many as 26% of persons with multiple sclerosis (MS) and can be associated with cognitive function. The present study examined the relationships between RLS symptoms (severity, frequency, occurrence) and cognitive function in adults with MS who have RLS.
METHODS
Twenty-two participants attended 1 laboratory session and completed the International Restless Legs Syndrome Study Group Rating Scale (IRLS), the Restless Legs Syndrome-6 Scale, and then the Brief International Cognitive Assessment for Multiple Sclerosis battery consisting of the Symbol Digit Modalities Test; California Verbal Learning Test, Second Edition; and Brief Visuospatial Memory Test–Revised.
RESULTS
Nonparametric bivariate correlations indicated that worse IRLS total severity was associated with slower processing speed (ρ = −0.42), worse verbal memory (ρ = −0.63), and worse visual memory (ρ = −0.61); worse RLS severity at falling asleep was associated with worse verbal memory (ρ = −0.45) and worse visual memory (ρ = −0.55); and worse RLS severity during the day while active was associated with slower processing speed (ρ = −0.58), worse verbal memory (ρ = −0.52), and worse visual memory (ρ = −0.60).
CONCLUSIONS
These results suggest that those with more severe RLS, including worse symptoms at falling asleep and during the day while active, might experience worse cognitive function, particularly processing speed and memory. Future research should evaluate whether treatment of RLS symptoms can offer new opportunities for managing cognitive dysfunction in adults with MS.
Restless legs syndrome (RLS) is a sensorimotor sleep disorder that occurs in an estimated 26% of persons with multiple sclerosis (MS),1 and it may exacerbate the symptoms and consequences of MS,2 including cognitive function. Indeed, there is evidence that sleep disorders and cognitive impairment are prominent and debilitating consequences of MS,3,4 and markers of sleep quality have been associated with cognitive impairment in MS. There is recent evidence supporting the association between the presence and severity of RLS with perceived cognitive impairment in adults with MS.5 In this recently published study, adults with MS and RLS perceived their cognitive impairment as significantly worse than adults with MS alone5; however, the relationship between RLS symptoms and neuropsychological measures of cognitive function has not been evaluated in adults with MS. This is important because there is a strong degree of uncertainty regarding the meaning of scores from measures of perceived cognitive function in MS whereby self-reported scores often correlate with psychological symptoms (ie, mood and emotion) more so than neuropsychological tests of cognitive function.6–8
The present pilot study extends previous research5 and explores the relationships between RLS symptoms (ie, overall severity, frequency, and occurrence) and objective cognitive function in adults with MS and RLS. We use neuropsychological testing to assess different domains of cognitive function, including processing speed, working memory, verbal memory, and visual memory. We expect that worse overall RLS severity, greater frequency of RLS symptoms, and worse RLS symptoms when falling asleep and during the night will be associated with slower processing speed as well as worse working, verbal, and visual memory based on previous research in sleep impairment and cognitive function in adults with MS.9 Because RLS symptoms can be particularly distracting, we further expect that worse RLS severity during the day, while active and while resting, will be associated with worse cognitive function as outcomes of cognitive function are captured during the day.
METHODS
Participants
The present study involves a secondary analysis of data from a subsample of participants who enrolled in a center-based study validating the Suggested Immobilization Test in MS; the data are not part of our previous mail-based study on patient-reported cognitive difficulty and RLS.5 To that end, no sample size calculations were performed because this secondary analysis was exploratory. Participants were recruited by sending letters and flyers to persons identified through a search of the University of Alabama at Birmingham’s i2b2 database for persons with MS residing in the Birmingham area. Interested persons were asked to contact research staff for a brief description of the study followed by screening for the following inclusion/exclusion criteria: age 18 years or older, diagnosis of MS, and a positive screen for the presence of RLS based on confirmatory responses to the 5 diagnostic criteria for RLS10 using the Cambridge-Hopkins Restless Legs Syndrome Short Form Diagnostic Questionnaire.11 Participants were excluded from the study based on mimics of RLS (eg, positional discomfort, muscle cramps) and diagnosis of radiculopathy, peripheral edema, peripheral neuropathy, anemia, renal disease, or diabetes.
RLS Severity
The International Restless Legs Syndrome Study Group Rating Scale (IRLS) is a validated 10-question survey that provides a global score regarding the severity and frequency of a patient’s RLS symptoms during the previous week.12,13 The severity is determined by summing the individual item scores into a total score ranging from 0 to 40, with higher scores indicating greater severity. Severity scores are categorized as no symptoms (score of 0), mild (scores of 1–10), moderate (scores of 11–20), severe (scores of 21–30), and very severe (scores of 31–40). Of note, item 7 on the IRLS provides a measure of RLS frequency on a 5-point scale ranging from 0 (never) to 4 (very often, ie, 6 to 7 days per week).
The Restless Legs Syndrome-6 Scale (RLS-6) is a validated scale for assessing a patient’s sleep satisfaction (item 1), RLS severity during different periods of a 24-hour day (items 2–5), and daytime sleepiness (item 6) during the previous week.14 The RLS-6 assesses the severity of RLS symptoms on a scale from 0 (none) to 10 (very severe) in 4 domains: at falling asleep (item 2), at night (item 3), during the day while resting (item 4), and during the day while active (item 5). The RLS-6 also contains items for assessing sleep satisfaction (item 1) and daytime sleepiness (item 6) on a scale from 0 to 10, whereby higher scores reflect worse sleep satisfaction and daytime sleepiness. We further calculated a sleep composite score by taking the average of items 1 and 6.
Cognitive Function
The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) battery was administered as a valid and reliable method for detecting cognitive impairment in the MS population15 and is composed of the Symbol Digit Modalities Test (SDMT); the California Verbal Learning Test, Second Edition (CVLT-II); and the Brief Visuospatial Memory Test–Revised (BVMT-R). The BICAMS offers a feasible, cost-effective method of assessing cognitive function in MS across cultures.16 The first author (K.L.J.C) administered all the neuropsychological outcomes as she is thoroughly trained and has 4 years of experience in administering the BICAMS battery in adults with MS. We included the raw scores for the analyses because this was a nonclinical application of BICAMS and not for making judgments regarding detection of cognitive impairment.
The SDMT provides a measure of visuospatial processing speed and working memory; it consists of 9 single-digit numbers paired with 9 particular abstract symbols. Participants were provided with a key that included the number and symbol pairings at the top of the page, followed by rows of pseudorandomly aligned symbols. Participants were asked to call out each number associated with the symbol according to the way they are paired in the key at the top of the page, completing as many as possible in 90 seconds. Scores are presented as the total number of correct pairings identified by the participant in 90 seconds, with lower scores indicative of worse visuospatial processing speed and working memory.
The learning trial of the CVLT-II is an assessment of immediate verbal memory and memory difficulties often used in adults with MS. The test is composed of a 16-item word list with 4 words associated with 4 categories (eg, animals, foods) that are randomly arranged. Participants were read the list of words at a rate of 1 word per second and then were asked to recall as many items as possible, in any order, across 5 learning trials. The total number of correct words in each trial was summed to provide a score ranging from 0 to 16, and the learning trial scores were summed for a total score ranging from 0 to 80, with lower scores indicative of worse recall.
The learning trial of the BVMT-R is an examination of immediate visuospatial memory. The test is composed of 3 recall trials wherein participants are presented with a display of 6 abstract geometric figures for 10 seconds; the display is hidden from view while participants attempt to draw each figure as accurately as possible and in the correct position on the page from memory. For each trial, the figures receive a score of 0, 1, or 2 based on the accuracy and position of the figure on the page. Thus, each trial results in a score ranging from 0 to 12, and the trial scores are summed for a total score ranging from 0 to 36, with lower scores indicative of worse visual memory and visuospatial memory.
Demographic and Clinical Characteristics
Participants completed a questionnaire for age, sex, race, years of education, employment status, MS type, MS disease duration (ie, time since diagnosis), time since MS symptom onset, previous diagnosis of RLS, time since RLS symptom onset, and family history of RLS. Participants provided a list of all current medications, including over-the-counter medications, for assessment of what might exacerbate or mitigate RLS symptoms. Participants further underwent a brief neurologic assessment for scoring the Expanded Disability Status Scale (EDSS) as a valid measure of disability status in persons with MS 17 ; the first author (K.L.J.C.) is Neurostatus-certified for EDSS administration and scoring.
Procedure
The institutional review board of the University of Alabama at Birmingham approved the study procedures, and all participants provided written informed consent. Participants with MS were screened for RLS diagnosis over the phone and were scheduled for a single, 90-minute session in the laboratory. The present study was part of a larger testing session wherein a rater performed a brief neurologic examination for scoring the EDSS. Participants then completed the questionnaires, followed by the BICAMS battery (ie, SDMT, CVLT-II, and BVMT-R). Participants and researchers were not blinded regarding the aims of the larger study, and participants were remunerated $25 for completing all the measures.
Statistical Analyses
All statistical analyses were conducted using SPSS Statistics for Windows, version 26.0 (IBM Corp), and descriptive data are presented as means (SDs) or as frequency (percentage) of individuals, unless otherwise specified. Skewness and kurtosis values along with assessment of frequency distributions were inspected for establishing normality of the variables.18–20 Based on the distribution and varying measurement characteristics (ie, continuous and categorical) of the outcome variables, we adopted nonparametric analyses and a P < .05 for interpreting statistical significance with the inferential analyses. We provide raw scores for BICAMS outcomes alongside calculated z scores using regression-based norms adjusting for age, sex, and years of education.21 The associations among IRLS total score, RLS frequency (ie, IRLS item 7), RLS occurrence (ie, RLS-6 items 2 through 5), and cognitive function (ie, SDMT, CVLT-II, and BVMT-R raw and z scores) were examined using bivariate Spearman rho (ρ) correlation coefficients. Values of 0.1, 0.3, and 0.5 were interpreted as small, moderate, and large, respectively.22 We further evaluated the relationship between outcomes of RLS symptom severity, sleep, and neuropsychological impairment that were significantly associated using multivariate linear regressions (α = 0.05). We regressed BVMT-R (raw and norm-corrected) scores on significantly associated outcomes of RLS severity (ie, IRLS total score and RLS-6 items 2 and 5 for raw scores and IRLS total score and RLS-6 items 2, 4, and 5 for norm-corrected scores) in step 1, and RLS severity plus the sleep component score, which was significantly correlated with both BVMT-R and RLS severity scores in bivariate correlation analyses, in step 2. We examined the change in the standardized beta coefficient for RLS severity predicting BVMT-R scores between steps 1 and 2 for judging the variables that might be intermediary factors in the associations between RLS and neuropsychological function in persons with MS and RLS.
RESULTS
Participant Characteristics
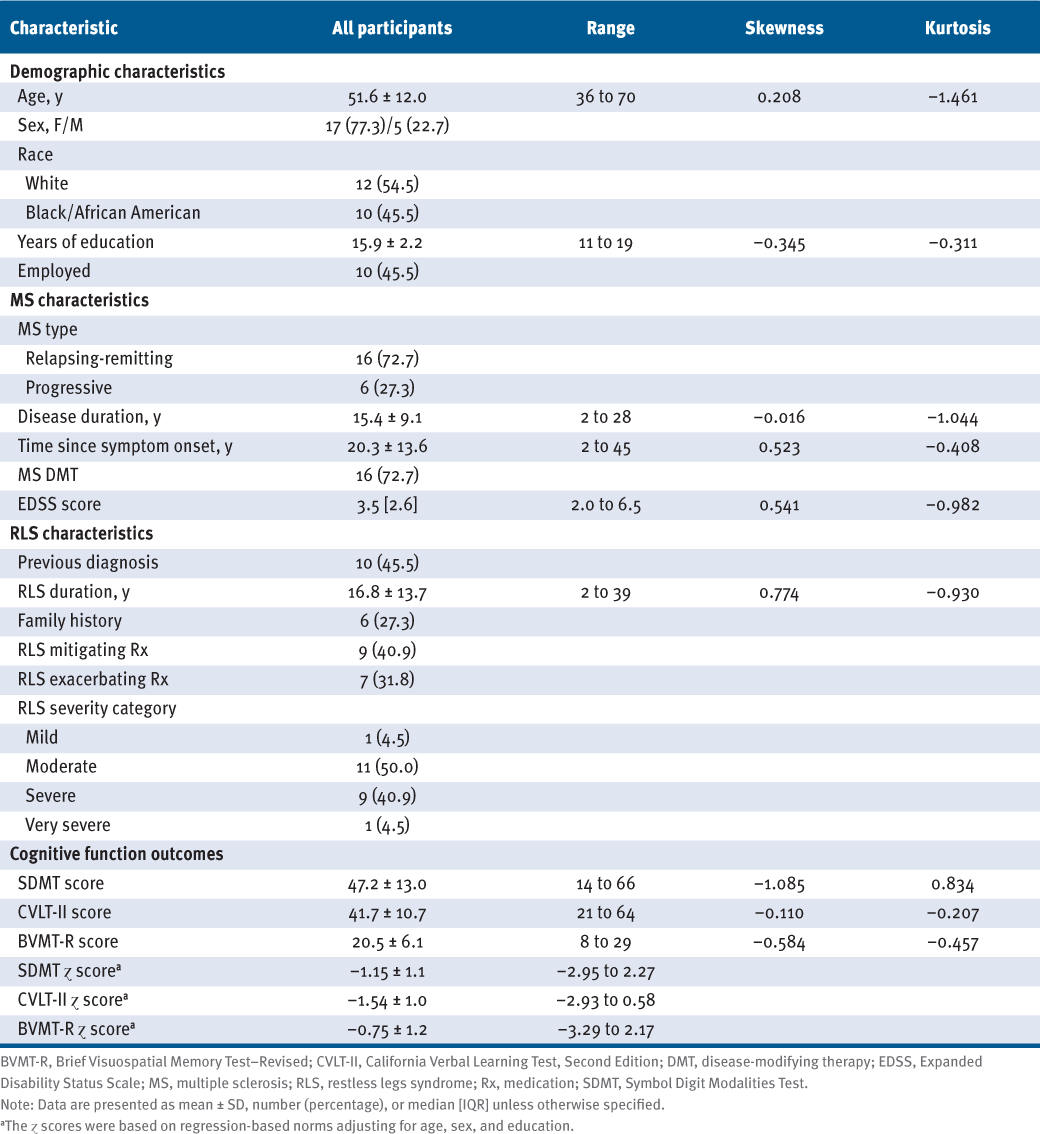
Characteristics of the 22 adults with MS and RLS in the study sample are presented in TABLE 1. The sample had a mean (SD) age of 51.6 (12.0) years and was primarily White (55%) and female (77%) with relapsing-remitting MS (73%), a mean (SD) disease duration of 15.4 (9.1) years, and moderate disability (median [interquartile range] EDSS score = 3.5 [2.6]). The sample had RLS symptoms for a mean (SD) of 16.8 (13.7) years, and only 46% of participants had received a previous diagnosis of RLS, with 27% reporting an immediate family member with RLS. Approximately 73% of individuals were receiving disease-modifying therapy for managing MS, including 41% of participants taking 1 or more medications that could improve RLS symptoms (eg, pramipexole, gabapentin, and rotigotine23) and 32% taking 1 or more medications that could worsen RLS symptoms (eg, antidepressants and first-generation antihistamines24). Regarding cognitive impairment, mean (SD) scores were 47.2 (13.0) for the SDMT, 41.7 (10.7) for the CVLT-II, and 20.5 (6.1) for the BVMT-R, and all were comparable with a recent systematic review of validation studies for the BICAMS battery.16 Scores for the BICAMS battery were slightly above the suggested cutoff scores of 44 for the SDMT, 39 for the CVLT-II, and 17 for the BVMT-R for identifying impaired cognitive performance for adults with MS.25 Mean (SD) regression-based norm-corrected z scores adjusting for age, sex, and years of education were −1.15 (1.1) for the SDMT, −1.54 (1.0) for the CVLT-II, and −0.75 (1.2) for the BVMT-R.
Demographic and Clinical Characteristics of the 22 Adults With MS and RLS
RLS Symptoms
A summary of RLS symptoms, including severity, frequency, and occurrence, is presented in TABLE S1, which is published in the online version of this article at IJMSC.org. The sample reported having moderate-to-severe RLS symptoms (mean [SD] IRLS total score = 20.4 [6.1]) with symptoms at least 2 days per week and most participants (63.6%) reporting symptoms on 4 or more days per week. Participants reported that mean (SD) RLS severity was worst at the time of falling asleep (5.0 [2.4]), followed by during the day while resting (4.6 [2.0]), during the night (4.5 [2.5]), and during the day while active (3.9 [2.7]).
Relationship Between RLS Symptoms and Cognitive Function
The summary of bivariate Spearman rho correlation analysis for RLS severity, frequency, and occurrence with cognitive function in 22 participants with RLS and MS is presented in TABLE 2. Higher IRLS total scores were associated with slower processing speed and worse working memory (ρ = −0.42 and −0.50) as well as worse immediate visuospatial memory based on raw scores (ρ = −0.61) and norm-corrected scores (ρ = −0.69), and worse immediate verbal memory based on raw scores only (ρ = −0.63). Worse RLS severity at falling asleep (ie, RLS-6 item 2) was associated with worse immediate verbal memory difficulties based on raw scores (ρ = −0.45) and worse immediate visuospatial memory based on raw scores (ρ = −0.55) and norm-corrected scores (ρ = −0.47). There was no association between RLS severity at falling asleep and processing speed and working memory. There were no significant associations between RLS severity during the night (ie, RLS-6 item 3) and neuropsychological outcomes. Worse RLS severity during the day while resting (ie, RLS-6 item 4) was associated with norm-corrected scores for immediate visuospatial memory (ρ = −0.44). Worse RLS severity during the day while active (ie, RLS-6 item 5) was associated with slower processing speed and worse working memory (ρ = −0.58 and −0.67) as well as worse immediate visuospatial memory (ρ = −0.60 and −0.63) based on raw and norm-corrected scores and worse immediate verbal memory based on raw scores only (ρ = −0.52). Associations between cognitive function outcomes and RLS symptoms in adults with MS and RLS are presented in FIGURE S1.
Summary of Bivariate Spearman Rho Correlation Analysis for RLS Severity, Frequency, and Occurrence With Cognitive Function in 22 Participants With MS and RLS
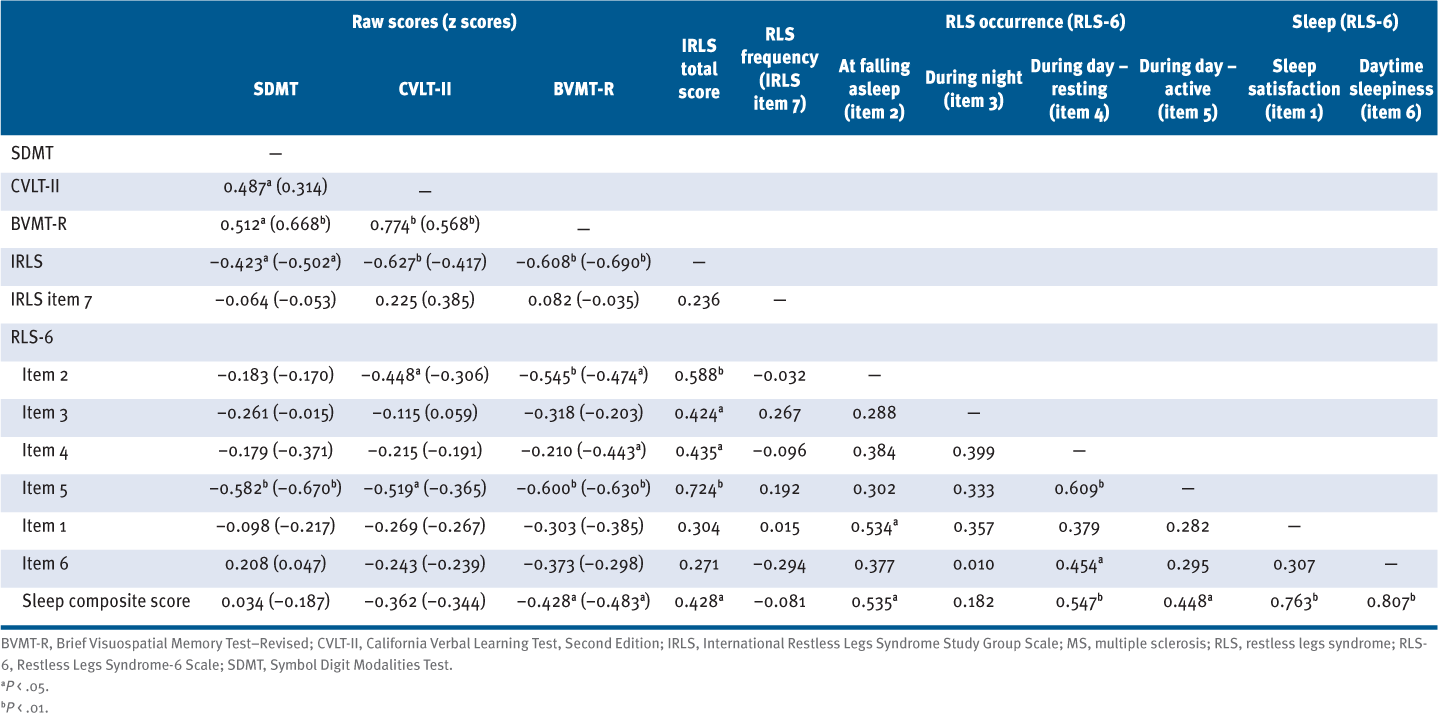
There were no significant associations between sleep satisfaction (ie, RLS-6 item 1) or daytime sleepiness (ie, RLS-6 item 6) and scores from the SDMT, CVLT-II, or BVMT-R. Worse sleep quality, based on the sleep composite score, was associated with worse immediate visuospatial memory (ρ = −0.43 and −0.48), based on raw and norm-corrected scores. The summary of linear regression analyses for further evaluating the relationships among RLS, sleep, and immediate visuospatial recall performance is presented in TABLE S2. Severity of RLS explained a significant amount of the variance in step 1 (raw scores: F = 6.17, P < .01, R2 = 0.507; z scores: F = 3.01, P < .05, R2 = 0.414), and the inclusion of the sleep composite score did not affect this relationship in step 2 (raw scores: F = 4.37, P < .05, ΔR2 = 0.000; z scores: F = 2.40, P = .08, ΔR2 = 0.014).
DISCUSSION
The present pilot study was the first, to our knowledge, to evaluate RLS symptoms and performance on neuropsychological measures of cognition in adults with MS. These results indicated that (1) worse IRLS total severity was associated with slower processing speed and working memory, worse verbal memory, and worse visuospatial memory; (2) worse RLS severity at falling asleep was associated with worse verbal memory and worse visuospatial memory; and (3) worse RLS severity during the day while active was associated with slower visuospatial processing speed and working memory, worse verbal memory, and worse visuospatial memory. When adjusting neuropsychological outcomes for age, sex, and education, associations between RLS severity and processing speed, working memory, and immediate visuospatial memory strengthened, whereas associations between RLS severity and immediate verbal memory weakened. The cross-sectional design of this pilot study precludes any inferences regarding the directionality of the relationship between RLS and performance on neuropsychological measures of cognition, and these relationships could be associated with other structural or functional disease-related outcomes in MS. Nevertheless, the possible interpretation that worse RLS symptoms yield reduced cognitive function, particularly memory and processing speed in adults with MS who have RLS requires confirmation using longitudinal designs that control for other variables as confounders.
The present results suggest that worse RLS severity, specifically higher IRLS total scores and worse severity at falling asleep and during the day while active, is associated with slower processing speed and worse visual and verbal memory in adults with MS who have RLS. When adjusting for age, sex, and education, the associations between RLS severity and processing speed and working memory and immediate visuospatial memory strengthened, whereas associations between RLS severity and immediate verbal memory weakened and were no longer significant. These results suggest that age, sex, and education might be accounting for the relationship between immediate verbal memory and IRLS total scores, RLS severity at falling asleep, and RLS severity during the day while active. In addition, when adjusting for age, sex, and education, there was a significant association between RLS severity during the day while resting and immediate visuospatial memory. Although this seems to be the first study to examine the association between RLS symptoms and neuropsychological outcomes in adults with MS, studies outside of MS have demonstrated similar relationships between RLS severity and cognitive function. One study reported that worse RLS severity was associated with worse attention and verbal fluency in 23 adults with untreated RLS in the general population,26 and another study suggested that severe RLS can result in more impaired working memory in 13 adults with untreated RLS in the general population.27 One potential explanation for this relationship could be related to the effect of RLS symptoms on concentration, as 1 study in the general population suggested that sensory symptoms related to RLS may lead to a lack of concentration.28 This suggests that participants who experience greater severity of RLS symptoms during the day while active might experience a reduced capacity to concentrate, therefore decreasing performance on neuropsychological outcomes, including learning and memory as well as processing speed.
Another possible explanation for the relationship between greater RLS severity and impaired neuropsychological outcomes may be related to chronic sleep loss due to RLS rather than a direct effect of RLS pathology. Outside of RLS, a study in adults with MS observed a reduction in objective cognitive function as a result of sleep disturbances, namely, visual memory, verbal memory, executive function, attention, processing speed, and working memory.4 This suggests that chronic sleep loss as a result of RLS symptoms might affect neuropsychological function in adults with MS. Chronic sleep loss from RLS symptoms at night combined with the aforementioned reduced concentration due to worse RLS severity during the day while active could yield reduced performance on neuropsychological outcomes. The present study demonstrated no significant associations between sleep satisfaction or daytime sleepiness with outcomes of neuropsychological function; however, there was a significant association between worse immediate visuospatial memory and worse sleep based on the sleep composite score. These results suggest that reduced performance on neuropsychological outcomes may not be a result of individual aspects of poor sleep but rather a function of overall sleep quality; however, linear regression analyses suggest that the sleep composite score did not account for the relationship between RLS and immediate visuospatial recall. Importantly, this pilot study might not be adequately powered to estimate such relationships with precision, and future research should evaluate the relationships among RLS symptoms, sleep dysfunction, and neuropsychological outcomes in a larger sample of adults with MS who have RLS.
There are important limitations to consider when interpreting these results. The small sample limited the power for detecting smaller correlations and the generalizability of the results. The cross-sectional design precludes any inferences of causality between RLS and cognitive function. The provision of longitudinal analyses of these relationships may provide additional insight into causality. There were no adjustments for multiple comparisons in this exploratory study, which may increase the likelihood of false alarms given the high number of correlations. A large percentage of the sample was taking medications that could mitigate (41%) or exacerbate (32%) RLS symptoms, and this could influence the associations between RLS and neuropsychological outcomes. Adverse effects of these medications on cognitive function are not well understood. Future research should consider comparing groups based on medication use as well as those who are untreated because medication could have secondary effects on cognition. The outcomes related to RLS symptoms were self-reported; thus, future research should evaluate objective measures of RLS symptoms, such as electromyography or actigraphy, in the relationship between RLS and cognitive function. Outcome measures of other symptoms and consequences of MS that may be related to both RLS and cognition (eg, sleep, fatigue, depression5,29–32) were not included. Neither participants nor researchers were blinded regarding the study design and outcomes, although the main study was designed to examine the validity of the Suggested Immobilization Test rather than to focus on RLS severity and cognition.
This pilot study was the first study to examine the relationship between RLS symptoms and neuropsychological outcomes in persons with MS. These results suggest that those with more severe RLS, including worse IRLS total severity and worse symptoms at falling asleep and during the day while active, may experience worse cognitive function, particularly slower processing speeds and more memory difficulties. Future research should examine (1) whether these relationships are present over time in a larger sample of adults with MS and RLS and (2) whether managing symptoms of RLS improves neuropsychological function, as treatment of RLS might offer new opportunities for managing cognitive dysfunction in adults with MS.
PRACTICE POINTS
» In this study examining the relationships between restless legs syndrome (RLS) symptoms and cognitive function in adults with multiple sclerosis and RLS, worse overall RLS severity was associated with slower processing speed and worse memory.
» Worse RLS severity at falling asleep was associated with worse memory.
» Worse RLS severity during the day while active was associated with slower processing speed and worse memory.
References
Ning P, Hu F, Yang B, . Systematic review and meta-analysis of observational studies to understand the prevalence of restless legs syndrome in multiple sclerosis: an update. Sleep Med. 2018;50:97–104. doi: 10.1016/j.sleep.2018.05.039
Cederberg KL, Motl RW. Restless legs syndrome in multiple sclerosis: a call for better understanding and non-pharmacological management. Curr Trends Neurol. 2016;10:65–73.
Macías Islas MÁ, Ciampi E. Assessment and impact of cognitive impairment in multiple sclerosis: an overview. Biomedicines. 2019;7(1):22. doi: 10.3390/biomedicines7010022
Braley TJ, Kratz AL, Kaplish N, Chervin RD. Sleep and cognitive function in multiple sclerosis. Sleep. 2016;39(8):1525–1533. doi: 10.5665/sleep.6012
Cederberg KLJ, Jeng B, Sasaki JE, Motl RW. Restless legs syndrome, sleep quality, and perceived cognitive impairment in adults with multiple sclerosis. Mult Scler Relat Disord. 2020;43:102176. doi: 10.1016/j.msard.2020.102176
Benedict RH, Cox D, Thompson LL, Foley F, Weinstock-Guttman B, Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler. 2004;10(6):675–678. doi: 10.1191/1352458504ms1098oa
Akbar N, Honarmand K, Feinstein A. Self-assessment of cognition in multiple sclerosis: the role of personality and anxiety. Cogn Behav Neurol. 2011;24(3):115–121. doi: 10.1097/WNN.0b013e31822a20ae
O’Brien A, Gaudino-Goering E, Shawaryn M, Komaroff E, Moore NB, DeLuca J. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol. 2007;22(8):933–948. doi: 10.1016/j.acn.2007.07.002
Braley TJ, Boudreau EA. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep. 2016;16(5):50. doi: 10.1007/s11910-016-0649-2
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8
Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10(10):1097–1100. doi: 10.1016/j.sleep.2008.10.007
Walters AS, LeBrocq C, Dhar A, . Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7
Abetz L, Arbuckle R, Allen RP, . The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med. 2006;7(4):340–349. doi: 10.1016/j.sleep.2005.12.011
Kohnen R, Martinez-Martin P, Benes H, . Rating of daytime and nighttime symptoms in RLS: validation of the RLS-6 scale of restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2016;20:116–122. doi: 10.1016/j.sleep.2015.10.014
Walker LA, Osman L, Berard JA, . Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): Canadian contribution to the international validation project. J Neurol Sci. 2016;362:147–152. doi: 10.1016/j.jns.2016.01.040
Corfield F, Langdon D. A systematic review and meta-analysis of the Brief Cognitive Assessment for Multiple Sclerosis (BICAMS). Neurol Ther. 2018;7:287–306. doi: 10.1007/s40120-018-0102-3
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444
Trochim WM, Donnelly JP. The Research Methods Knowledge Base . Atomic Dog; 2006.
Field A. Discovering Statistics Using SPSS . SAGE Publications Ltd; 2009.
Gravetter F, Wallnau L. Essentials of Statistics for the Behavioral Sciences . Wadsworth; 2014.
Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2010;16(1):6–16. doi: 10.1017/S1355617709990750
Cohen J. Statistical Power Analysis for the Behavioral Sciences . Lawrence Earlbaum Associates; 1988.
Medications. Restless Legs Syndrome Foundation. Accessed May 28, 2018. https://www.rls.org/treatment/medications
Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9(4):776–790. doi: 10.1007/s13311-012-0139-4
Beier M, Gromisch ES, Hughes AJ, . Proposed cut scores for tests of the Brief International Cognitive Assessment of Multiple Sclerosis (BICAMS). J Neurol Sci. 2017;381:110–116. doi: 10.1016/j.jns.2017.08.019
Fulda S, Beitinger ME, Reppermund S, Winkelmann J, Wetter TC. Short-term attention and verbal fluency is decreased in restless legs syndrome patients. Mov Disord. 2010;25(15):2641–2648. doi: 10.1002/mds.23353
Kim SM, Choi JW, Lee C, . Working memory deficit in patients with restless legs syndrome: an event-related potential study. Sleep Med. 2014;15(7):808–815. doi: 10.1016/j.sleep.2014.03.010
Wagner ML, Walters AS, Fisher BC. Symptoms of attention-deficit/hyperactivity disorder in adults with restless legs syndrome. Sleep. 2004;27(8):1499–1504. doi: 10.1093/sleep/27.8.1499
Hughes AJ, Bhattarai JJ, Paul S, Beier M. Depressive symptoms and fatigue as predictors of objective-subjective discrepancies in cognitive function in multiple sclerosis. Mult Scler Relat Disord. 2019;30:192–197. doi: 10.1016/j.msard.2019.01.055
Giannaki CD, Aristotelous P, Stefanakis M, . Restless legs syndrome in multiple sclerosis patients: a contributing factor for fatigue, impaired functional capacity, and diminished health-related quality of life. Neurol Res. 2018;40(7):586–592. doi: 10.1080/01616412.2018.1454719
Kaminska M, Kimoff RJ, Schwartzman K, Trojan DA. Sleep disorders and fatigue in multiple sclerosis: evidence for association and interaction. J Neurol Sci. 2011;302(1–2):7–13. doi: 10.1016/j.jns.2010.12.008
Hornyak M, Kopasz M, Berger M, Riemann D, Voderholzer U. Impact of sleep-related complaints on depressive symptoms in patients with restless legs syndrome. J Clin Psychiatry. 2005;66(9):1139–1145. doi: 10.4088/jcp.v66n0909
FINANCIAL DISCLOSURES: The authors declare no conflicts of interest.
FUNDING/SUPPORT: This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (grant F31HD097903).
DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no involvement in the study design; data collection, analysis, or interpretation; writing of the report; or decision to submit the article for publication.
PRIOR PRESENTATION: Aspects of this study have been presented in abstract form at the Virtual Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC); May-June 2020.







