Publication
Research Article
Safety, Feasibility, and Efficacy of an Eccentric Exercise Intervention in People with Multiple Sclerosis with Ankle Contractures
Author(s):
Abstract
Background:
The primary aim of this study was to investigate the safety and feasibility of an eccentric exercise program for people with multiple sclerosis (MS) who have ankle contractures, ie, reduced ankle range of motion (ROM). Secondary aims were to explore the efficacy of this eccentric exercise on ankle joint ROM and functional mobility.
Methods:
Five adults with MS with ankle contractures (three women and two men; mean ± SD age, 50.8 ± 9.4; MS duration, 7.6 ± 5.6 years) completed two eccentric exercise training sessions (10–45 minutes) per week for 12 weeks. The training involved walking backward downhill on an inclined treadmill (gradient, 10°–14°) at a self-selected pace. The intervention was assessed for safety (adverse events), feasibility (recruitment rates, adherence rates, enjoyment levels, difficulty, and discomfort), and clinical outcomes, including passive/active ankle ROM and distance walked in 6 minutes.
Results:
There were no adverse events during or after the eccentric exercise training. There was a 100% adherence rate. All participants enjoyed the training and experienced low levels of muscle soreness/discomfort. The training program improved passive/active ankle ROM in all participants; however, improvements did not translate to improvements in walking for all participants.
Conclusions:
Walking backward and downhill is a safe and feasible training modality for people with MS with ankle contractures. Clinical outcomes (greater passive/active ankle ROM) after this eccentric exercise training were evident. However, translation to clinically meaningful changes in walking function requires further examination.
People with multiple sclerosis (MS) often present with sensory and motor deficits that limit their functional mobility and physical independence.1 Symptoms of MS often result in muscle weakness and other soft tissue consequences, such as joint contractures. A recent population-based study of the prevalence of joint contractures in MS reported that approximately 80% of people with MS who are still ambulant (with or without assistive devices) have developed joint contractures, with the ankle joint as the most common site.2 A significant consequence of ankle contractures is the debilitating effect on gait and functional mobility, such as impaired heel-to-toe progression during gait.3 Similarly, ankle contractures may also contribute to the high frequency of falls in people with MS4 due to reduced foot clearance during the swing phase of gait, with consequent trips and slips, which account for 48% of falls in people with MS.5 Therefore, interventions that can reduce ankle contractures should improve gait and functional mobility as well as reduce trips, slips, and falls.
Currently, the most common interventions for the prevention and treatment of joint contractures are various types of muscle stretching. However, a Cochrane systematic review of 35 randomized trials that examined the effects of stretching interventions concluded that there is moderate- to high-quality evidence that stretching does not have clinically useful immediate, short-term, or long-term effects on joint range of motion (ROM).6 Therefore, there is a need to develop alternative interventions to prevent and treat joint contractures in populations with neurologic conditions.
Recent developments in the field of eccentric exercise may be promising in this regard.7–13 Eccentric exercise requires a muscle to generate force by actively contracting while it is forcefully lengthened. This type of exercise often causes delayed muscle soreness due to disrupted short muscle fibers. However, experimental data from animal14 and human15 studies suggest that levels of soreness diminish with repeated bouts of eccentric exercise because muscle remodeling increases muscle length. Furthermore, some studies have shown that eccentric exercise can increase joint ROM and lengthen muscle fascicles via stimulating muscle remodeling and increasing the length and extensibility of a muscle.7–13 Although these studies were conducted on people without contractures, they provided some evidence that eccentric exercise can increase the extensibility of muscles in healthy adults. However, to date, no study has examined the use of eccentric exercise intervention to treat ankle contractures in populations with neurologic conditions, such as people with MS, spinal cord injury, or stroke.
Therefore, the primary aim of this pilot study was to investigate the safety and feasibility of an eccentric exercise program in people with MS who have ankle contractures. Secondary aims were to explore the efficacy of this eccentric exercise on ankle joint ROM and on mobility in people with MS who have developed ankle contractures.
Methods
Design and Participants
This study used an exploratory research design that aimed to determine whether walking backward (downhill) on an inclined treadmill was safe and feasible in adults with MS with ankle joint contractures.
Participants with MS were recruited from an outpatient MS rehabilitation setting (Sydney, Australia). Inclusion criteria were a confirmed diagnosis of MS by a neurologist; an Expanded Disability Status Scale (EDSS) score of 6 or less (EDSS score was calculated using the Toronto EDSS Calculator, developed by NeuroApps Toronto, scored by the therapists who screened potential participants); an ankle contracture (defined as <90° of passive ankle dorsiflexion ROM when a dorsiflexion torque of 10 Nm was applied to the forefoot of the relaxed ankle)16; no relapses in the preceding 12 weeks; free of any other disease, injury, or illness preventing the participant from exercising; and age 18 years or older. People who were not mobile or who had fixed contractures in the ankle were excluded. The study was approved by the Human Studies Ethics Committee at the University of New South Wales (Sydney). Informed consent was obtained from all participants before study participation.
Assessments
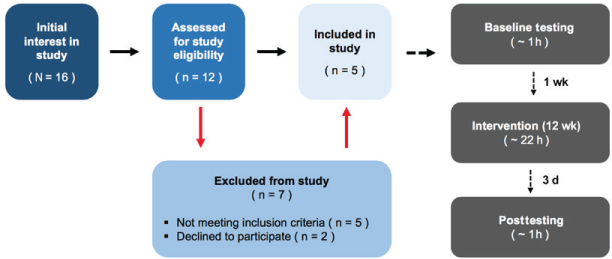
Baseline assessments were conducted 7 days before the first training session. Repeated assessments were conducted within 3 days of the last training session (Figure 1).
Study recruitment and approximate intervention time investment
Safety and Feasibility
Safety was described by the number of adverse events (accidents or injuries related to the intervention) reported by participants during and after each treatment session. Aspects of feasibility were assessed by recruitment rates, acceptability (based on adherence rates and enjoyment levels), and practicality (intervention difficulty and discomfort).17 Recruitment rates were calculated by dividing the number of participants included in the study by the number of participants assessed for eligibility. Enjoyment levels were assessed using the original 18-item Physical Activity Enjoyment Scale (PACES)17 at the conclusion of the study.18 Respondents were asked to rate “how you feel at the moment about the physical activity you have been doing” using a 7-point bipolar rating scale. Eleven items are reverse scored. Higher PACES scores reflect greater levels of enjoyment (maximum score = 126). The perceived difficulty of each session was monitored using the rating of perceived exertion (RPE) scale where participants rated how difficult they perceived the training to be. Participants were asked to give an overall (session) RPE after resting for 10 minutes (after the session) from 0 (nothing at all) to 10 (very heavy).19 Participants reported their muscle soreness and discomfort (delayed onset of muscle soreness [DOMS]) using a visual analogue scale from 0 (no pain) to 10 (unbearable pain) after each session and over a 72-hour period. Peak DOMS was recorded for the 24 sessions.
Ankle ROM
Passive and active ROM were measured on the more affected ankle of potential participants with MS. Participants were placed in a long sitting position using a chair and foot stool with the knees extended. To minimize the effects of thixotropy on muscle, the ankle was moved through six cycles before data were collected.16 Passive ROM was measured by applying 100 N of pulling force (~10 kg on an analogue spring-loaded force gauge) to the heads of the metatarsals (of the affected ankle) and pulled toward the knee (parallel to the shank), and the ankle angle was measured using an inclinometer.16 To measure active ROM, participants were required to actively dorsiflex the ankle as far as they could, and again the ankle angle was measured using an inclinometer.
Functional Mobility
The 6-Minute Walk Test (6MWT) was chosen to evaluate whether the improvement in ankle ROM after eccentric exercise helped improve functional mobility. Participants completed a 6MWT at self-selected walking speeds on a 20-m marked walking pathway. Participants were given standard instructions to “walk as far as possible for 6 minutes.” Participants were reminded that they would be walking for 6 minutes and could stop and rest if needed. Participants were given standardized encouragement after each minute.20 The distance walked in the 6-minute period was recorded.
Intervention
Eccentric exercise training (twice a week for 12 weeks = 24 sessions) was conducted under the supervision of an accredited exercise physiologist. Participants performed eccentric exercise by walking backward downhill on an inclined (10°–14°) treadmill (pulsar 3p; h/p/cosmos) using a previously published protocol.21 Each training session included a 5-minute warm-up consisting of slow (self-selected) forward walking on a treadmill followed by ten concentric/eccentric contractions of the plantar flexor muscles (calf raise/lower) of the leg with ankle joint contracture. After the warm-up, the belt of the treadmill was turned in the reverse direction. Participants were then instructed to step as far backward as possible with the affected leg in a toe-to-heel pattern while keeping the knee straight (to maximize the stretch on the gastrocnemius). After each step with the affected leg, the unaffected leg stepped backward in a normal pattern. Participants were instructed to use hand rails for support only if needed. The first two sessions were considered as an introductory training period where participants were trained for 10 to 20 minutes at a self-selected speed. After these sessions, participants were encouraged to walk continuously, at the speed they were comfortable with (ie, self-selected), for as long as they could tolerate, with the maximal time being 60 minutes.21 An overhead support harness was offered as an additional level of safety. The chest-belt (harness) was connected to an emergency stop button on the treadmill to cut power if more than 8 kg of pulling force was applied to the harness (Video S1, which is published in the online version of this article at ijmsc.org, shows a participant with MS performing the intervention).
Data Analysis
This study aimed to explore the efficacy of an eccentric exercise training program on reduced ankle ROM (secondary aim). Therefore, we report only the magnitude (95% CI) of the differences before and after the intervention. No formal statistical analysis was performed.
Results
Participants, Safety, and Feasibility
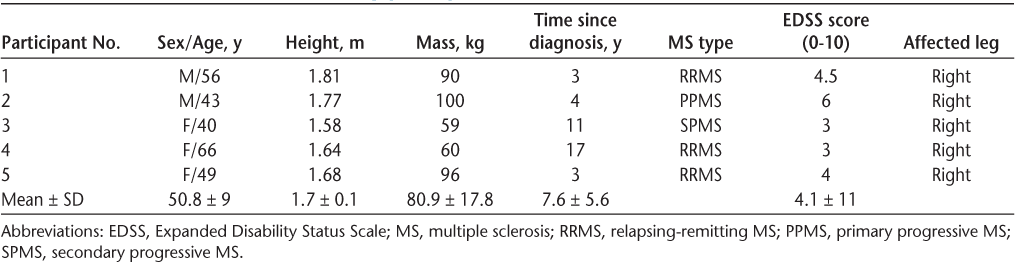
Sixteen individuals indicated an initial interest in the study (Figure 1), but only 12 (75%) followed up and were assessed for study eligibility. Five of the 12 assessed patients (42%) did not meet the inclusion criteria of having less than 90° of passive ankle ROM, and two (17%) declined the offer to participate due to lack of time. Recruitment uptake was, therefore, 42% (n = 5; two men, three women). See Table 1 for baseline characteristics of study participants.
Baseline characteristics of study participants
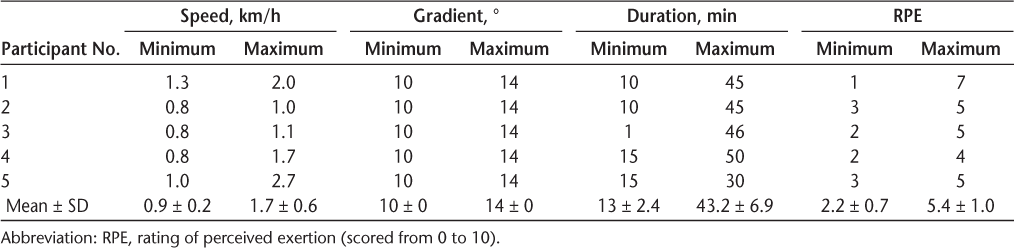
There were no adverse events recorded during or after the intervention. Each participant spent an average of 24 (supervised) hours to complete the intervention. There was a 100% adherence rate for all study participants. Participant intervention data (speed, duration, gradient, and RPE) recorded over 24 sessions are shown in Table 2. All participants enjoyed the intervention, with a mean ± SD PACES score of 105 ± 15. In addition, participants reported a low level of discomfort/soreness, with a mean ± SD peak DOMS of 2.7 ± 2. Participants perceived the difficulty of the intervention to range from 2.2 (easy) to 5.4 (hard) of 10.
Participant intervention data recorded over 24 sessions
Ankle ROM and Functional Mobility
All participants had greater mean passive ankle ROM after the intervention (93°) compared with baseline (82°) (mean difference, 11°; 95% CI = 5°–17°). Similarly, all participants had greater active ROM after the intervention (77°) compared with baseline (67°) (difference, 10°; 95% CI = 1.2°–19.2°) (Table 3 and Figure 2). According to a recent Cochrane review,22 the difference in this study (on average) was greater than that of a clinically meaningful improvement (~5°).
Enjoyment, soreness/discomfort, ankle range of motion, and functional mobility before and after intervention
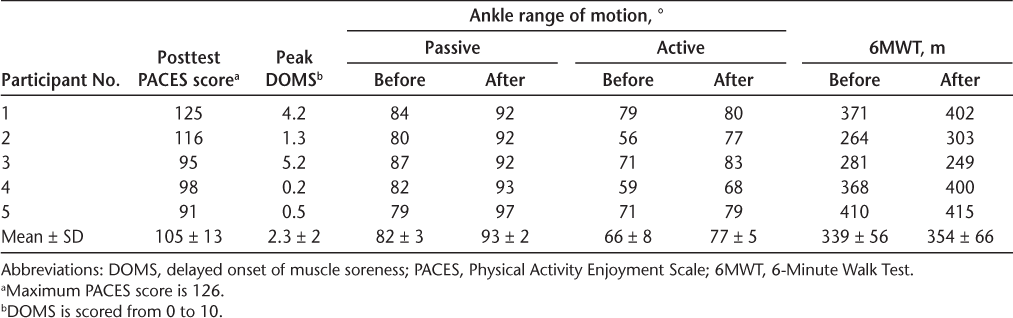
Passive range of motion (ROM) of ankle (A), active ROM of ankle (B), and 6-Minute Walk Test (6MWT) distance (C)
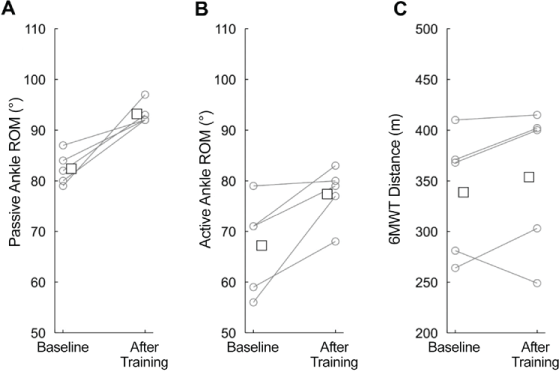
The 6MWT distance was slightly greater after training (mean, 354 m) than at baseline (mean, 339 m; difference, 15 m; 95% CI = −21.5 to 51.4 m) (Table 3 and Figure 2). According to a European multicenter study,23 the difference in functional mobility in this study was slightly short of a clinically meaningful improvement (~22 m).
Discussion
In the present study, backward walking on an inclined treadmill was safe and feasible for patients with MS who have ankle contractures. In addition, preliminary observations support the hypothesis that this intervention could improve ankle ROM and functional outcomes in patients with MS.
Backward walking is an emerging rehabilitation strategy in patients with neurologic conditions such as stroke24 and children with cerebral palsy.25 Yang et al24 reported that adding backward walking training to walking forward on a (flat) treadmill could improve the asymmetrical gait pattern in patients after stroke. Kim et al25 reported that backward walking on an inclined treadmill may strengthen the rectus femoris and tibialis anterior in walking training for children with cerebral palsy. In both studies, researchers used backward walking tasks to improve forward walking impairments. In MS, it has been suggested that backward walking tests (with or without a cognitive task) are an effective way to examine walking difficulties in individuals with relatively minimal walking impairment.26 Extending on previous literature, we hypothesized that backward downhill walking on an inclined treadmill (a form of eccentric exercise) could result in improvements in ankle contractures in people with MS who are mobile via changes in the soft tissues around the ankle. This, in turn, could improve mobility.
It was observed that ankle joint ROM (passive and active) improved by approximately 10° in participants after eccentric exercise training. A recent Cochrane review22 comparing traditional stretching interventions in people with and without neurologic conditions highlighted that any possible benefit (in ROM) achieved from stretching did not exceed 4°. Therefore, eccentric exercise may provide greater improvements in ROM than traditional (stretching) interventions.
Previous research suggests that increases in ROM after eccentric exercise are due to muscle damage and subsequent remodeling at longer lengths.7–13 However, levels of DOMS reported in the present study were low, suggesting that observed changes in passive and active ROM in this study may not be solely related to muscle remodeling and could be due to changes in nonmuscle stiffness, such as a reduction in capsular stiffness. Significant changes in active ROM may be attributed to a combination of factors, including an increase in tibialis anterior activation in conjunction with less overall (passive) ankle dorsiflexion stiffness. Previous research in patients with cerebral palsies who walked backward downhill at a gradient of 10% showed a significant increase in tibialis anterior muscle activation.25 These results, in conjunction with improved passive ankle ROM, provide a plausible explanation for the overall improvements in active ROM in participants with MS who have developed ankle contractures.
The present results demonstrated that, on average, participants improved 6MWT distance by only 15 m. In a large multicenter study, it was suggested that a clinically meaningful change in 6MWT distance in people with MS is approximately 22 m.23 In older adults a small meaningful change in 6MWT distance is 19 to 22 m and a substantial meaningful change is 47 to 49 m.27 In populations with lung diseases the clinically meaningful change in 6MWT distance was from 14.0 to 30.5 m.28 Taking all these criteria together, only three participants in the present study had a small clinically meaningful change in function. It is possible that the concomitant motor fatigue that is common in people with MS may have limited improvements in the 6MWT distance. Future research may consider comparison of 10-m walk test distances with fast and slow speeds, which may reveal greater results.
Fatigue is an important issue in people with MS and needs to be considered regarding safety during exercise, especially in relation to the type of eccentric exercise applied in this study. Accordingly, we gradually trained the participants and closely monitored them during the exercise by monitoring their sessional RPE and adjusting the intensity in the next session if needed. In addition, we monitored adverse events (ie, tripping or reduced balance or falls) during and after the exercise sessions: none were reported by the study participants. In this study we set a maximal target of 60 minutes of backward walking per session; none of the participants could achieve this. Future studies may consider shorter periods or breaks during the session so that participants can exercise longer and, therefore, get more benefits from this type of exercise.
We acknowledge several limitations and future recommendations of this study. First, the small sample size of this study limits the interpretation and generalization of data. Second, because the participants were only “moderately” affected by MS (average EDSS score = 4.1), the intervention may be limited to those who are more ambulant and not for people with advanced MS. Third, the outcome measures were collected only twice over a 3-month period. Due to the high day-to-day variability in people with MS, a single assessment occurring over a 3-month period may be misrepresentative of the participant’s performance throughout the intervention.29 More frequent testing of outcome measures throughout an intervention may serve to overcome this limitation in future studies. Finally, postintervention measurements were taken within 3 days after the intervention. It is not known whether these results are transient and can be maintained after the intervention. Future studies should consider longer-term follow-up to investigate the effectiveness of this type of intervention with more regular intervals of testing, which may help track changes in people with MS.
Although the backward walking training applied in this study was found to be safe and feasible, several recommendations need to be addressed in preparation for a larger study based on the criteria suggested by Thabane et al.30 First, 42% of participants assessed were excluded from the study because they had greater than 90° of passive ankle ROM. Given that normal ambulation requires approximately 10° of dorsiflexion,31 our criteria may be too strict and require modification to reduce exclusion rates. Second, the low levels of DOMS reported in the present study may be attributed to the low intensity (speed), poor muscle activation (coordination), and knee hyperextension during the intervention. Previous research in healthy participants using a similar protocol reported high levels of DOMS and resulted in one participant incurring a grade II muscle tear.21 Participants in this study achieved higher levels of intensity (speed), proposing a plausible link between the intensity of the intervention and the magnitude of muscle damage. A lack of muscle activation while stepping backward on the treadmill may also reduce the amount of eccentric load and, presumably, reduce the amount of muscle damage. For eccentric exercise to be effective, patients must actively contract the muscle (triceps surae complex) with sufficient force while it is forcibly lengthened (stepping backward downhill) to facilitate muscle damage.15 Lower limb paresis and muscular weakness most likely limited the intensity (speed) and duration (time) of the intervention, thereby potentially lessening its effect. In addition, we noted that all participants demonstrated various amounts of knee hyperextension during the intervention. Knee hyperextension places additional load through the posterior knee capsule and may decrease the load on the triceps surae complex. Therefore, future studies should consider using a brace to control knee hyperextension throughout the intervention to maximize the eccentric load placed on the triceps surae complex.
In conclusion, the present study demonstrates that backward downhill walking is a safe and feasible training modality in people with MS with ankle contractures. Clinical outcomes (passive and active ROM) after backward downhill walking are promising. However, translation to clinically meaningful changes in walking function requires further examination.
PRACTICE POINTS
Backward downhill walking on a treadmill is a safe and feasible eccentric exercise training modality for people with MS who have ankle contractures.
Clinical outcomes—greater passive and active ankle range of motion—were evident after backward downhill walking. However, translation to clinically meaningful changes in walking function requires further examination.
Acknowledgments
The authors thank Dr Peter Stubbs for assisting with data collection and providing comments on the manuscript.
References
Coote S, Garrett M, Hogan N, Larkin A, Saunders J. Getting the balance right: a randomised controlled trial of physiotherapy and exercise interventions for ambulatory people with multiple sclerosis. BMC Neurol. 2009; 9: 34.
Hoang PD, Gandevia SC, Herbert RD. Prevalence of joint contractures and muscle weakness in people with multiple sclerosis. Disabil Rehabil. 2014; 36: 1588– 1593.
Psarakis M, Greene D, Moresi M, . Impaired heel to toe progression during gait is related to reduced ankle range of motion in people with multiple sclerosis. Clin Biomech (Bristol, Avon). 2017; 49: 96– 100.
Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil. 2014; 95: 480– 486.
Matsuda PN, Shumway-Cook A, Bamer AM, Johnson SL, Amtmann D, Kraft GH. Falls in multiple sclerosis. PM R. 2011; 3: 624– 632; quiz 632.
Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev. 2010; ( 9): CD007455.
Duclay J, Martin A, Duclay A, Cometti G, Pousson M. Behavior of fascicles and the myotendinous junction of human medial gastrocnemius following eccentric strength training. Muscle Nerve. 2009; 39: 819– 827.
Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol. 2007; 103: 1565– 1575.
Mahieu NN, McNair P, Cools A, D’Haen C, Vandermeulen K, Witvrouw E. Effect of eccentric training on the plantar flexor muscle-tendon tissue properties. Med Sci Sports Exerc. 2008; 40: 117– 123.
Potier TG, Alexander CM, Seynnes OR. Effects of eccentric strength training on biceps femoris muscle architecture and knee joint range of movement. Eur J Appl Physiol. 2009; 105: 939– 944.
Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol. 2009; 94: 825– 833.
Nelson RT, Bandy WD. Eccentric training and static stretching improve hamstring flexibility of high school males. J Athl Train. 2004; 39: 254– 258.
O’Sullivan K, McAuliffe S, DeBurca N. The effects of eccentric training on lower limb flexibility: a systematic review. Br J Sports Med. 2012; 46: 838– 845.
Lynn R, Morgan DL. Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J Appl Physiol. 1994; 77: 1439– 1444.
Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001; 537( pt 2): 333– 345.
Kwah LK, Herbert RD, Harvey LA, . Passive mechanical properties of gastrocnemius muscles of people with ankle contracture after stroke. Arch Phys Med Rehabil. 2012; 93: 1185– 1190.
Bowen DJ, Kreuter M, Spring B, . How we design feasibility studies. Am J Prev Med. 2009; 36: 452– 457.
Measuring enjoyment of physical activity in children: validation of the Physical Activity Enjoyment Scale. J Appl Sport Psychol. 2009; 21( suppl 1): S116– S129.
Foster C, Florhaug JA, Franklin J, . A new approach to monitoring exercise training. J Strength Cond Res. 2001; 15: 109– 115.
Steffen TM, Hacker TA, Mollinger L. Age-and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002; 82: 128– 137.
Hoang PD, Herbert RD, Gandevia SC. Effects of eccentric exercise on passive mechanical properties of human gastrocnemius in vivo. Med Sci Sports Exerc. 2007; 39: 849– 857.
Harvey LA, Katalinic OM, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev. 2017; 1: CD007455.
Baert I, Freeman J, Smedal T, . Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multi-center study. Neurorehabil Neural Repair. 2014; 28: 621– 631.
Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clin Rehabil. 2005; 19: 264– 273.
Kim WH, Kim WB, Yun CK. The effects of forward and backward walking according to treadmill inclination in children with cerebral palsy. J Phys Ther Sci. 2016; 28: 1569– 1573.
Wajda DA, Sandroff BM, Pula JH, Motl RW, Sosnoff JJ. Effects of walking direction and cognitive challenges on gait in persons with multiple sclerosis. Mult Scler Int. 2013; 2013: 859323.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006; 54: 743– 749.
Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-Minute Walk Test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017; 23: 377– 381.
Albrecht H, Wötzel C, Erasmus L, Kleinpeter M, König N, Pöllmann W. Day-to-day variability of maximum walking distance in MS patients can mislead to relevant changes in the Expanded Disability Status Scale (EDSS): average walking speed is a more constant parameter. Mult Scler. 2001; 7: 105– 109.
Thabane L, Ma J, Chu R, . A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010; 10: 1.
Weir J, Chockalingam N. Ankle joint dorsiflexion: assessment of true values necessary for normal gait. Int J Ther Rehabil. 2007; 14: 76– 82.







