Publication
Research Article
International Journal of MS Care
Step-Length Variability in Minimally Disabled Women with Multiple Sclerosis or Clinically Isolated Syndrome
Gait is one of the most frequently impaired bodily functions in multiple sclerosis (MS). Determining abnormal parameters of gait in early MS could influence MS treatment and rehabilitation. The purpose of this study was to determine whether increased step-length variability could be detected in minimally disabled patients with MS or clinically isolated syndrome (CIS) using a sensored walkway gait analysis system. Nine participants with MS/CIS and nine age- and gender-matched controls were recruited for this study. MS/CIS participants underwent a neurologic examination, and all participants completed a screening interview. Each participant completed three walks at a self-selected pace and three walks at a brisk pace across the GAITRite walkway (MAP/CIR Inc, Havertown, PA). Mean values for step-length variability, step length, and velocity were calculated for each participant's self-selected and brisk trials. Independent t tests were used to compare MS/CIS participants with controls, and effect sizes were calculated. Step-length variability in the left leg at the self-selected pace was found to be greater in participants with MS/CIS than in controls, although no significant differences were found in velocity or step length. Step-length variability measurement shows promise in detecting subtle gait dysfunction. Larger, prospective studies exploring step-length variability may determine its clinical viability for detecting subtle gait dysfunction and could lead to improved prognostication of disability progression in MS.
The execution of gait involves a complex interaction between motor and sensory systems frequently affected in multiple sclerosis (MS). Neurologic deficits of the lower limbs leading to gait dysfunction, including weakness, spasticity, and ataxia, are among the most common neurologic symptoms of MS.1 Patients with MS perceive lower-limb function to be among the most highly valued and most frequently impaired bodily functions.2 In the search for improved outcome measures in MS, the Timed 25-Foot Walk has been recommended as part of a composite assessment.3 A limitation of the Timed 25-Foot Walk is that it may not be a particularly sensitive measure in early disease.4 5 Quantitative gait analysis allows the measurement of other parameters of gait, which may be more sensitive to subtle change and could influence MS treatment and rehabilitation decision making.6
Gait analysis includes the measurement of the movement of the body in space (kinematics) and the forces involved in producing the movement (kinetics). Prior research investigating kinematic parameters of gait in MS has found decreased gait velocity,1 7–11 cadence, step length, and stride length1 7–9 and increased time spent in the double limb support phase of the gait cycle.1 6 8 Also reported are abnormalities in the joint angles of the hip, knee, and ankle1 8 12 and kinetic abnormalities demonstrated by altered patterns of electromyography (EMG) activity during regular gait.1 8 13
Of the multitude of abnormal gait parameters reported in MS, those that are most reliable and sensitive to subtle, clinically meaningful change may be of greatest value. Parameters related to gait variability may show promise in meeting these criteria. Regulation of the consistency of gait is not entirely understood, but it is thought to be automated, requiring little cognitive input.14 Crenshaw and colleagues11 described increased variability of the hip, knee, and ankle joint angles during regular gait in a sample of 20 individuals with MS compared with healthy controls. The mean ± SD Expanded Disability Status Scale (EDSS) score was 3.1 ± 1, suggesting the occurrence of increased joint angle variability even when disability is in the moderate range. The reliability of the findings was demonstrated by a similar increase in joint angle variability under fatigued conditions later the same day. The increased joint angle variability in this study was present despite a lack of a significant increase in kinetic variability (joint force variability) between the MS participants and controls. The findings may suggest that increased kinematic variability is a more subtle abnormality of gait, occurring prior to the onset of increased kinetic variability. Increased kinematic variability has also been associated with cognitive decline and the development of dementia in non-demented older adults, suggesting prognostic value.14 In the presence of subclinical central nervous system disease, automation regulating gait consistency may be disrupted early.
Increased kinematic variability may also be of functional significance in terms of predicting falls. Guimares and Isaacs15 compared kinematic gait parameters between older adult hospitalized fallers and nonfallers. Individuals with neurologic disease, fractures, or abnormalities of gait on clinical examination were excluded from the study. Among the kinematic gait parameters reported, greater variability in step length was one of the most clearly defined outcomes distinguishing the group of fallers from the nonfallers. Step length is the distance between the heel point of the current (eg, left) footfall to the heel point of the previous (eg, right) footfall.
A full traditional gait laboratory is not required to measure step-length variability. Without attaching any devices to the individual, step-length variability can be measured with a portable, sensored walkway system connected to a computer. From the captured footfalls, the coefficient of variability for step length may be calculated in real time. This system may be advantageous over gait laboratory analysis, which is more cumbersome, time-consuming, and costly.
To our knowledge, there are no reports in the literature specifically addressing step-length variability in people with MS with minimal disability compared with controls. With the use of a portable sensored walkway system, the aim of this pilot study was to determine whether abnormal step-length variability occurs in people with MS compared with age-matched controls. We hypothesized that step-length variability would be increased in minimally impaired participants with MS or clinically isolated syndrome (CIS) compared with controls.
Methods
Participants
Volunteer control, CIS, and MS participants were recruited from the Saskatoon MS Clinic. Inclusion criteria consisted of one attack of neurologic disturbance lasting a minimum of 24 hours strongly suggestive of a demyelinating event (CIS) or a diagnosis of MS according to the McDonald 2005 revised diagnostic criteria.16 Exclusion criteria included arthritis, orthopedic conditions, heart and lung disease, neurologic disease other than MS, and abnormal gait on clinical examination. For the controls, additional exclusion criteria were any neurologic disease and having a first-degree relative with MS. Controls with first-degree relatives with MS were excluded to minimize the chances of controls having subclinical gait impairment associated with undiagnosed MS, as there is a 20- to 40-fold increase in lifetime risk of MS among first-degree relatives of people with MS.17 For the MS/CIS group, exclusion criteria also included an EDSS score18 of 3.5 or greater and a neurologic diagnosis other than MS. Normal controls were age- (±2 years) and gender-matched to the MS/CIS group. The study was approved by the University of Saskatchewan biomedical research ethics board.
Measures
Step-length variability (step-length standard deviation; cm), step length (cm), and velocity (cm/s) were evaluated using an electronic 10-m walkway system (GAITRite, version 3.8; MAP/CIR Inc, Havertown, PA).
Procedure
Participants with CIS or MS underwent a neurologic examination followed by a screening interview. Control participants completed an abbreviated screening interview. All testing was performed in stocking feet to control for any variability associated with footwear. Participants did not have any practice walks on the walkway prior to their trials. Participants had a lead-in and lead-off of 2 m at either end of the mat. For the first three walks, they were instructed to walk at their most comfortable self-selected walking pace. For the next three walks, they were instructed to walk as fast as safely possible without running. All participants were given the same cues for conducting these trials. All walking trials were conducted on the same day for each individual. A 2-minute seated rest was scheduled after the completion of the three self-selected paced walking trials and before the start of the three brisk walking trials. Otherwise, the walking trials occurred consecutively with no rest periods.
Statistical Analysis
Mean values for step-length variability, step length, and velocity for the three self-selected pace trials and three brisk pace trials were calculated. For each walk, the measurement of the temporospatial gait parameters started with the first full footfall and ended with the last footfall detected on the walkway. For the main analyses, in order to compare step-length variability between the MS/CIS and control groups, independent-samples t tests were run using SPSS (version 19.0; SPSS, Chicago, IL). Effect size calculations were performed using the following formula: Cohen's d = (Mean 1 – Mean 2)/standard deviationpooled; where standard deviationpooled = [(standard deviation1 + standard deviation2)/2].19 Secondary analyses were conducted in order to evaluate alternate explanations for any findings in the main analyses. In order to compare step length and velocity between groups, independent-samples t tests were run. These analyses also involved SPSS (version 19.0). P values of ≤.05 were considered to indicate statistical significance.
Results
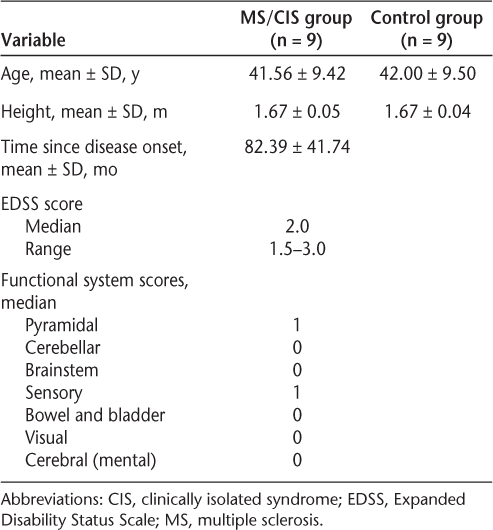
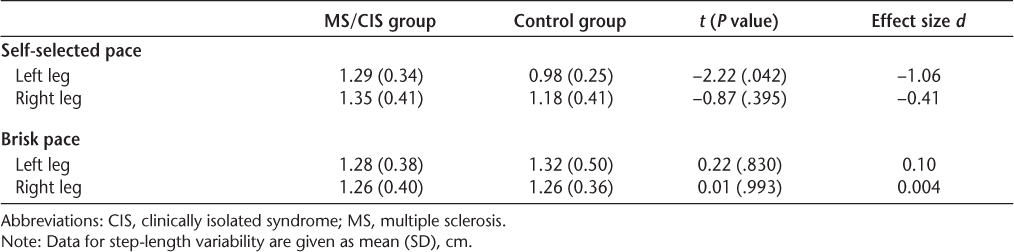
Eighteen females participated in the study: nine in the MS/CIS group and nine in the control group. Participant demographics are displayed in Table 1. In terms of main analyses, a statistically significant difference was found between the two groups for step-length variability in the left leg at the self-selected pace, with the MS/CIS group showing significantly greater variability than the control group. The effect size for this difference was large (Table 2).
Demographics of study participants
Step-length variability results
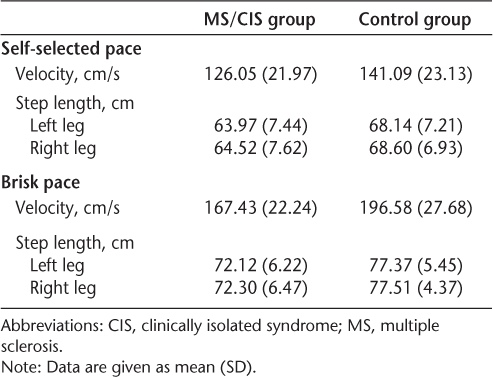
Despite the low EDSS scores, five of the nine MS/CIS participants already had predominantly left-sided lower-limb impairments in strength, tone, sensation, coordination, and/or upper motor neuron signs; three had bilateral impairments; and only one had no lower-extremity impairments on neurologic examination. Participants averaged eight left footfalls (8.26 MS/CIS; 7.70 control) for the self-selected pace and seven left footfalls (7.26 MS/CIS; 6.44 control) for the brisk pace to traverse the walkway. Mean velocities and step lengths appear in Table 3. The MS/CIS group walked significantly more slowly for the brisk pace trials compared with the controls (P = .025). There was no significant difference in speed between groups for the self-selected pace trials (P > .05). There were no significant differences in step length at either speed (P > .05).
Velocity and step length results
Discussion
Our results partially support our hypothesis that step-length variability is increased in people with MS/CIS with minimal disability compared with healthy controls. At a self-selected walking pace, step-length variability was greater in the MS/CIS group than in the healthy control group despite the fact that the self-selected walking velocities were similar. In healthy controls, it has been shown that step-length variability is influenced by walking speed.20 Sekiya and colleagues20 found that step-length variability followed a U-shaped curve with the minimum amount of variability generally observed at preferred walking speeds. Interestingly, for the brisk walk trials in the present study, step-length variability did not appear to increase from that in the self-selected pace trials in the MS/CIS group, but it did appear to increase in the control group, as might be expected with a U-shaped curve. Our findings must be interpreted with caution given the small sample size of this pilot study. It is possible that step-length variability at preferred walking speeds and change in expected step-length variability with changes in gait velocity are altered in MS.
Previous research has found decreased gait velocity in people with MS compared with controls at self-selected walking speeds.1 7–9 In our study, velocities were not significantly different for the self-selected walking trials between participants with MS/CIS and controls. The discrepancy between our findings and those of previous studies may be partly explained by the instructions given to our participants. In order to minimize perceived performance expectation effects for the self-selected paced trials, participants were specifically instructed to walk at the pace most comfortable for them. As would be anticipated, differences in velocity were readily apparent when participants were asked to perform at their maximum walking velocities.
The lack of significant difference between groups for velocity at the self-selected walking pace may also be explained by our small sample size. Despite the small sample size, step-length variability on the left side was significantly greater in the MS/CIS sample for the self-selected pace trials. It is possible that this finding is a spurious one. However, the effect size was large, suggesting that increased step-length variability at a self-selected walking speed may be a sensitive marker of early, subtle gait abnormality in people with minimal disability.
Step-length variability is also influenced by the number of steps used to calculate the variability coefficients; larger numbers of steps yield greater accuracy. Step-length variability may have been overestimated in this study in both groups because each walk trial involved a relatively small number of footfalls. The accuracy of our estimates is improved by employing mean values of three trials at each speed, but a longer sensored walkway capturing more footfalls is recommended for calculating variability coefficients.
Because options for early disease intervention exist and axonal damage may begin before any clinical signs of MS are evident,21 improved and conveniently captured subclinical markers of disease may be helpful. Although our study is the first to report increased step-length variability in people with MS with minimal disability, we do not know if step-length variability might be increased before the onset of any neurologic impairment on examination. In our study, all but one participant had some form of minimal lower-extremity impairment, with the left side more frequently affected.
Gait analysis has previously revealed other abnormal gait parameters in study samples in which participants had minimal abnormal neurologic findings,7 8 but details concerning gait parameters involving MS study samples with normal neurologic examination results are surprisingly lacking. It remains to be determined if a particular gait parameter may be especially sensitive to subclinical disease activity and whether this could be easily recorded at a bedside assessment. In a small sample of MS patients with no pyramidal involvement, but still with other mild abnormal findings on examination, Martin et al.8 reported differences in stride length, EMG activity of the tibialis anterior and medial gastrocnemius, and ankle joint angles compared with controls. A potential technical advantage of step-length variability measurement is that no EMG recordings or attachments to the participant are required.
Our preliminary data suggest that step-length variability at a self-selected walking speed is increased in female MS patients with minimal disability. The increased step-length variability may be explained by the minimal impairment observed on neurologic examination. We are not able to draw conclusions from our study regarding the sensitivity of altered step-length variability in the absence of neurologic findings. Future prospective and longitudinal studies should aim to explore parameters of gait over the course of the disease and include larger samples of individuals with normal neurologic examination findings. It is possible that subtle abnormalities of gait detected even before the onset of neurologic impairment on examination could improve early disability prognostication and influence treatment decision making.
PracticePoints
Increased step-length variability may be a sign of early gait dysfunction.
Gait analysis detected increased step-length variability at submaximal walking speeds in people with MS without gait dysfunction on physical examination.
Future study may determine the assessment feasibility and the prognostic value of subclinical gait abnormalities in MS.
Acknowledgments
The authors are grateful to Dr. Larry Brawley for expertise and use of the Russ Kisby Physical Activity and Health-Promotion Laboratory, Drs. Walter Hader and Chris Voll for assistance with recruitment, and Kit Beyer for technical expertise.
References
Benedetti M, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler. 1999; 5: 363–368.
Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold S. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008; 14: 988–991.
Polman C, Rudick R. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010; 74: S8–S15.
Goldman M, Marrie R, Cohen J. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2007; 14: 383–390.
Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000; 6: 286–290.
Gianfrancesco M, Triche E, Fawcett J, Labas M, Patterson T, Lo A. Speed- and cane-related alterations in gait parameters in individuals with multiple sclerosis. Gait Posture. 2011; 33: 140–142.
Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture. 2009; 29: 138–142.
Martin C, Phillips B, Kilpatrick T, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006; 12: 620–628.
Morris M, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002; 72: 361–365.
Thoumie P, Mevellec E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002; 73: 313–315.
Crenshaw S, Royer T, Richards J, Hudson D. Gait variability in people with multiple sclerosis. Mult Scler. 2006; 12: 613–619.
Gehlsen G, Beekman K, Assmann N, Winant D, Seidle M, Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Arch Phys Med Rehabil. 1986; 67: 536–539.
Kelleher K, Spence W, Solomonidis S, Apatsidis D. The characterisation of gait patterns of people with multiple sclerosis. Disabil Rehabil. 2010; 32: 1242–1250.
Verghese J, Wang C, Lipton R, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007; 78: 929–935.
Guimares R, Isaacs B. Characteristics of the gait in old people who fall. Int Rehabil Med. 1980; 2: 177–180.
Polman C, Reingold S, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol. 2005;58:840–846.
Sadovnick A, Dyment D, Ebers G, Risch N, Group CCS. Evidence for genetic basis of multiple sclerosis. Lancet. 1996; 347: 1728–1730.
Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452.
Cohen J. A power primer. Psychol Bull. 1992; 112: 155–159.
Sekiya N, Nagasaki H, Ito H, Furuna T. Optimal walking in terms of variability in step length. J Orthop Sports Phys Ther. 1997; 26: 266–272.
Goodin D, Bates D. Review: treatment of early multiple sclerosis: the value of treatment initiation after a first clinical episode. Mult Scler. 2009; 15: 1175–1182.
Financial Disclosures: The authors have no conflicts of interest to disclose.







