Publication
Research Article
International Journal of MS Care
Differentiation of Neuromyelitis Optica from Multiple Sclerosis on Spinal Magnetic Resonance Imaging
In order to examine the accuracy of magnetic resonance imaging (MRI)–based diagnosis of neuromyelitis optica (NMO) versus multiple sclerosis (MS), we performed a retrospective, rater-blinded review of 29 cases of NMO and 30 cases of MS using the criteria of long (more than three vertebral levels), continuous lesions with a central cord location for NMO and more peripheral and patchy lesions for MS. Using these criteria, two raters were able to distinguish the two conditions with a good degree of confidence, particularly when the imaging was performed at the time of an acute cord attack. The sensitivity and specificity for diagnosis of NMO were 86.2% and 93.3%, respectively, for Rater A and 96.4% and 78.6%, respectively, for Rater B, with a kappa value of 0.72. Thus there are significant differences in lesion characteristics that allow the distinction on spinal cord imaging between MS and NMO with a moderately high degree of confidence. The location of the lesion as evident on MRI of the spine can be regarded as a distinguishing diagnostic feature between MS and NMO.
Neuromyelitis optica (NMO) is a demyelinating disease of the central nervous system (CNS) that meets all formal criteria for an autoimmune etiology,1 with clinical manifestations resembling those of multiple sclerosis (MS).2–4 The advent of NMO antibody has permitted clearer differentiation between NMO and MS. It has increased the accuracy of diagnosis, allowing differentiation of the two disorders in many cases where it was not previously possible.5 6 New diagnostic criteria have been developed for NMO, which have extended our understanding of the disease and its characteristics. Before the introduction of NMO antibody, the presence of cerebral lesions would change a diagnosis of NMO to MS. It is now known that the presence of cerebral demyelinating lesions on magnetic resonance imaging (MRI) does not rule out NMO. Since the introduction of NMO antibody, some patients followed for long periods with a diagnosis of MS have been found to have NMO. The differentiation of the two diseases has become increasingly important, as some treatments for MS are totally ineffective in NMO, and evidence has emerged that beta-interferons can actually make NMO worse.7
Background, Clinical Features, and Diagnostic Criteria of NMO
The coexistence of optic nerve and spinal cord dysfunction was first described by Albutt in the late 19th century. In 1894, Gault used the term neuromyélite optique aiguë (acute optic neuromyelitis) to describe 17 cases collected from the literature and personal experience by his mentor, Eugène Devic. From then on, the disorder was also known as Devic disease or Devic syndrome.8 9 The disorder consists of one or more clinical episodes of optic neuritis (ON) in combination with myelitis. These clinical events also occur commonly in typical MS; however, in NMO they are usually more acute (sometimes fulminant) and severe, raising initial diagnostic suspicion of NMO. Paraclinical measures, such as MRI of the brain and spinal cord and cerebrospinal fluid (CSF) examination, also frequently reveal findings that differ from those in typical MS. In retrospective and small prospective series, most patients with NMO have been found to have no or very few nonspecific white matter lesions on brain MRI. Spinal cord MRI also shows distinctive findings: a majority of patients have longitudinally extensive lesions covering three or more vertebral segments. Furthermore, NMO patients frequently have CSF pleocytosis of more than 50 leukocytes, with or without the presence of neutrophils.10
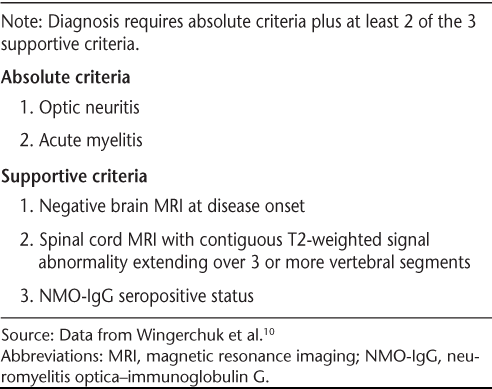
Revised diagnostic criteria for NMO proposed by Wingerchuk et al.10 are shown in Table 1. The disorder may follow either a monophasic or a relapsing course.2 In monophasic NMO, patients experience both unilateral or bilateral ON and a single episode of myelitis, typically within a very short interval. In contrast, patients with a relapsing course continue to have discrete exacerbations of ON and/or myelitis after they meet NMO diagnostic criteria.
Proposed diagnostic criteria for neuromyelitis optica
Epidemiology
Neuromyelitis optica affects young adults, as does MS, but has been reported from infancy through the ninth decade. The reported mean age of onset, especially for the relapsing type, may be greater than for typical MS. The mean onset ages were 35 and 47 years in two series of patients with relapsing NMO.11 12 Wingerchuk et al.2 reported a mean age of onset of 29 years (range, 1–54 years) for monophasic patients and 39 years (range, 6–72 years) for relapsing patients. The ratio of women to men may differ according to disease course. Most reports suggest a ratio of approximately 1.4:1 to 1.8:1; rates of 83% to 100% women have been reported in recent case series consisting predominantly of patients with a relapsing course. The incidence and prevalence of NMO are unknown. The disorder appears to be more common in nonwhites including African Americans, Japanese, and other Pacific Islanders. Demyelinating disease in Asia and India is often restricted to the optic nerves and spinal cord. Reports exist of identical twins or siblings with NMO. Despite these clues, the role of genetic factors in NMO is not known.
Materials and Methods
This was a retrospective case-control study designed to examine the accuracy of MRI-based diagnosis of NMO versus MS. A list of NMO and MS patients was generated from hospital discharge codes over a 10-year period; medical records were reviewed to determine whether the patient had received a diagnosis of NMO and whether he or she had myelopathy. The records of about 70 randomly selected MS patients were reviewed for the presence of spinal cord symptoms and at least one cord lesion on spinal MRI. All MS patients selected met McDonald criteria for the diagnosis. Of the 70 MS patients, 30 were randomly selected for the study based mainly on the availability of spinal MRI scans. Among the NMO patients, some of the antibody-negative cases were diagnosed clinically by other physicians, and we were not able to definitively determine whether they met Wingerchuk criteria for NMO. Although not all of the MS patients had NMO antibody testing, of those who were tested, nearly half (14 of 30) were antibody-negative. Neuromyelitis optica antibody status was recorded for those who had undergone antibody testing. This study was approved by the institutional review board at the University of Mississippi Medical Center.
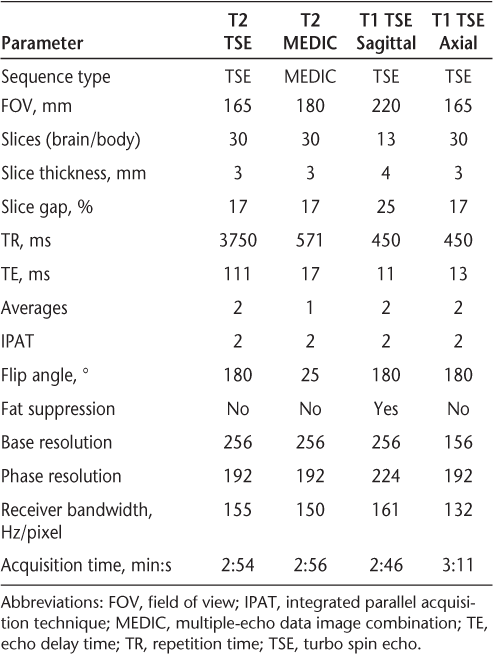
All records were checked for the availability of spinal cord imaging. Images for review were initially selected in a blinded fashion; however, because some of the images selected were remote from the time of the attack, the procedure was revised to provide the available image closest to the spinal cord attack. This revision was necessary because changes over time obscure some of the imaging findings present immediately after an acute attack. One NMO patient who had symptoms of myelopathy was rejected because there was no visible lesion in the available cord images. Another NMO antibody–positive patient's MR images could not be used because the patient did not have radiologic abnormality in the spinal cord despite some symptoms attributable to cord involvement. The scans were obtained on 1.5-T GE and Siemens MR Magnets with scan parameters of the relevant T2-weighted images as shown in Table 2.
Imaging parameters used for spinal magnetic resonance imaging
The final participants in the study were 29 patients diagnosed with NMO and 30 patients diagnosed with MS with spinal cord involvement. The spinal cord lesions were independently reviewed in a blinded fashion by two CAQ (certificate of added qualification)–certified neuroradiologists at our institution who were asked to try to determine whether the patient had MS or NMO based on imaging alone. The two raters made comments on the location, cross-sectional area of involvement, length of cord abnormality corresponding to number of vertebral bodies involved, whether continuous or discontinuous segments were involved, type of cord (cervical, thoracic, or lumbar) involvement with or without contrast enhancement, and the sequence best demonstrating the abnormality. They were then asked to make a blinded diagnosis based mainly on the criteria of centricity of the lesions and length of the segments involved. Rater B did not comment on three of the cases because he felt there was insufficient information on the MRI scans to support any diagnosis. The data were then collected and compiled, and the rater's blinded diagnosis was compared with the actual clinical diagnosis. The results were then statistically evaluated by a statistician. Sensitivity was calculated as the percentage of true NMO cases diagnosed as NMO by Rater A (or B); specificity was calculated as the percentage of true MS cases diagnosed as MS by Rater A (or B). Interrater agreement was measured using Cohen's kappa statistic.13
Results
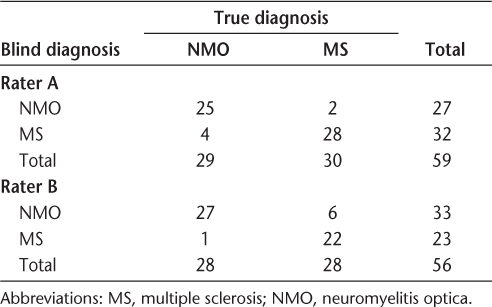
The findings of Rater A are as follows: A total of 13 of 29 NMO patients had central lesions, 4 had peripheral lesions, and the remaining 12 had both central and peripheral lesions, with a propensity toward the central location. Meanwhile, 25 of 30 MS patients had peripheral lesions, 2 had central lesions, and the remaining 3 had both central and peripheral lesions, with a propensity toward the peripheral location. The findings for Rater B are as follows: A total of 21 of 29 NMO patients had central lesions, none had peripheral lesions, and the remaining 8 had both central and peripheral lesions, with a propensity toward the central location. Meanwhile, 7 of 30 MS patients had peripheral lesions, 3 had central lesions, and the remaining 20 had both central and peripheral lesions, with a propensity toward the peripheral location.
The blinded diagnosis of NMO or MS given by the raters was based on the length of the spinal lesion (number of vertebral segments) and, most importantly, the centricity or the peripheral nature of the lesion. The statistical results are published in Tables 3A and 3B and Table 4. As shown in Table 3A, the sensitivity and specificity for diagnosis of NMO were 86.2% and 93.3%, respectively, for Rater A and 96.4% and 78.6%, respectively, for Rater B. The two raters agreed on 48 of 56 readings, yielding a kappa value of 0.72, which is good but not excellent agreement (Table 3B).
Radiologic diagnosis versus clinical diagnosis for each rater
Raters' diagnosis and judgment of location of lesions within the cord
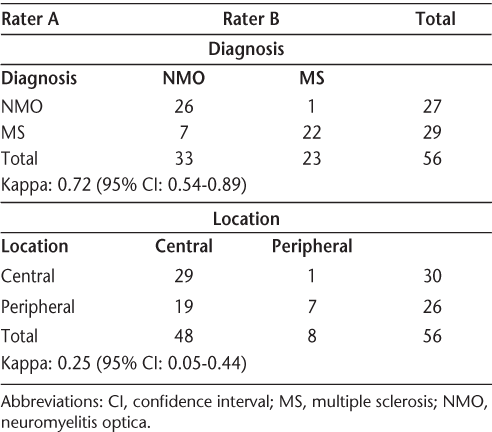
Lesion location in antibody-positive and antibody-negative NMO
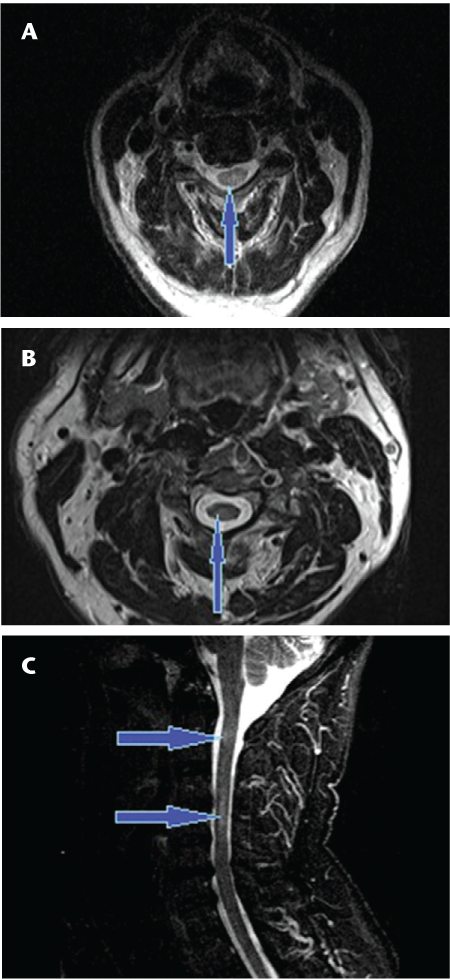
If we restrict the results to NMO antibody–positive patients as shown in Table 4, the accuracy rates are 12 of 14 for Rater A and 13 of 14 for Rater B. In these NMO cases, 12 of 14 NMO antibody–positive cases were read as having central lesions in the cord by Rater A, whereas 13 of 14 NMO antibody–positive cases were read as having central lesions in the cord by Rater B. In the case in which there was agreement between the two raters on noncentricity of the lesion, the interval between myelopathy and imaging was 8 years.
Discussion
Although NMO and MS were once thought to be different manifestations of a single autoimmune disease entity, we now know that they are different entities in many respects, and must be treated with different therapies. For instance, some authors have suggested that a standard therapy for MS (interferon beta) may actually promote NMO progression and increase relapses.14 15 Therefore, it is necessary to differentiate between the two diagnostically—specifically, between antibody-negative NMO with brain lesions and MS.
This study has several limitations indicating that the overall accuracy figures should be considered a lower limit for the accuracy of radiologic diagnosis of NMO using the criteria of longitudinal extension and central location of the cord lesion. First, the clinical diagnosis of both MS and NMO almost certainly includes some errors for both the NMO antibody–negative patients and the MS patients who had not undergone antibody testing. In several cases diagnosed as MS before NMO antibody became available, diagnosis was based on earlier criteria in which any evidence of the presence of intracerebral involvement led to a diagnosis of MS. Neuromyelitis optica antibody was available in only 33 of 59 cases. It is now known that intracerebral lesions are fairly common in NMO; thus some of the cases diagnosed as MS may in fact be NMO. Also, in some of these cases the initial diagnosis was made at another institution, and records to verify the diagnosis were not available.
In the NMO antibody–positive cases, when there was a disagreement between the two raters (in one instance), the MRI was performed 8 years after the occurrence of the patient's acute transverse myelitis. No MRI performed close to the time of the myelitis was available. Because the changes that occur in MRI after the acute phase of the illness often obscure the initial changes, we would expect better accuracy with MRI performed during or immediately after the attack.
Long-segment linear T2-hyperintense lesions in the spinal cord have been shown to be characteristic of NMO myelopathy.16 17 Additionally, in our experience the typical lesions are symmetrical and centrally located in the cord (Figures 1 and 2). Those seen in MS myelopathies are usually less extensive on cross-sectional imaging and are typically asymmetrical and located at or near the periphery of the cord (Figure 3).
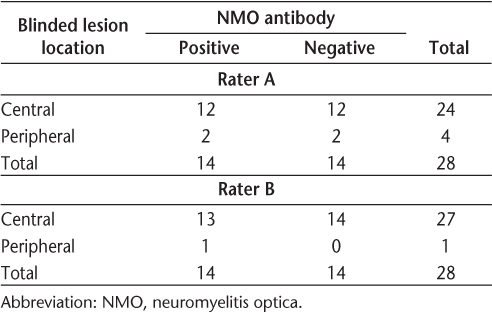
Axial T2 fast spin echo (A) and axial T2 gradient (B) images of the cervical cord showing central T2 hyperintensity (arrows) in neuromyelitis optica involving central portions of the spinal cord with signal changes involving more than 50% of the cross-sectional area
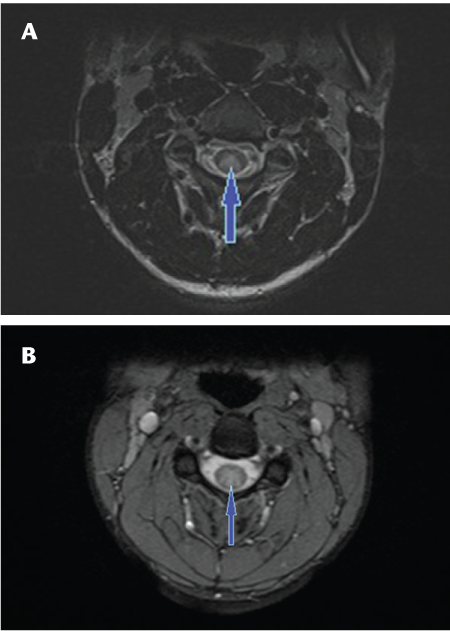
Sagittal T2 fast spin echo images of the cervical (A, B) and thoracic (C) spine showing continuous long-segment linear T2 signal hyperintensity (arrows) involving the spinal cord extending from the cervicomedullary junction to the T8-9 level with predominantly central involvement of the cord in a patient with neuromyelitis optica
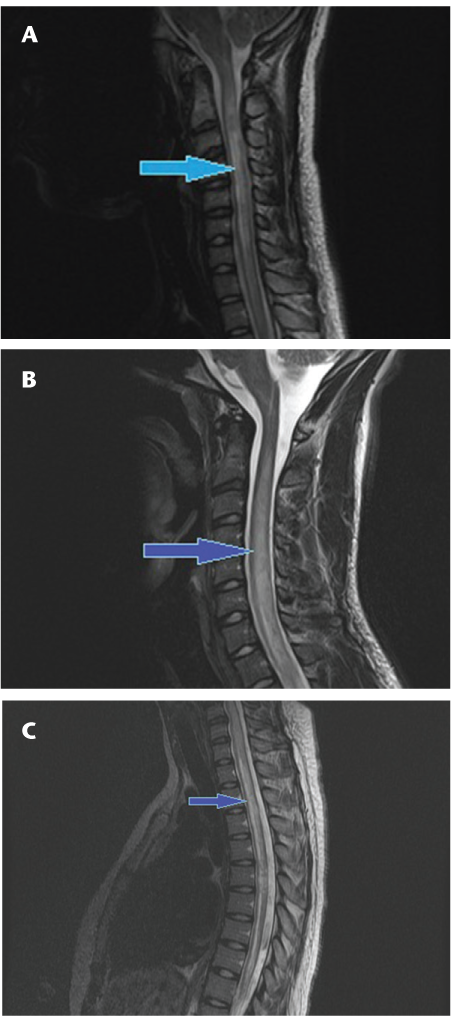
Axial T2 fast spin echo (A, B) and sagittal short tau inversion recovery (STIR) (C) sequences showing the typical peripheral signal changes on axial images (A, B) and discontinuous high T2 signal (arrows) involving short segments of the spinal cord (C) in a patient with multiple sclerosis
The central localization of the lesions in NMO and the peripheral localization of the vast majority of the lesions in MS have some interesting implications related to disease mechanism. In MS, the peripheral localization along with the recent emphasis on cortical lesions that are present early in the disease course suggests that the CSF is somehow involved in initiating the peripheral lesions. On the other hand, the central localization in NMO and the preferential localization of lesions within the spinal cord suggest possible differences in blood flow, in the concentration of aquaporin-4 around the blood vessels, or in the blood-brain barrier in the cord.
While NMO antibody has helped us to define the disease and distinguish it from MS, it is clear that there are many antibody-negative cases that are otherwise typical both clinically and in terms of imaging characteristics. Whether another, as yet unidentified antibody is involved or a different immunologic mechanism is involved remains to be determined. However, we believe that the clinical picture and the imaging characteristics discussed in this article are sufficient to identify the condition as distinct from MS, and experience suggests that the antibody-negative cases respond better to the immunosuppressive approach used in antibody-positive NMO than to typical MS medications. We believe that the imaging features characteristic of NMO and discussed in this article may be sufficient to define the disease and thus call for a treatment program different from that typical for MS.
Conclusion
Significantly different lesion parameters allow the distinction on spinal cord imaging between MS and NMO with a moderately high degree of confidence. The location of the lesion as evident on MRI of the spine can now be considered a distinguishing diagnostic feature between the two disorders. Certainly, MRI examination performed as we have described seems to be specific and sensitive for an NMO diagnosis. When MRI is performed acutely, the characteristics we have described may have even better interrater reliability for the diagnosis of NMO than we found in this retrospective series. Larger-scale studies in patients who are NMO antibody–positive or NMO antibody–negative may reveal greater sensitivity and specificity.
PracticePoints
On spinal magnetic resonance imaging, neuromyelitis optica (NMO) is strongly suggested by acute continuous longitudinal lesions covering three or more vertebral levels, while MS is suggested by patchy lesions that are rarely continuous over more than one vertebral segment.
In NMO, spinal cord lesions tend to be centrally located, rarely extending to the surface of the cord, whereas in MS such lesions are usually located peripherally.
Chronic cord lesions in NMO often change over time, becoming patchier in appearance, making these distinguishing criteria less applicable to older lesions.
References
Rodriguez M. Have we finally identified an autoimmune demyelinating disease? Neurology. 2009; 66: 572–573.
Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999; 53: 1107–1114.
Cree BAC, Goodin DS, Hauser SL. Neuromyelitis optica [review]. Semin Neurol. 2002; 22: 105–122.
Wingerchuk DM. Neuromyelitis optica: current concepts. Front Biosci. 2004; 9: 834–840.
Weinshenker BG, Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA. NMO-IgG: a specific biomarker for neuromyelitis optica. Dis Markers. 2006; 22: 197–206.
Paul F, Jarius S, Aktas O, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007; 4:e133.
Palace J, Leite MI, Nairne A, Vincent A. Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010; 67: 1016–1017.
Gault F. De la neuromyélite optique aiguë [thesis]. Lyon; 1894.
Devic C. Myélite subaiguë compliquée de nevrite optique. Bull Med. 1894; 35: 18–30.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006; 66: 1485–1489.
Mandler RN, Davis LE, Jeffery DR, Kornfeld M. Devic's neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol. 1993; 34: 162–168.
O'Riordan JI, Gallagher HL, Thompson AJ, et al. Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry. 1996; 60: 382–387.
Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 2006; 20: 37–46.
Warabi Y, Matsumoto Y, Hayashi H. Interferon beta-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J Neurol Sci. 2007; 252: 57–61.
Tanaka M, Tanaka K, Komori M. Interferon-beta (1b) treatment in neuromyelitis optica. Eur Neurol. 2009; 62: 167–170.
Bizzoco E, Lolli F, Repice AM, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol. 2009; 256: 1891–1898.
Krampla W, Aboul-Enein F, Jecel J, et al. Spinal cord lesions in patients with neuromyelitis optica: a retrospective long-term MRI follow-up study. Eur Radiol. 2009; 19: 2535–2543.
Financial Disclosures: The authors have no conflicts of interest to disclose.







