Publication
Research Article
International Journal of MS Care
Depression Levels and Interferon Treatment in People with Multiple Sclerosis
Multiple sclerosis (MS) is the most common cause of neurologic disease in young and middle-aged adults, and 75% of patients are female. Nearly one in two patients with MS will experience clinically significant depression—approximately three times the prevalence rate in the general population. This study used a cross-sectional approach to examine the link between depression levels and use of interferon medications among individuals with MS. Data were collected from 694 patients of the Multiple Sclerosis Comprehensive Care Center at Holy Name Medical Center in Teaneck, New Jersey. Analysis of variance was used to compare depression scores between patients taking and not taking interferons. Regression analyses with depression scores as the dependent variable were also conducted. The Beck Depression Inventory (BDI) was reduced to a set of dimensions by principal components analysis. Two components were identified, reflecting somatic and cognitive symptoms. The results showed no significant relationship between depression levels and interferon treatment. Significant associations were observed between depression and both age and disability status, with younger, more disabled patients tending to be more depressed.
Multiple sclerosis (MS) is the most common cause of neurologic disease in young and middle-aged adults, with approximately 75% of cases occurring in females.1 Nearly one in two patients with MS will experience clinically significant depression—approximately three times the prevalence rate in the general population.1 Various theories about the relationship between MS and depression have been offered. It has been proposed that depression in MS may develop in reaction to the vicissitudes of having a chronic, progressive, incurable illness that makes the future unpredictable.2 3 Chronic activation of the immune system may also precipitate the development of depressive disorders.4 5 There is growing evidence that major depression is associated with significant elevations in circulating levels of proinflammatory cytokines. Increases in proinflammatory cytokines and inflammation may be markers for major depressive disorder.5–8 In addition, the term “sickness behavior” has been used to denote the specific psychological and behavioral components of illness that together represent a way for an organism to fight infection.6 Some of these ways of thinking and behaving overlap with clinical depression.
It has been suggested that interferon medications may also be related to increased risk for depression.2 9 Soon after the introduction of interferon beta-1b (IFNβ-1b) as a disease-modifying treatment (DMT) for MS patients in the early 1990s, concern about depression as a possible side effect emerged.10 In a study on the efficacy of IFNβ-1b in secondary progressive MS, three suicide attempts were made in the group assigned the interferon medication.11 Although interferon may inhibit cytokines and the activation of monocytes, side effects include flu-like symptoms, increased body temperature, a general feeling of illness, fatigue, headaches, muscle pain, numbness, tingling, and depression.12–14 Depressive symptoms were associated with physical worsening during interferon treatment, emotional disturbances were noted in some at the beginning of treatment, and those patients treated with interferon over a long period of time (4 years) were more emotionally reactive to transient stressors.15
Several reported studies have not found a relationship between interferon treatment and depression in MS. According to these studies, factors intrinsic to the individual are correlated with depression levels. Predictors cited include a history of psychiatric illness prior to starting interferon treatment,2 15 16 female sex,17 18 lower age,18 severity of disability,17 19 and presence of depressive symptoms at baseline (measured at the start of the investigation).19 Interferon use was not associated with depressive symptoms.
The specific aims of this study were 1) to compare the depression levels of patients taking interferon-based DMTs versus those taking noninterferon DMTs, and 2) to evaluate predictors of depression in a cross-sectional sample. The study used cross-sectional between-groups and correlational design features. Analysis of variance (ANOVA) was used for the first aim. Patients taking interferons were compared with patients taking other DMTs on Beck Depression Inventory–II (BDI-II) depression scores while controlling for appropriate covariates. Hierarchical multiple regression was used as a second approach for the first aim, as well as for the second aim.
Methods
Measures
Beck Depression Inventory–II
The BDI-II is a 21-item multiple-choice questionnaire designed to measure the presence and severity of depressive symptoms. Each item is scored from 0 to 3 in terms of intensity, with the total possible score ranging from 0 to 63.20 The BDI-II has been widely used in MS research and has shown high internal consistency among psychiatric-clinic outpatients (Cronbach α = 0.92).20 21 The instrument's Cronbach α for the MS population is also high (0.86).22 The measure has shown adequate content validity and diagnostic utility in discriminating outpatients with mood disorders from those with other disorders.20 One-week test-retest reliability is 0.93.20 The BDI has a correlation coefficient of 0.71 with respect to the Hamilton Rating Scale for Depression.21
Incapacity Status Scale (ISS)
The ISS is a 16-item, 5 point (0–4) ordinal rating scale of functional disability in MS. The ISS was developed as part of the Minimal Record of Disability in MS23 and has been widely used.24 ISS ratings were assigned by an MS specialty neurologist or nurse and extracted from the patient's medical record. A summary score across the 16 rated items was used to measure overall disability level.
Sample
Participants recruited for the study were patients attending the Multiple Sclerosis Comprehensive Care Center at Holy Name Medical Center in Teaneck, New Jersey. Consecutive patients attending the MS center for medical appointments were approached by MS center nurses or research assistants to participate in a longitudinal natural history study of depression in MS. Patients with a definite diagnosis of MS who were taking DMTs and consented to participate were included in the study. Although the majority of patients attending the center participated, the exact percentage of patients approached who participated, and the characteristics of those who refused, are unknown. The sample reported on here consisted of 694 individuals from the first cohort (Time 1) in the longitudinal study. At the time of their initial visits, patients completed informed consent forms that described the purpose of the study, the procedure, means of ensuring confidentiality, and risks and benefits for participants. The participants were not screened for cognitive impairment. The study received institutional review board approval and oversight from the Albert Einstein College of Medicine.
Procedure
Each patient completed the BDI-II. The patient's neurologist completed the ISS during the visit.
Currently prescribed antidepressants and DMTs were extracted from the medical record on the day of the visit. These DMTs included IFNβ-1a (Rebif, EMD Serono, Rockland, MA), IFNβ-1a (Avonex, Biogen Idec, Weston, MA), IFNβ-1b (Betaseron, Bayer Health-care, Berlin, Germany), glatiramer acetate (Copaxone, Teva Pharmaceuticals, Tikva, Israel), and mitoxantrone (Novantrone, Immunex, Seattle, WA). To address the first study aim, the sample was divided into two groups: those who were taking interferon medication and those who were not. In order to determine whether the data were suitable for parametric statistics, a Levene test of variance was performed, which indicated that the dependent variable was suitable for ANOVA. Next, a univariate ANOVA was conducted. The dependent variable was the total BDI score; the fixed variables consisted of gender and interferon use. ISS score and age were entered as covariates. In addition, the BDI was reduced to a set of dimensions by principal components analysis (PCA). Ultimately, two components (eigenvectors) were identified and used to produce two subscales, which were then used as dependent variables in subsequent analyses. Hierarchical multiple linear regression analysis with demographic variables, disease severity, and the two interferon groups (taking interferon medication and not taking interferon medication) as independent variables for each factor (dependent variables) was performed using two separate equations.
Results
Demographic data for the study participants are presented in Table 1. A total of 694 patients (499 women, 195 men) completed the study protocol. Because of missing data, the number of participants in the tables and in each analysis varies somewhat. The interferon group included 77 (30.1%) males and 179 (69.9%) females. The mean (SD) age for the total sample was 44.95 (9.80) years. The mean total BDI score was 13.05 (10.11) for patients taking interferon medication and 14.48 (10.55) for patients not taking interferon medication. The mean ISS score was 8.71 (5.50) for patients taking interferon medication and 9.78 (7.70) for patients not taking interferon medication.
Participant characteristics by gender (N = 694)
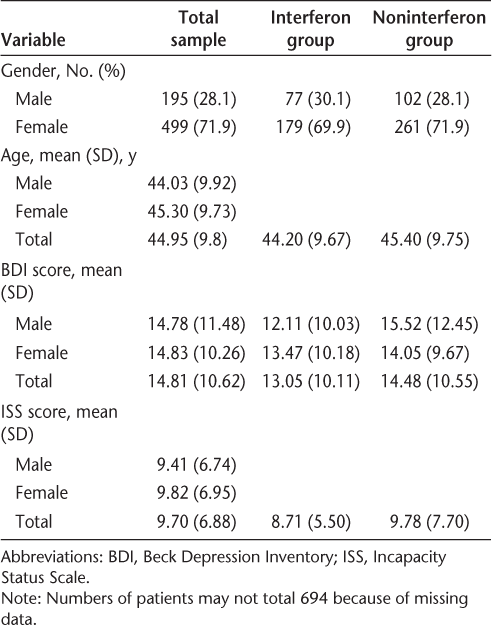
The ANOVA data are presented in Table 2. The results show that there was no difference between the two groups of patients on depression scores when controlling for age and disability level (F 2,1 = 0.42; P > .1). In addition, no difference was found in depression levels between men and women (when controlling for age and disability level), nor were there any gender × interferon group interaction effects (F 2,1 = 0.20; P > .1).
Results of analysis of variance tests of between-subjects effects
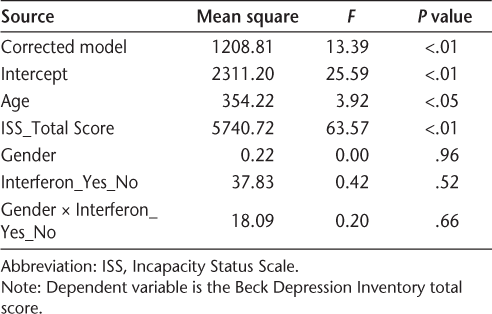
Exploratory PCA was performed on the BDI in order to determine whether there were items that grouped into different subscales/dimensions. The Kaiser-Meyer-Olkin measure of sampling adequacy was 0.946, which supports performing a PCA on the correlation matrix. An oblique (promax) rotation was used given that the components were moderately correlated (r = 0.65). The decision to extract two components was based on the eigenvalues and an examination of the scree plot.
The results of the PCA indicated that two components best described the BDI, explaining 47.9% of the total variance. A pattern matrix was used to extract loadings. Items were included in a component if the item-loading was 0.45 or higher (Table 3). Component 1 (cognitive-affective) included self-criticalness, guilty feelings, past failure, punishment feelings, self-dislike, worthlessness, sadness, suicidal thoughts/wishes, pessimism, and crying. Component 2 (somatic) included tiredness or fatigue, loss of energy, concentration difficulty, loss of interest in sex, changes in sleep pattern, indecisiveness, changes in appetite, loss of interest, and irritability. The rotated sum of squared loadings associated with component 1 (cognitive-affective) was 7.54, and that associated with component 2 was 7.04. Thus the two components are nearly equal with respect to the amount of variability explained.
Results of principal components analysis
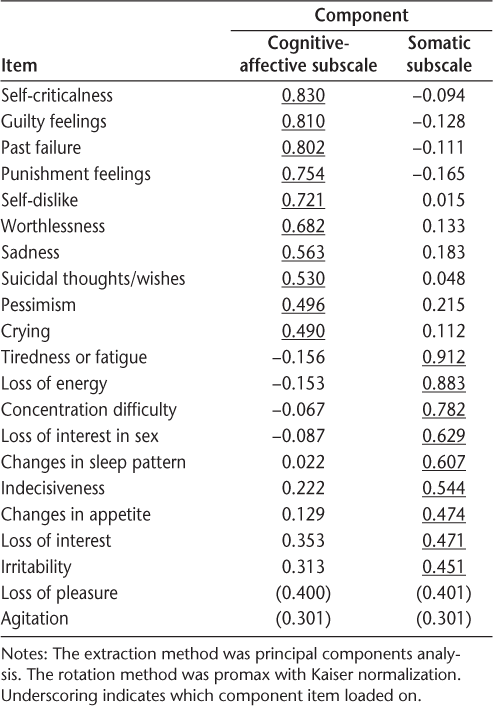
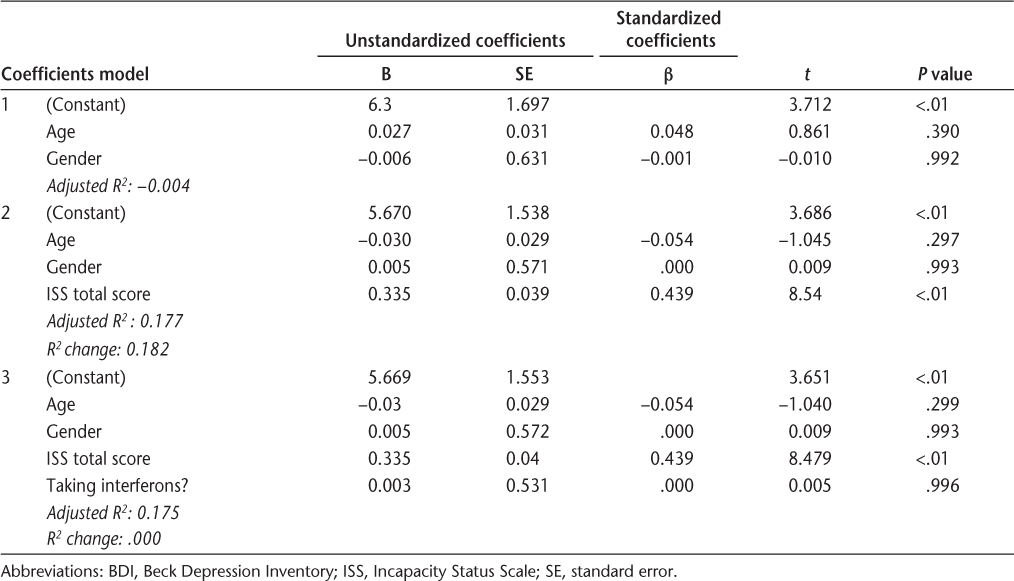
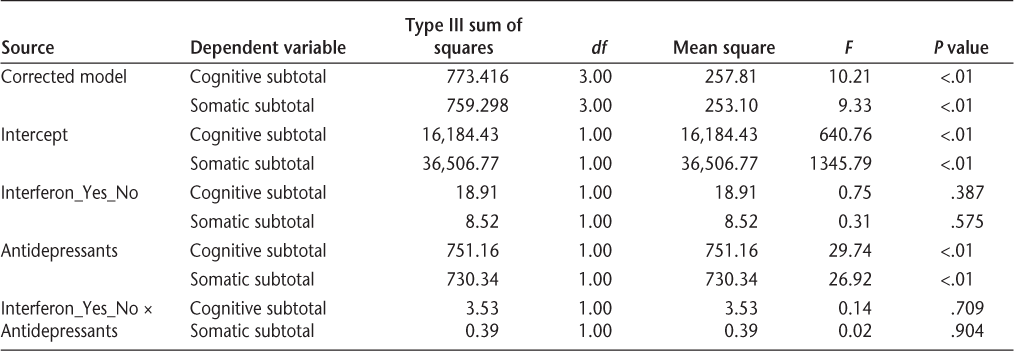
Cognitive-affective and somatic subscale items were summed to create subscale scores. The subscale scores were then used as dependent variables in two hierarchical multiple linear regressions. In the first step of each, demographic variables (age and gender) were entered. In the second step, disability (total ISS score) was entered. Group affiliation—whether the patient was taking interferon or not—was entered in the third step. The results showed that taking interferon medication was not associated with either the cognitive-affective or the somatic subscale of the BDI. Level of disability (higher ISS score) and age (younger) were predictive of higher BDI scores (Tables 4 and 5). Finally, a multivariate test was performed to assess whether the subgroup of patients taking antidepressants, who are more vulnerable to depression by implication, would be more depressed if they also took interferon medications. The BDI total scores and BDI subscales scores (cognitive-affective and somatic subscales as defined by the PCA) were entered as dependent variables; the antidepressant and interferon usage were entered as fixed factors. According to the results of the multivariate analysis (Wilks Λ = 0.99), there was no interaction effect between interferon and antidepressant use (F 2,582 = 0.197; P = .82). Taking interferon in addition to antidepressants did not significantly affect patients' total BDI score or their subscale BDI scores (Table 6). Patients taking antidepressants, regardless of whether they took interferon, were significantly more depressed than those not taking antidepressants (F 2,582 = 16.94; P < .001).
Results of BDI cognitive-affective items hierarchical linear regression

Results of BDI somatic items hierarchical linear regression
Results of multivariate analysis tests of between-subjects effects
Discussion
This study provides further support for the idea that the use of interferon medication by individuals with MS is not associated with depression. The results of both the ANOVA and regressions showed significant relationships between both age and disability and depression levels, with younger, more disabled patients being more depressed. Furthermore, there was no interaction effect between gender and interferon medication use on levels of depression: taking interferon medication was not associated with higher levels of depression regardless of whether the patient was male or female.
Principal components analysis found two components in the BDI that reflected cognitive-affective and somatic symptoms. Previous literature suggests that disease manifestations may confound BDI total scores.25 In this case, however, following an oblique rotation, results demonstrated similar strength in component 1, the cognitive-affective factor, and component 2, the somatic factor. Thus the two components are nearly equal with respect to the amount of variability explained. In addition, although the somatic subscale BDI mean score was higher than the cognitive-affective subscale mean score (7.93 vs. 5.23) regardless of whether patients took interferon, these two components were highly correlated (r = 0.692; P < .01), which strongly suggests that the cognitive-affective and somatic components defined by the PCA are measuring the same construct. Both of these findings indicate that the overall BDI score is a good measure of depression for our patients. Nevertheless, using these subscores as separate dependent variables, we performed two separate hierarchical multiple linear regression analyses. Age and gender were entered in the first step, total ISS score in the second step, and interferon group in the third step as independent variables. When controlling for demographics and disability level, there was no association between interferon medication and depression. Severity of disability (ISS scores) emerged as a significant predictor of depression.
Limitations and Future Research
In this study, depression was evaluated with the BDI-II, which is a self-report measure. Although the BDI has been widely used as a measure of depression in MS, clinical interviews by a trained mental health professional represent the “gold standard” for detecting clinically significant levels of depression. Future studies might use clinical interviews such as the Structured Clinical Interview for DSM diagnosis (SCID) to assess depression. Studies have made distinctions between depression and delusion, and they indicate that a delusionally depressed patient is five times more likely to commit suicide than a nondelusional one.26 27 One study found that delusional depressives' risk for suicide attempts was much higher than that of nondelusional depressives.28 Another report described a case of a patient receiving IFNβ-1a therapy for MS who developed acute delirium, delusion, and depression that ceased with treatment discontinuation.15 This patient had a history of depression prior to interferon therapy. The authors highlight the psychiatric vulnerability of patients receiving IFNβ-1a therapy for MS, and they suggest that this medication may induce delirium or exacerbate preexisting psychotic symptoms. Perhaps the vulnerability of MS patients to becoming depressed while receiving interferon therapy, if it indeed exists, is restricted to a small group of patients with premorbid depression with psychotic features. Future research might focus on evaluating this group to see if there are any associations between interferon therapy and vulnerability to depression.
Because the current study was cross-sectional, involving a single point in time, we were unable to test prospective alteration of depression with these patient groups. In addition, the current study was not a clinical trial. The treating neurologists made their decisions about which DMT to prescribe based on a variety of individual and medical factors. It is, however, not known what influence these preexisting factors may have had on the results of the current study. In addition, the study was conducted at a single MS center, and the characteristics of patients who refused to participate in the study were not determined or compared with the characteristics of those who participated.
PracticePoints
Depression is common in MS, with a prevalence of approximately three times that of the general population.
Although it has long been believed that MS patients with a history of or vulnerability to depression should avoid interferon medications, this study found no association between treatment with interferon medication and depression.
Depression levels were associated with lower age and higher disability scores.
Acknowledgments
The authors acknowledge the support of Rebecca Miller and Mathew Bongardino for their help in creating and maintaining the depression database used in this study.
References
Goldman Consensus Group. The Goldman consensus statement on depression in multiple sclerosis. Mult Scler. 2005; 11: 328–333.
Goeb JL, Even C, Nicolas G, Gohier B, Dubas F, Garré JB. Psychiatric side effects of interferon-beta in multiple sclerosis. Eur Psychiatry. 2006; 21: 186–193.
Wallin MT, Wilken JA, Turner AP, Williams RM, Kane R. Depression and multiple sclerosis: review of a lethal combination. J Rehabil Res Dev. 2006; 43: 45–62.
Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005; 29: 891–909.
Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Neurol Clin. 2006; 24: 507–519.
Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006; 24: 441–460.
Minor TR, Huang Q, Witt AE. Cytokine-purine interactions in traumatic stress, behavioral depression, and sickness. CNS Neurol Disord Drug Targets. 2006; 5: 547–560.
Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007; 21: 153–160.
Neilley LK, Goodin DS, Goodkin DE, Hauser SL. Side effect profile of interferon beta-1b in MS: results of an open label trial. Neurology. 1996; 46: 552–554.
IFNB Multiple Sclerosis Study Group. Interferon beta-lb is effective in relapsing-remitting multiple sclerosis: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993; 43: 655–661.
Panitch H, Miller A, Paty D, Weinshenker B; North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004; 63: 1788–1795.
Simone IL, Ceccarelli A, Tortorella C, et al. Influence of interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes. 2006; 4: 1–7.
Zivadinov R, Zorzon M, Tommasi MA, et al. A longitudinal study of quality of life and side effects in patients with multiple sclerosis treated with interferon beta-1a. J Neurol Sci. 2003; 216: 113–118.
Arnaud P. The interferons: pharmacology, mechanism of action, tolerance and side effects. Rev Méd Interne. 2002;23(suppl 4): 449S–458S.
Feinstein A, O'Connor P, Feinstein K. Multiple sclerosis, interferon beta-1b and depression: a prospective investigation. J Neurol. 2002; 249: 815–820.
Mohr DC, Likosky W, Dwyer P, Van Der Wende J, Boudewyn AC, Goodkin DE. Course of depression during the initiation of interferon beta-1a treatment for multiple sclerosis. Arch Neurol. 1999; 56: 1263–1265.
Galeazzi GM, Ferrari G, Giaroli A, Mackinnon E, Motti L, Rigatelli M. Psychiatric disorders and depression in multiple sclerosis outpatients: impact of disability and interferon beta therapy. Neurol Sci. 2005; 26: 255–262.
Patten SB, Williams JV, Metz LM. Anti-depressant use in association with interferon and glatiramer acetate treatment in multiple sclerosis. Mult Scler. 2008; 14: 406–411.
Porcel J, Río J, Sánchez-Betancourt A, et al. Long-term emotional state of multiple sclerosis patients treated with interferon beta. Mult Scler. 2006; 12: 802–807.
Beck AT, Steer RA Brown GK. The Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
Beck A, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988; 8: 77–100.
Aikens JE, Reinecke MA, Pliskin NH, et al. Assessing depressive symptoms in multiple sclerosis: is it necessary to omit items from the original Beck Depression Inventory? J Behav Med. 1999:22:127–142.
Slater RJ, Raun N. Criteria and uses of the minimal record of disability in multiple sclerosis. Acta Neurol Scand. 1984; S101: 16–20.
Pittock SJ, Mayr WT, McClelland RL, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004; 62: 601–606.
Brown-DeGagne AM, McGlone J, Santor DA. Somatic complaints disproportionately contribute to Beck Depression Inventory estimates of depression severity in individuals with multiple chemical sensitivity. J Occup Environ Med. 1998; 40: 862–869.
Roose SP, Glassman AH, Walsh BT, Woodring S, Vital-Herne J. Depression, delusions, and suicide. Am J Psychiatry. 1983; 140: 1159–1162.
Hori M, Shiraishi H, Koizumi J. Delusional depression and suicide. Jpn J Psychiatry Neurol. 1993; 47: 811–817.
Lee TW, Tsai SJ, Yang CH, Hwang JP. Clinical and phenomenological comparisons of delusional and non-delusional major depression in the Chinese elderly. Int J Geriatr Psychiatry. 2003; 18: 486–490.
Financial Disclosures: Dr. Foley has been a consultant for Bayer Therapeutics and Biogen Idec. He has been on the speakers' bureau for Bayer, Biogen, and Teva Neuroscience. He has also received grant support from Bayer. The other authors have no conflicts of interest to disclose.
Funding/Support: This study was supported in part by a grant from the Multiple Sclerosis Foundation to Dr. Foley.







