Publication
Research Article
International Journal of MS Care
Effect of Vascular Comorbidity on Visual and Disability Outcomes in a Secondary Progressive Multiple Sclerosis Clinical Trial Cohort
Author(s):
ABSTRACT
BACKGROUND
Vascular comorbidity (VC) is associated with multiple sclerosis (MS) disease progression and visual dysfunction. The longitudinal effect of VC in people with secondary progressive MS (SPMS) is unclear. This study explored the impact of VC on standard clinical, MRI, and visual outcomes in people with SPMS enrolled in a clinical trial.
METHODS
Data were extracted from a 2-year randomized controlled trial (N = 51) testing the supplement lipoic acid in people with SPMS who underwent annual Expanded Disability Status Scales, Timed 25-Foot Walk tests, MRIs, visual acuity testing, and retinal nerve fiber layer (RNFL) and ganglion cell/inner plexiform layer (GCIPL) thicknesses per optical coherence tomography (OCT). Post hoc linear mixed-effects regression analysis compared baseline and annualized outcomes between participants without VC (VC−) and with 1 or more VCs (VC+) (hypertension, dyslipidemia, obesity, diabetes, peripheral or cardiovascular disease, tobacco use).
RESULTS
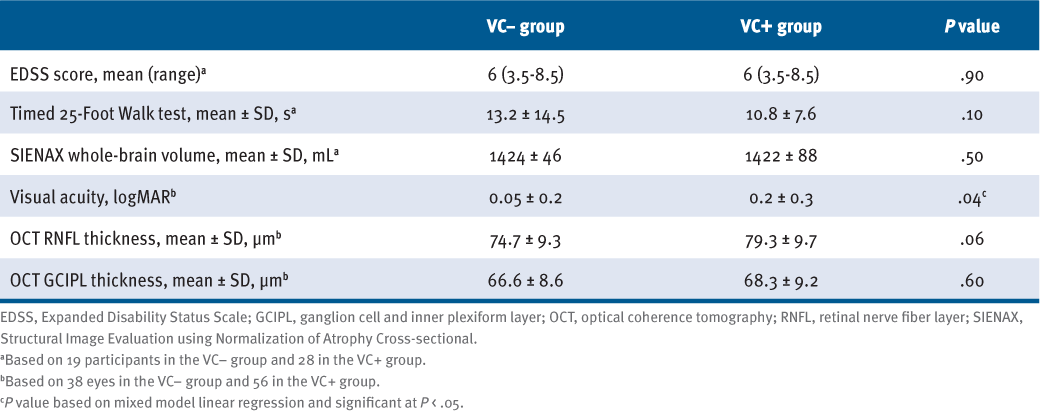
The VC− (n = 19) and VC+ (n = 28) participants were similar in age, sex, and MS disease duration and had comparable MS disability, mobility, and brain atrophy at baseline and throughout the 2-year parent study. The VC+ participants had worse baseline visual acuity than those in the VC− group by 0.13 logMAR (P = .041). No significant differences were detected in RNFL or GCIPL baseline thickness or atrophy between groups.
CONCLUSIONS
In an SPMS cohort, VC had an inconsistent effect on standard clinical, MRI, and exploratory OCT outcomes, suggesting that the effect of VC may not be evident in smaller cohort studies. Using a more refined definition of VC in future, adequately powered investigations may help effectively elucidate and account for the interaction between vascular risk burden and MS disability.
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system characterized by chronic and recurrent inflammation, demyelination, and neuronal death. It affects more than 2.5 million people worldwide and is the leading cause of nontraumatic disability in young adults in the United States.1 Repetitive insult to both the myelin sheath surrounding neurons and the axons themselves results in slowed cell signaling. Over time, this damage can lead to permanent disability in people with MS.2
Although MS is traditionally considered an autoimmune disease, researchers have recently become interested in studying modifiable risk factors of MS-related disability, including the relationship between vascular comorbidity (VC) and disease-related outcomes in MS. Vascular comorbidity, or the co-occurrence of a vascular disease risk factor(s) and another medical condition, is highly prevalent in the general population as well as in people with MS.3 Although the definition of VC varies slightly, most studies include a current diagnosis of hypertension, hyperlipidemia, diabetes, obesity, cardiovascular disease, peripheral vascular disease, or tobacco use.4,5,6 A landmark 2010 study by Marrie et al4 investigated nearly 9000 patient responses from the North American Research Committee on Multiple Sclerosis Registry and revealed that the presence of 1 or more VCs at any point in a person’s MS disease course was associated with a 1.5-fold increased risk of disability progression. More recent evidence corroborates this finding by showing associations between VC and worsening health-related quality of life,7 ambulatory disability,4 brain volume,5 and visual disability6 in people with MS.
To date, most studies investigating the role of VC in MS are observational and cross-sectional in design and rely on large databases. Despite recommendations by the investigators of these studies to consider VC as a potential effect modifier in the design of future clinical trials,6 the impact of VC on disability outcomes in clinical trial cohorts remains unknown. Lack of investigations using prospective, longitudinal data from clinical trial cohorts precludes claims of causality between VC and MS disease progression.
In this retrospective analysis of a randomized controlled trial of lipoic acid in a secondary progressive MS (SPMS) cohort, we aimed to (1) evaluate the magnitude of the effect of VC on standard disability and MRI outcomes and (2) characterize the previously unreported correlation between VC and retinal nerve fiber layer (RNFL) and combined ganglion cell and inner plexiform layer (GCIPL) thicknesses as structural markers of visual disability. Furthermore, few investigations have examined the correlation between VC and common clinical and structural outcomes in prospective MS clinical trials. In addition, no studies of any design have explored the impact of VC on visual structural outcomes, abnormalities that are highly prevalent in MS.5,8
METHODS
Study Design
The present analysis was performed using data from a 2-year, randomized, placebo-controlled, double-blind trial of lipoic acid, 1200 mg daily, in participants with SPMS; the parent trial has been described previously in detail.9 The present study was approved by the institutional review boards of Oregon Health and Science University and Veterans Affairs Portland Health Care System.
Participants
Full inclusion and exclusion criteria of participants in the parent study have been described previously.9 Briefly, participants in the parent study were 40 to 70 years old with SPMS, defined as disease progression independent of clinical relapse in the preceding 5 years and a diagnosis of relapsing-remitting MS (per 2005 McDonald criteria10). Disease progression was defined as a change in Expanded Disability Status Scale (EDSS) score or a meaningful change in function per clinical investigators. Participants were assigned to receive lipoic acid or placebo in a 1:1 block randomization pattern based on EDSS score. Participants with insulin-dependent or uncontrolled diabetes or self-reported ocular disease that could confound optical coherence tomography (OCT) interpretation were excluded from the parent study. Participants were permitted to continue glatiramer acetate or interferon beta disease-modifying therapies during the study.
The present analysis also excluded participants with OCT scans of low quality (artifact, signal strength < 7 of 10, misalignment) or those observed to have confounding findings (macular edema, glaucomatous optic nerve cupping) (n = 4) based on post hoc image analysis per OSCAR-IB criteria by a trained neuro-ophthalmologist (K.W.). Participants lacking a baseline OCT scan or at least 2 of the 3 possible scans were excluded from baseline and annualized atrophy analyses, respectively.
Vascular Comorbidity Definition
Vascular comorbidity was defined as having at least 1 of the following active diagnoses at baseline (per comprehensive medical history): hypertension, dyslipidemia, obesity (body mass index > 30), diabetes mellitus, cardiovascular disease, peripheral vascular disease, or current tobacco use. This definition represents the most commonly included diagnoses in the existing associated literature.4,5,6
Outcome Measures
Detailed protocols for acquisition of all study data at baseline, 1-year, and 2-year visits from the parent study have been described previously.9 Vascular comorbidity status was obtained at baseline via participant self-report, comprehensive electronic health record review, and medication lists. Each VC was classified as present or absent. The OCT scans of each eye were collected by spectral-domain OCT (Cirrus HD-OCT model 5000, software version 7.0.1.290; Carl Zeiss Meditec, Inc) by a trained operator. Optic Disc Cube 200 × 200 and Macular Cube 512 × 128 scans were collected by the same machine after pharmacologic dilation at each visit. High-contrast visual acuity (VA) was tested with Early Treatment Diabetic Retinopathy Study charts at 3 m and measured by logMAR. MRIs were obtained using a single Philips Achieva 3.0T X-series with the Quasar Dual gradient system (Philips Electronics North America Corp.). Cross-sectional and annualized percentage change in brain volume were estimated from 3-dimensional sagittal T1-weighted images using Structural Image Evaluation using Normalization of Atrophy. The EDSS and the Timed 25-Foot Walk (T25FW) test provided measures of MS disability and mobility, respectively.
Statistical Methods
Data were analyzed using Stata 15.1 (StataCorp LLC). Summary statistics were calculated to describe participant demographic and clinical characteristics and VC among participants. Vascular comorbidities were characterized as present or not present as severity information was not available. Mean and standard deviation are reported for continuous variables. Frequency and percentage are reported for categorical variables.
Baseline participant EDSS score, T25FW test time, brain volume, VA, and RNFL and GCIPL thicknesses were compared between groups using linear mixed-effects regression models. In addition to VC as the primary covariate of interest, these models corrected for the fixed effects of age, sex, study arm assignment, and MS disease duration. History of optic neuritis was an additional fixed effect in models of visual outcomes. Serial correlation of repeated measures accounted for a cluster-specific random effect by nesting eye measurements (left/right) within each participant. Linear model assumptions and normality of dependent variable data were confirmed using residual and Q-Q plots and indicated no need for data transformation of model outcomes.
Annualized rates of change of all outcomes during the 2-year study were calculated using similar linear mixed-effects regression models using all observations in an intention-to-treat framework. An interaction term for presence of comorbidity and study visit (baseline, 1-year, or 2-year) was included in longitudinal analyses as the covariate of interest. Coefficient estimates and standard errors were calculated using restricted maximum likelihood estimation and allowed for missing data from 1 study visit using listwise deletion. Data were assumed missing-at-random without a need for imputation. No adjustments for multiplicity were performed given the exploratory nature of the investigation. A 2-sided P < .05 was used to determine statistical significance.
RESULTS
Forty-seven of 51 participants had sufficient data for analysis (n = 4 excluded for suspected glaucoma). The demographic characteristics of participants without VC (VC−) and those with 1 or more VCs (VC+) are summarized in TABLE S1, which is published in the online version of this article at IJMSC.org.
Overall, participant groups had similar baseline demographic and clinical characteristics. The VC− and VC+ groups had comparable mean ± SD ages (56.7 ± 6.8 years vs 60.3 ± 6.2 years), MS disease durations (28.1 ± 9.7 years vs 32.2 ± 8.5 years), and median EDSS scores (6 ± 2 vs 6 ± 2.25) (Table S1). At baseline, 9 VC− (47.4%) and 12 VC+ (42.9%) participants were taking glatiramer acetate or interferon beta disease-modifying therapy (Table S1). Twelve VC− (63%) and 12 VC+ (43%) participants were randomized to receive daily lipoic acid treatment. Eight VC− (42%) and 14 VC+ (50%) participants reported a history of optic neuritis (Table S1).
The VC+ participants had a mean ± SD of 1.5 ± 0.8 VCs at baseline. Most of these individuals presented with 1 VC (19 of 28), most commonly hypertension (64%) or dyslipidemia (54%) (FIGURES S1 and S2). Fourteen percent of VC+ participants presented with obesity, 7% with type 2 diabetes mellitus, and 4% with heart disease. None had peripheral vascular disease or were current smokers (Figure S2).
At baseline, there was no statistically significant difference between the VC groups’ EDSS scores, T25FW test times, and whole-brain volumes (TABLE 1). However, there were differences in visual outcomes between the 2 groups: VC+ participants demonstrated significantly worse VA by 0.13 logMAR at baseline (P = .041) (Table 1). The RNFL thickness of VC+ participants was 5.46 μm greater than that of VC− participants (P = .056), whereas there was no statistically significant difference in average GCIPL thickness between groups.
Comparison of Baseline Standard Clinical, MRI, and OCT Outcomes Between Participants Without (VC−) and With (VC+) Vascular Comorbidity (N = 47)
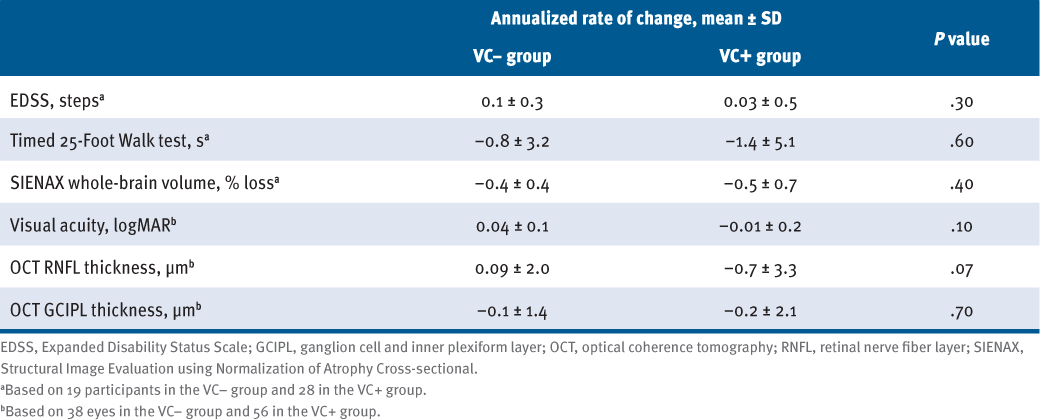
Across 2 years, there were no significant differences in annualized decline in EDSS score (P = .25), T25FW test time (P = .55), or whole-brain volume (P = .43) (TABLE 2). The RNFL thickness decreased by 0.7 μm annually in VC+ participants, whereas RNFL thickness largely remained unchanged in VC− participants (0.09 μm annually; P = .068) (Table 2). Annualized atrophy rates of GCIPL thickness (−0.1 μm vs −0.2 μm; P = .69) and VA (0.04 logMAR vs −0.01 logMAR; P = .12) did not differ between VC− and VC+ participants.
Comparison of Annualized Rates of Change of Standard Clinical, MRI, and OCT Outcomes Between Participants Without (VC−) and With (VC+) Vascular Comorbidity Using Outcomes From Baseline, 1-Year, and 2-Year Visits (N = 47)
DISCUSSION
In this secondary analysis from a randomized clinical trial of lipoic acid in SPMS, we did not detect a consistent VC impact on standard clinical, MRI, or exploratory OCT outcomes. Specifically, VC+ and VC− participants had comparable MS disability, mobility, and brain atrophy at baseline and during the 2-year parent study, even when controlling for age, sex, study arm assignment, and MS disease duration. Although VA and structural measures differed between VC− and VC+ participants, there was not a clear pattern. At baseline, VC+ participants demonstrated lower VA and greater RNFL thickness. Across 2 years, RNFL thickness declined faster in the VC+ group. No significant differences were detected in GCIPL baseline thickness or atrophy between groups.
The present finding that the presence of baseline VC did not influence MS disability, mobility, or MRI outcomes does not support the hypothesis, which was built on the existing evidence that VC is associated with worsening disability progression,4 ambulatory disability,4 and brain atrophy5 in people with MS. There are several explanations for this. The small sample size likely played a considerable role by limiting the power to detect previously established associations that used data from national databases or other large-scale sources. Using these results to guide a preliminary sample size calculation, we calculated that 200 (brain atrophy) to 4000 (OCT outcomes) participants would be needed per arm to observe a significant difference in the study outcomes using 80% power and P < .05. The power calculations suggest that the effect of VC may not be revealed in small clinical trials.
An additional explanation for these findings is the definition of VC. This investigation adopted the definition of VC that has been used in related literature. However, the severity, disease duration, and other VC disease characteristics were not taken into account in this categorization. This definition oversimplifies the complexity of VCs to a single indication, despite our knowledge that the neurovascular consequences of comorbidity can vary substantially. There is a broad spectrum of vascular pathology in any given VC (eg, hypertension) as well as between different VCs (eg, hypertension vs obesity). However, this definition of VC falsely implies that all VCs lend equal degrees of vascular pathology. Due to limited sample size, we were unable to analyze the impact of individual VCs on standard clinical, MRI, or exploratory OCT outcomes in this secondary analysis. However, a secondary analysis of the CombiRx trial found that comorbid dyslipidemia was associated with a 32% increased hazard of disease activity in MS, whereas there was no associated increase in disease activity in those with comorbid hypertension or diabetes.11 This finding supports the idea that individual VCs have variable effects on MS disease activity and disability. Thus, the working definition of VC used commonly in the literature and in this study may not be best suited to detect differences in smaller studies.
The present exploration of the relationship between VC and visual outcomes produced similarly unexpected results. We hypothesized that the presence of 1 or more VCs would be associated with thinner baseline RNFL and GCIPL and greater rates of atrophy across the study. This hypothesis was an extrapolation of previous findings by Marrie et al5 that VC increased the risk of visual disability 3-fold in people with MS and that visual disability is associated with thinner RNFL and GCIPL. Unexpectedly, we found that people with MS with VC had greater RNFL thickness but worse VA at baseline. In addition, although we did anticipate correctly that participants with VC would have greater RNFL atrophy resulting in a loss of approximately 1.4 μm over 2 years, this finding runs contrary to previous studies that show that RNFL thinning correlates with worse VA in MS.12–14 It is possible that the present results are influenced by the plateau effect of RNFL atrophy described in the parent trial,9 indicating that RNFL atrophy is nonlinear such that participants with less than 75 μm at baseline demonstrate less atrophy than those with greater than 75 μm. This plateau effect may explain the present findings that people with MS with VC concomitantly demonstrated greater mean baseline RNFL thickness and a greater rate of atrophy. Notably, as the thickest layer in the macula, the GCIPL is reported to be less prone to the plateau effect.15 Results of early studies also suggest that GCIPL thickness demonstrates better correlation with visual and overall disability than RNFL thickness in people with MS.15,16 These studies suggest that compared with the GCIPL, the rate of RNFL atrophy may be less meaningful as a marker of neurodegeneration, particularly in those with thin layers at baseline. Thus, we suggest caution when interpreting the RNFL findings in the present study.
Study limitations, including the limited sample size and the definition of VC, have been discussed previously. In addition, participants with insulin-dependent or uncontrolled diabetes were excluded from the parent study and thus also from this secondary analysis, thereby potentially eliminating a significant and representative sector of those with a more pronounced vascular risk burden. Furthermore, participants were not monitored for development of VCs or disease-modifying therapy discontinuation during the study. This study is also limited by its retrospective nature.
The findings of this analysis provide valuable takeaways for future investigations into VC and MS disease-related outcomes. First, investigators may require a large sample size for prospective clinical trials to detect a potential effect modification by VC. As noted previously, the sample size of the cohort differs vastly from the sample sizes of previous studies of VC in MS, which assessed large-scale populations using national and international registries. In addition, future trials may consider characterizing VC more thoroughly by including disease duration and severity to more accurately account for vascular risk burden. This will help determine whether there is a dose-response relationship between vascular disease severity and MS disability. In addition, future investigations that are powered to characterize the impact of individual VCs on MS disability, as opposed to grouping all VCs together, would provide valuable and more nuanced insight into this emerging area of research. Finally, further research should investigate whether structural markers of MS disease progression, such as MRI and OCT, are sensitive or specific enough to characterize VC. Future studies may consider emerging outcome measures that may be better suited to directly measure vascular integrity. For example, OCT angiography (OCTA) is a new technique that noninvasively measures changes in the retinal microvasculature in addition to the structural data from OCT.17 Early studies show promise for using OCTA as a vascular biomarker of MS disease progression,18,19 indicating its utility in future explorations of VC in people with MS.
In summary, VC did not affect standard MS disease-related outcomes in a prospective, longitudinal clinical trial cohort of people with SPMS. Similarly, VC did not affect RNFL or GCIPL thickness cross-sectionally or longitudinally in this first correlative analysis of VC and OCT parameters in people with MS. Although cross-sectional, population-based studies suggest that VC is an important factor in MS-related disability, the present results suggest that the effect of VC may not be as evident in smaller cohort studies. Additional consideration of population-based findings of VC will be important in the design and analysis of future clinical trials. Using a more refined definition of VC in future, adequately powered investigations may help effectively elucidate and account for the interactions between vascular risk burden and MS disability.
PRACTICE POINTS
» Vascular comorbidity (VC), including hypertension, dyslipidemia, obesity, diabetes mellitus, cardiovascular disease, peripheral vascular disease, and current tobacco use, is highly prevalent in people with multiple sclerosis (MS).
» The relationship between VC and MS-related disability is critical to understand to optimally manage the growing number of people with MS with comorbidities.
» Results of population-based studies suggest that VC is an important factor in MS-related disability. The present results suggest that the effect of VC may not be as evident in smaller clinical MS cohorts.
» Using a more refined definition of VC that accounts for disease severity and duration may help effectively elucidate the interaction between vascular risk burden and MS disability in future clinical trials.
ACKNOWLEDGMENTS
We thank Andrea Hildebrand for her contribution to data analysis and interpretation.
References
GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–897. doi: 10.1016/S1474-4422(18)30499-X
Compston A, Coles A. Multiple sclerosis. Lancet . 2008: 372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7
Marrie RA, Reider N, Cohen J, . A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015;21(3):318–331. doi: 10.1177/1352458514564485
Marrie RA, Rudick R, Horwitz R, . Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–1047. doi: 10.1212/WNL.0b013e3181d6b125
Pichler A, Khalil M, Langkammer C, . The impact of vascular risk factors on brain volume and lesion load in patients with early multiple sclerosis. Mult Scler. 2019;25(1):48–54. doi: 10.1177/1352458517736149
Marrie RA, Cutter G, Tyry T. Substantial adverse association of visual and vascular comorbidities on visual disability in multiple sclerosis. Mult Scler. 2011;17(12):1464–1471. doi: 10.1177/1352458511414041
Warren SA, Turpin KVL, Pohar SL, Jones CA, Warren KG. Comorbidity and health-related quality of life in people with multiple sclerosis. Int J MS Care. 2009;11(1):6–16. doi: 10.7224/1537-2073-11.1.6
Ma S-LL, Shea JA, Galetta SL, . Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133(5):686–692. doi: 10.1016/S0002-9394(02)01337-5
Spain R, Powers K, Murchison C, . Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374. doi: 10.1212/NXI.0000000000000374
Polman CH, Reingold SC, Edan G, . Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol . 2005;58(6):840–846. doi: 10.1002/ana.20703
Salter A, Kowalec K, Fitzgerald KC, . Comorbidity is associated with disease activity in MS: findings from the CombiRx trial. Neurology. 2020;95(5):e446–e456. doi: 10.1212/WNL.0000000000010024
Fisher JB, Jacobs DA, Markowitz CE, . Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–332. doi: 10.1016/j.ophtha.2005.10.040
Alonso R, Gonzalez-Moron D, Garcea O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult Scler Relat Disord. 2018;22:77–82. doi: 10.1016/j.msard.2018.03.007
Britze J, Frederiksen JL. Optical coherence tomography in multiple sclerosis. Eye. 2018;32(5):884–888. doi: 10.1038/s41433-017-0010-2
Petzold A, Balcer LJ, Calabresi PA, . Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797–812. doi: 10.1016/S1474-4422(17)30278-8
Saidha S, Syc SB, Durbin MK, . Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve layer thickness. Mult Scler. 2011;17(12):1449–1463. doi: 10.1177/1352458511418630
Wang Y, Bower BA, Izatt JA, . In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12(4):041215. doi: 10.1117/1.2772871
Lanzillo R, Cennamo G, Criscuolo C, . Optical coherence tomography angiography retinal vascular network assessment in multiple sclerosis. Mult Scler. 2018;24(13):1706–1714. doi: 10.1177/1352458517729463
Murphy OC, Kwakyi O, Iftikhar M, . Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Mult Scler. 2020;26(7):815–828. doi: 10.1177/1352458519845116
FINANCIAL DISCLOSURES: The authors declare no conflicts of interest.
FUNDING/SUPPORT: None.







