Publication
Research Article
Social Cognitive Theory and Physical Activity in Older Adults with Multiple Sclerosis
Author(s):
Abstract
Background:
The expanding population of older adults with multiple sclerosis (MS) likely experiences many of the same benefits of physical activity (PA) as younger and middle-aged adults with MS. However, participation in PA is exceedingly low in this segment of the MS population. This study examined variables from social cognitive theory (SCT) as correlates of PA in older adults with MS to inform the subsequent development of behavioral interventions.
Methods:
Older adults with MS (≥60 years of age, N = 180) completed an online survey including demographic and clinical characteristics, SCT variables (exercise self-efficacy, exercise goal setting, social support, and outcome expectations), and PA (total PA [TPA] and moderate-to-vigorous PA [MVPA]).
Results:
Bivariate correlation analyses indicated that all SCT variables were significantly associated with TPA and MVPA (all P ≤ .001). Hierarchical linear regression analyses indicated that disability status was a significant correlate of TPA (β = −0.48, R2 = 0.23) and MVPA (β = −0.44, R2 = 0.19) in step 1; disability and self-efficacy were significant correlates of TPA (disability β = −0.20, self-efficacy β = 0.59, R2 = 0.50) and MVPA (disability β = −0.16, self-efficacy β = 0.60, R2 = 0.47) in step 2; and disability, self-efficacy, and exercise goal setting were significant correlates of TPA (disability β = −0.21, self-efficacy β = 0.50, exercise goal setting β = 0.14, R2 = 0.55) and MVPA (disability β = −0.17, self-efficacy β = 0.51, exercise goal setting β = 0.15, R2 = 0.51) in step 3.
Conclusions:
These results suggest that behavioral interventions focusing on self-efficacy and exercise goal setting as targets from SCT may be appropriate for increasing PA in older adults with MS.
Of the nearly 1 million adults with multiple sclerosis (MS) in the United States, an estimated 43% are 55 years or older.1 This finding reflects a graying of the population of adults with MS that coincides with the demographic shift of adults in the general population, and it portends considerable risk of negative effects of MS and aging on walking performance2,3 and physical3,4 and cognitive2,5 function as well as health-related quality of life.6
To date, there are few options for managing the consequences of aging with MS,7 but some data support physical activity (PA) behavior as being associated with better walking performance,2,3,8 physical function,2,3 and cognitive function2 in this particular segment of the MS population. Nevertheless, the level of PA is exceedingly low among older adults with MS.9 Older adults with MS consistently engage in less moderate-to-vigorous PA (MVPA) than do younger and middle-aged adults with MS,8,10 and the MS population as a whole is considerably less physically active than is the general population of adults without MS.11 This observation supports the importance of identifying theory-based, modifiable correlates of PA behavior for informing the design of behavioral interventions in older adults with MS.
Social cognitive theory (SCT)12 represents a common approach for identifying modifiable variables as correlates of PA in those with MS.13 It posits self-efficacy as a proximal determinant of health behaviors and further posits a causal structure among self-efficacy, outcome expectations, perceived impediments/facilitators, and goal setting with health behaviors.14 Furthermore, SCT recognizes key sources or factors for targeting self-efficacy, including mastery experiences and vicarious experiences, that may translate into health behavior change. There is a wealth of cross-sectional and longitudinal data indicating that SCT variables correlate with PA in young and middle-aged adults with MS.15–20 Importantly, SCT has further informed the design and delivery of behavioral interventions that have increased PA participation in young and middle-aged adults with MS.21,22 This suggests that adopting SCT for examining correlates of PA represents an important first step in the subsequent design and delivery of behavioral interventions for older adults with MS.
The present study examined variables from SCT as correlates of PA in older adults with MS. We first examined SCT variables as correlates of total PA (TPA) and MVPA in the sample of older adults. We then examined the hierarchical associations among SCT variables and TPA and MVPA. We expected bivariate associations among SCT variables and PA outcomes and further expected that self-efficacy would be the primary correlate of PA, followed by other SCT variables, even after controlling for disability status as a confounder.
Methods
Participants
This cross-sectional study recruited participants from across the United States via an e-mail distribution from the National Multiple Sclerosis Society. Participants were invited to complete an online survey that focused on correlates of PA behavior. To be included, participants self-reported a diagnosis of MS and age of 60 years or older.
Measures
Demographic and Clinical Characteristics
Participants self-reported sex, age, race (selected from a predetermined list), type of MS, and disease duration (years). Disability status was measured using the Patient-Determined Disease Steps (PDDS) scale.23
Physical Activity
Participants completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ) as a validated self-measure of PA for persons with MS.24–27 Participants recorded the number of bouts (≥15 minutes) of mild (eg, easy walking, yoga), moderate (eg, fast walking, easy bicycling or swimming), and strenuous (eg, jogging, running, vigorous swimming or bicycling) activity completed during a typical week. The number of bouts was multiplied by weights of 3, 5, and 9 for mild, moderate, and strenuous activity, respectively, and then summed into a total leisure-time PA score (GLTEQ-total); higher scores reflect more TPA. The GLTEQ was further scored by summing the weighted values for moderate and strenuous activity only, which provided a health contribution score (GLTEQ-HCS) that corresponds with health-promoting PA (ie, MVPA); higher scores reflect more MVPA.
Self-efficacy
Self-efficacy was measured by the Exercise Self-Efficacy Scale (EXSE). The EXSE contains six items regarding one’s confidence for accumulating 30 minutes or more of MVPA on most days of the week in 1-month increments across the next 6 months.20,28 The items were rated on a scale ranging from 0 (not confident at all) to 100 (highly confident) and were averaged into a total exercise self-efficacy score, with higher scores reflecting greater self-efficacy.
Outcome Expectations
The Multidimensional Outcome Expectations for Exercise Scale (MOEES) assessed beliefs about the benefits of regular participation in exercise and PA.29 The MOEES contains 19 items across three subdomains of physical, social, and self-evaluative outcome expectations. The items were rated on a 5-point scale ranging from 1 (strongly disagree) to 5 (strongly agree), and item scores were summed, yielding a total score reflecting perceptions of benefits of regular exercise and PA.
Social Support
The abbreviated Social Provisions Scale (SPS) measured social support for PA.30,31 This version of the SPS contains six-items that measure current relationships/support for PA behaviors, and the items were rated on a 4-point scale ranging from 1 (strongly disagree) to 4 (strongly agree). The item scores were summed into a total score, with higher scores reflecting greater perceived support for PA.
Goal Setting
Goal setting for PA was measured using the ten-item Exercise Goal-Setting Scale (EGS).32 The items reflect goal-setting behaviors for PA and include statements such as “I often set exercise goals,” “I usually set dates for achieving my exercise goals,” and “I usually achieve the exercise goals I set for myself.” Items were rated on a 5-point scale ranging from 1 (does not describe) to 5 (completely describes), and the scores for each item were summed into a total score reflecting goal setting for exercise and PA engagement.
Procedures
All study procedures were approved by the University of Alabama at Birmingham institutional review board. After receiving the initial e-mail invitation that provided a brief overview of the survey, interested participants were prompted to access the questionnaire online. Participants provided informed consent and confirmed both age and MS diagnosis before accessing the survey. Participants then completed the questionnaires online using survey software (Qualtrics, Provo, UT) within 1 week of starting the research process. The use of internet-based self-report measures increases the accessibility of the survey and, therefore, the generalizability of the data. In addition, all measures included in the survey were validated for use in persons with MS. Participants who were interested in receiving compensation provided an address for receipt of remuneration ($10 USD).
Data Analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp, Armonk, NY), and descriptive statistics are presented as mean ± SD unless otherwise noted (eg, number [%]). We examined the associations between SCT variables (EXSE, EGS, SPS, MOEES) and PA (GLTEQ-total, GLTEQ-HCS) using Spearman rank-order correlations. We further examined the association between disability status (PDDS scale) and PA (GLTEQ-total, GLTEQ-HCS) because previous research has demonstrated a relationship between these two variables18 and disability might represent a confounder. The magnitude of correlations was interpreted as small, moderate, and large based on values of 0.1, 0.3, and 0.5, respectively.33 We then conducted hierarchical linear regression analyses whereby we regressed PA (GLTEQ-total and GLTEQ-HCS) on disability status (PDDS scale) in step 1, exercise self-efficacy (EXSE) in step 2, and goal setting (EGS), social support (SPS), and outcome expectations (MOEES) in step 3. We examined the β-coefficients for identifying the independent contributions of the variables in the model as well as model fit based on R2 and change in R2 (ΔR2) per step of the model.
Results
Participant Characteristics
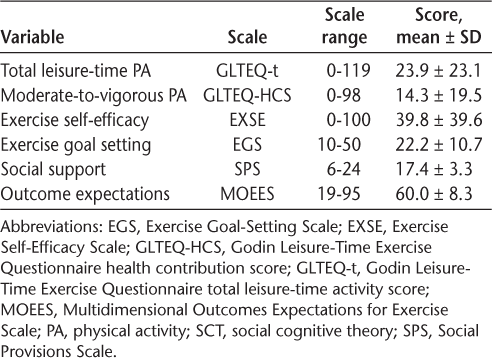
The demographic and clinical characteristics of the 180 participants are presented in Table 1. The mean ± SD age of the sample was 65.2 ± 4.7 years, and the participants were mostly female and White. The sample had a mean ± SD disease duration of 20.8 ± 9.9 years and presented with a relapsing-remitting course of MS with moderate disability (median PDDS scale score = 4.0). This sample is generally consistent with the demographic characteristics of older adults with MS who are primarily female and White race.1,34 Descriptive statistics for the measures of PA and SCT variables are presented in Table 2.
Demographic and clinical characteristics of the 180 study participants
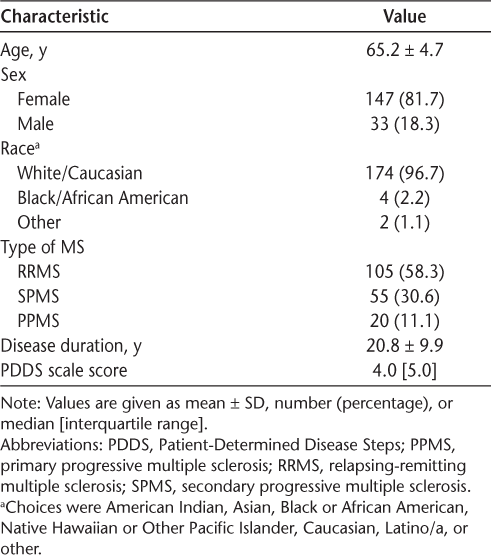
Mean scores of PA and SCT variables
Associations Between PA and SCT Variables
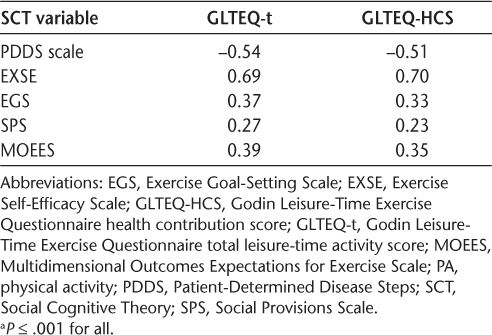
Results from the Spearman rank-order correlation analyses are presented in Table 3. Disability status and all SCT variables were significantly associated with TPA (GLTEQ-total) and MVPA (GLTEQ-HCS) (all P ≤ .001). The associations between exercise self-efficacy (EXSE) and disability status with PA were large in magnitude, whereas all other associations were between small and moderate in magnitude.
Correlations between PA and SCT variables a
Regressions by Outcome Measure
Total Physical Activity (GLTEQ-Total)
Results of the regression are presented in Table S1, which is published in the online version of this article at ijmsc.org. Disability status (PDDS scale) was included in step 1 of the hierarchical linear regression as a possible confounding variable18 and accounted for 23% of the variance in TPA (R 2 = 0.23, β = −0.48). Exercise self-efficacy was included in step 2 and explained an additional 28% of the variance in TPA (R2 = 0.50, ΔR2 = 0.28, β = 0.59). The remaining SCT variables (EGS, SPS, and MOEES) were included in step 3. This model explained an additional 4% of the variance (R2 = 0.55, Δ R2 = 0.04), and disability status (β = −0.21), exercise self-efficacy (β = 0.50), and exercise goal setting (β = 0.14) were significant correlates of TPA scores.
Moderate-to-Vigorous Physical Activity (GLTEQ-HCS)
Results of the regression are presented in Table S2. Variables were entered into the regression in the same manner as GLTEQ-total. Disability status initially explained 19% of the variance in MVPA (R2 = 0.19, β = −0.44). Exercise self-efficacy explained an additional 28% of the variance in MVPA (R2 = 0.47, ΔR2 = 0.28, β = 0.60), and including all SCT variables explained a total of 51% of the variance in MVPA (R2 = 0.51, ΔR2 = 0.04). The final model included disability status (β = −0.17), exercise self-efficacy (β = 0.51), and exercise goal setting (β = 0.15) as significant correlates of MVPA scores.
Discussion
Older adults with MS are an expanding segment of the MS population,1 and these individuals experience a fast rate of disability progression35 that manifests as problems with walking2,3,8 and reduced physical3,4 and cognitive2,5 function. There are few options available for managing the consequences of aging with MS. There is evidence of an association between PA and improved symptoms in this particular population,2,3,8 yet levels of PA are exceedingly low in older adults with MS.9 We conducted bivariate correlation and linear regression analyses based on SCT for examining modifiable correlates of PA in older adults with MS. The analyses indicated that self-efficacy was significantly and independently associated with PA and consistently explained the largest portion of variance in PA outcomes, even after accounting for disability status and other SCT variables. The only other SCT variable explaining variance in PA was goal setting. These results support continued research on self-efficacy, as well as goal setting, for informing behavioral interventions that target PA and its secondary benefits in older adults with MS.
The results of the present study align with previous research on associations between self-efficacy and PA participation in comparatively younger adults with MS.15–17,20 Importantly, self-efficacy is a modifiable construct, and SCT identifies specific sources or influences of self-efficacy that can be targeted with behavioral interventions.12 The most effective source of self-efficacy is mastery experiences. By experiencing success, an individual’s confidence in their ability to make a change and adopt the new health behavior is increased. Vicarious experiences, or seeing someone similar to you succeed, is another strategy for increasing self-efficacy via social support. Another major source of self-efficacy is social persuasion and verbal encouragement, which suggests that developing interventions that provide strong social support are a powerful precursor and driver of behavior change. Last, a person’s emotional state or affective experiences can influence self-efficacy. To positively affect self-efficacy, it is important to reduce stress and minimize negative emotional responses.12 Specific behavior change techniques that target these sources of self-efficacy include action planning that involves explicit instructions of how to achieve goals, providing positive feedback, and focusing on small successes.36 Future research should consider focusing on the sources of self-efficacy using theory-based approaches to inform the design of behavioral interventions for increasing PA in older adults with MS.13
According to the causal model proposed in SCT, self-efficacy may be associated with PA through goal setting; however, goal setting itself influences health behavior independent of self-efficacy.14 This is supported by the present results that demonstrated a relationship between goal setting and PA (TPA and MVPA) independent of disability status, self-efficacy, and other SCT variables. Together with previous research in MS that has demonstrated an independent association between goal setting and PA,17,19,21,37 this finding suggests that goal setting may be another target of behavioral interventions aimed at increasing PA in older adults with MS. Goals set the course for behavior change and provide incentives and motivation.14 Important characteristics of successful goal-setting behavior include setting goals that are clearly defined, attainable, measurable, and time limited.38 Behavioral interventions that incorporate goal-setting practices may have a positive effect on PA behavior in older adults with MS.
The present study is not without important limitations. The cross-sectional research design precludes any inferences of causality. Another limitation is the homogeneity of the primarily female and White sample; this may limit the generalizability of the results to males and other races with MS. Future research may benefit from including a more diverse sample. Also note that all data were based on self-report measures and, therefore, are inherently associated with certain limitations compared with objective measures. In addition, the present study considered only variables from SCT as possible correlates of PA. Future studies may consider examining determinants of PA proposed by alternative behavior change models (eg, the transtheoretical model39 or the health action process approach40).
Overall, the primary result of this cross-sectional study indicated that self-efficacy was significantly and independently associated with both total leisure-time PA and health-promoting PA (ie, MVPA) in older adults with MS. Exercise goal setting was further identified as an independent correlate of PA. These SCT variables are modifiable through focal intervention and may represent targets of behavioral change for increasing PA in older adults with MS. Future research may design and test behavioral interventions based on SCT for increasing PA in the expanding segment of older adults living with MS.
PRACTICE POINTS
The expanding population of older adults with MS likely experiences many of the same benefits of physical activity (PA) as younger and middle-aged adults with MS. However, participation in PA is exceedingly low in this particular segment of the MS population.
The present results suggest that behavioral interventions focusing on self-efficacy and exercise goal setting as targets from social cognitive theory may be appropriate for increasing PA in older adults with MS.
Financial Disclosures
The authors declare no conflicts of interest.
References
Wallin MT, Culpepper WJ, Campbell JD, . The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019; 92: e1029– e1040.
Bollaert RE, Motl RW. Physical and cognitive functions, physical activity, and sedentary behavior in older adults with multiple sclerosis. J Geriatr Phys Ther. 2019; 42: 304– 312.
Cederberg KL, Motl RW, McAuley E. Physical activity, sedentary behavior, and physical function in older adults with multiple sclerosis. J Aging Phys Act. 2018; 26: 177– 182.
Motl RW, Chaparro G, Hernandez ME, Balto JM, Sandroff BM. Physical function in older adults with multiple sclerosis: an application of the short physical performance battery. J Geriatr Phys Ther. 2018; 41: 155– 160.
Bollaert RE, Balto JM, Sandroff BM, Chaparro G, Hernandez ME, Motl RW. Preliminary evidence for the effects of aging and multiple sclerosis on cognitive performance: an analysis based on effect size estimates. Exp Aging Res. 2017; 43: 346– 354.
Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am. 2010; 21: 299– 308.
Vaughn CB, Jakimovski D, Kavak KS, . Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019; 15: 329– 342.
Baird JF, Cederberg KL, Sikes EM, . Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Mult Scler Relat Disord. 2019; 35: 36– 41.
Motl RW, Sebastião E, Klaren RE, McAuley E, Stine-Morrow EA, Roberts B. Physical activity and healthy aging with multiple sclerosis—literature review and research directions. US Neurol. 2016; 12: 29– 33.
Klaren RE, Sebastiao E, Chiu C-Y, . Levels and rates of physical activity in older adults with multiple sclerosis. Aging Dis. 2016; 7: 278– 284.
Kinnett-Hopkins D, Adamson B, Rougeau K, Motl R. People with MS are less physically active than healthy controls but as active as those with other chronic diseases: an updated meta-analysis. Mult Scler Relat Disord. 2017; 13: 38– 43.
Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998; 13: 623– 649.
Motl RW, Pekmezi D, Wingo BC. Promotion of physical activity and exercise in multiple sclerosis: importance of behavioral science and theory. Mult Scler J Exp Transl Clin. 2018; 4: 2055217318786745.
Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004; 31: 143– 164.
Suh Y, Weikert M, Dlugonski D, Sandroff B, Motl RW. Social cognitive correlates of physical activity: findings from a cross-sectional study of adults with relapsing-remitting multiple sclerosis. J Phys Act Health. 2011; 8: 626– 635.
Suh Y, Joshi I, Olsen C, Motl RW. Social cognitive predictors of physical activity in relapsing-remitting multiple sclerosis. Int J Behav Med. 2014; 21: 891– 898.
Suh Y, Weikert M, Dlugonski D, Balantrapu S, Motl RW. Social cognitive variables as correlates of physical activity in persons with multiple sclerosis: findings from a longitudinal, observational study. Behav Med. 2011; 37: 87– 94.
Streber R, Peters S, Pfeifer K. Systematic review of correlates and determinants of physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2016; 97: 633– 645.e629.
Casey B, Coote S, Shirazipour C, . Modifiable psychosocial constructs associated with physical activity participation in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017; 98: 1453– 1475.
Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006; 32: 154– 161.
Motl RW, Dlugonski D, Wójcicki TR, McAuley E, Mohr DC. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler. 2011; 17: 116– 128.
Pilutti L, Dlugonski D, Sandroff B, Klaren R, Motl R. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler. 2014; 20: 594– 601.
Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013; 13: 37.
Godin G, Shephard RJ. Godin Leisure-Time Exercise Questionnaire. Med Sci Sports Exerc. 1997; 29: 36– 38.
Godin G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fitness J Canada. 2011; 4: 18– 22.
Sikes EM, Richardson EV, Cederberg KJ, Sasaki JE, Sandroff BM, Motl RW. Use of the Godin Leisure-Time Exercise Questionnaire in multiple sclerosis research: a comprehensive narrative review. Disabil Rehabil. 2019; 41: 1243– 1267.
Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 2006; 28: 1151– 1156.
Motl RW, Balto JM, Ensari I, Hubbard EA. Self-efficacy and walking performance in persons with multiple sclerosis. J Neurol Phys Ther. 2017; 41: 114– 118.
McAuley E, Motl RW, White SM, Wójcicki TR. Validation of the multidimensional outcome expectations for exercise scale in ambulatory, symptom-free persons with multiple sclerosis. Arch Phys Med Rehabil. 2010; 91: 100– 105.
Konopack JF, McAuley E. Efficacy-mediated effects of spirituality and physical activity on quality of life: a path analysis. Health Qual Life Outcomes. 2012; 10: 57.
McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med. 2003; 25: 1– 7.
Rovniak LS, Anderson ES, Winett RA, Stephens RS. Social cognitive determinants of physical activity in young adults: a prospective structural equation analysis. Ann Behav Med. 2002; 24: 149– 156.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; 1988.
Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013; 80: 1734– 1739.
Trojano M, Liguori M, Bosco Zimatore G, . Age-related disability in multiple sclerosis. Ann Neurol. 2002; 51: 475– 480.
Williams SL, French DP. What are the most effective intervention techniques for changing physical activity self-efficacy and physical activity behaviour—and are they the same? Health Educ Res. 2011; 26: 308– 322.
Suh Y, Motl RW, Olsen C, Joshi I. Pilot trial of a social cognitive theory-based physical activity intervention delivered by nonsuper-vised technology in persons with multiple sclerosis. J Phys Act Health. 2015; 12: 924– 930.
Mann T, De Ridder D, Fujita K. Self-regulation of health behavior: social psychological approaches to goal setting and goal striving. Health Psychol. 2013; 32: 487– 498.
Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psy-chol. 1983; 51: 390– 395.
Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psy-chol. 2008; 57: 1– 29.







