Publication
Research Article
International Journal of MS Care
Magnetic Resonance Imaging of Spinal Cord Lesions in Patients with Multiple Sclerosis in Saskatchewan, Canada
Author(s):
Abstract
Background:
Spinal cord lesions (SCLs) contribute to disability in multiple sclerosis (MS). Data in Saskatchewan, Canada, concerning SCLs and their association with disability levels in patients with MS are lacking. The study objectives were to identify clinicodemographic profiles of patients with MS with respect to spinal cord magnetic resonance imaging (MRI) involvement; determine the frequency of individuals with MRI SCLs; and explore differences between patients with MS with and without SCLs with respect to disability and disease-modifying therapy status.
Methods:
A monocentric, cross-sectional, retrospective review of prospectively collected data from 532 research-consented patients seen at Saskatoon MS Clinic was performed. Data were collected from a database and electronic medical records.
Results:
Of the 356 patients (66.9%) with an SCL, 180 (50.6%) had only cervical cord lesions. Median Expanded Disability Status Scale (EDSS), ambulation, and pyramidal scores of patients with SCLs were higher than those of patients without SCLs. Of patients with EDSS scores of at least 6, those with SCLs were younger than those without SCLs (P = .01). Patients with SCLs were 55% less likely to have been on continuous disease-modifying therapy since diagnosis than patients without SCLs (adjusted odds ratio, 0.45; 95% CI, 0.25–0.81; P = .008).
Conclusions:
Prevalence and association with disability of SCLs in patients with MS are comparable with existing literature. Patients with MS with SCLs have higher levels of disability and attain EDSS scores of at least 6 at a younger age.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system.1 Spinal cord lesions (SCLs) in patients with MS are considered to be a major source of clinical disability and are detectable by magnetic resonance imaging (MRI).1 2 Spinal cord lesions correspond to areas of demyelination in the spinal cord, neuroaxonal loss, and gliosis, affecting the normal anatomy and physiology of the spinal cord.3 Spinal cord abnormalities as evident from MRI have been associated with ambulatory, pyramidal, and bowel/bladder dysfunction.2 Previous studies have reported SCLs in 68% to 92% of patients with MS.4–6 In MS, SCLs often present in the cervical spinal cord and less frequently in the lower thoracic spinal cord.7
The Saskatoon MS Clinic, located in the Saskatoon City Hospital, Saskatoon, Saskatchewan, Canada, has a multidisciplinary team consisting of MS neurologists, a physiatrist, nurse educators, a clinic coordinator, researchers, and administrative/support staff. Traditionally it had a more rehabilitative focus, with more disabled and progressive patients visiting the clinic. Although basic epidemiologic data for MS in Saskatchewan are published,8–10 there is a lack of information on disease specifics such as spinal MRI lesions and their effect on disease course. This prospective MS database collects clinical and radiographic information and further describes a clinic cohort. In the present study, we analyzed data collected from the large cohort of patients visiting the Saskatoon MS Clinic. The aim of this study was to describe the prevalence and patterns of SCLs in a cohort of patients with MS with the main goal of improving our understanding of the importance of SCLs in MS patients from a real-world clinic setting where people with advanced disease are also followed up.
Methods
Study Design and Sample
A monocentric, cross-sectional, and retrospective review of prospectively collected data from patients seen at the Saskatoon MS Clinic was performed. This study included a cross-sectional sample of 532 patients who had consented to participate in research. Inclusion criteria included a confirmed diagnosis of MS or clinically isolated syndrome (CIS), age 18 years or older, and an available spinal MRI report. Those who were not able to consent for themselves and those with diseases/conditions other than MS/CIS that preclude their participation were excluded. Ethics approval for this study was obtained from the University of Saskatchewan’s Behavioral and Biomedical Research Ethics Board.
Data Sources
The clinical and demographic variables, including month/year of birth; sex; year of first symptom; year of diagnosis; smoking status; MS phenotype; number of relapses; Expanded Disability Status Scale (EDSS) scores and ambulation, pyramidal, and bowel/bladder subscores at last visit; and MS-specific treatments, were extracted from the Saskatoon MS Clinic’s database, as entered in iMed. Most patients in this study cohort have a clinical examination at the Saskatoon MS Clinic and a follow-up MRI at least annually. The MRI data were obtained from electronic medical records (Accuro, QHR Technologies), which include a summary of a radiologist’s report and the neurologist’s impression regarding SCL presence. We had collected clinical variables from the last clinic visit and had looked at the most recent spinal MRI report. Spinal cord MRI was completed within 6 months before or after the clinic visit, with some exceptions for nonactive progressive patients, where scans were completed within 2 years of the clinic visit. A qualified (MD, MPH) research associate involved with the study extracted whether each patient had an SCL and which section of the cord was involved (cervical and/or thoracic), as evident from the most recent spinal cord MRI. Number and specific locations of SCLs were recorded for a subset of the study population (n = 68) who were newly diagnosed between January 1, 2017, and October 22, 2018 (data extracted for analysis). In addition, for this newly diagnosed subset of the population we also looked at baseline MRI SCLs, baseline EDSS scores, and baseline ambulation, pyramidal, and bowel/bladder subscores. The lesions in that subset were classified by location as high cervical (C1–C4), low cervical (C5–C7), high thoracic (T1–T5), and low thoracic (T6–T12) and by number as one, two, three, and four or more lesions. The extracted data were then reviewed and cross-verified by a neurologist (M.C.L.) involved in the study.
Statistical Analysis
Descriptive and inferential statistics were used to establish clinicodemographic profiles and association between SCLs and disability outcomes. The MS study population was divided into two groups: patients with SCLs and patients without SCLs based on the presence or absence of SCLs as evident from their latest spinal MRI reports. Groups were compared using the independent two-sample t test, Mann-Whitney U test, and χ2 test for independence as appropriate. To evaluate the influence of SCLs on the clinicodemographic profiles of patients with significant walking impairment, we subdivided the study population into patients with SCLs with EDSS scores of at least 6 and patients without SCLs with EDSS scores of at least 6 and compared those subgroups using the independent two-sample t test and the χ2 test for independence as appropriate. To evaluate the significance of the burden and the distribution of SCLs, we analyzed a subset of the study population (n = 68) using the Kruskal-Wallis test and Spearman rank correlation. Logistic regression was used to calculate odds ratios (ORs) and 95% CIs of having SCLs and disease-modifying therapy (DMT) use/smoking status. Use of DMT was classified into three categories: continuous DMT—those who were on DMT since diagnosis without any interruption for more than 90 days; never on DMT—those who had never been on DMT since diagnosis; and interrupted DMT—those who had a history of DMT use with more than 90 days of interruption. A discontinuation of any DMT was defined as more than 90 days of treatment interruption based on a previous population-based study on adherence and persistence to drug treatment for MS.11 The patients in the continuous DMT group started DMT within 1 year of diagnosis (mean time to DMT initiation from MS diagnosis, 3.95 months). Current and former smokers were grouped under one category of those who had ever smoked during their lifetime; this group was compared with those who had never smoked during their lifetime. The ORs were adjusted for age, sex, disease duration (time since symptom onset), and MS phenotype. All the statistical analyses were performed using SPSS Statistics for Windows, version 25.0 (IBM Corp), and significance was set at α = .05.
Results
Clinicodemographic Profile of Study Population
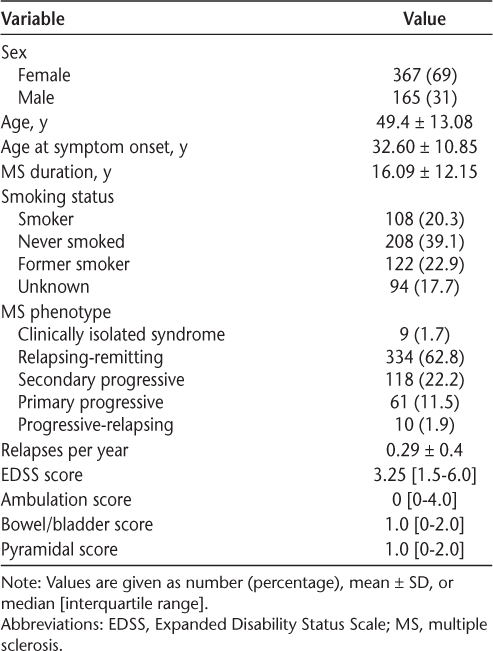
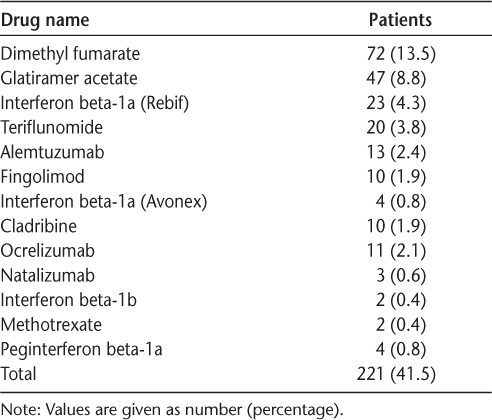
The total study population of 532 patients had a female to male ratio of 2.2:1. Table 1 shows the clinicodemographic profile of the study population. In terms of MS phenotypes, the highest frequency was of relapsing-remitting MS (RRMS) (334 patients [62.8%]), followed by secondary progressive MS (118 patients [22.2%]). Of the study population, 200 (37.6%) were continuously on DMT since diagnosis, 146 (27.4%) were never on DMT, and 186 (35.0%) were in the interrupted DMT group. The most common DMTs prescribed were dimethyl fumarate (n = 72 [13.5%]), followed by glatiramer acetate (n = 47 [8.8%]) (Table 2). A moderate negative correlation was found between age and number of relapses per year (rs = −0.608, P < .001), indicating that relapses per year were decreasing with increasing patient age in this study cohort. However, moderate positive correlations were found between age and EDSS scores (rs = 0.504, P < .001) and between disease duration and EDSS scores (rs = 0.454, P < .001).
Characteristics of the 532 study patients
Disease-modifying therapies used by the 532 study patients
Spinal Cord Lesions
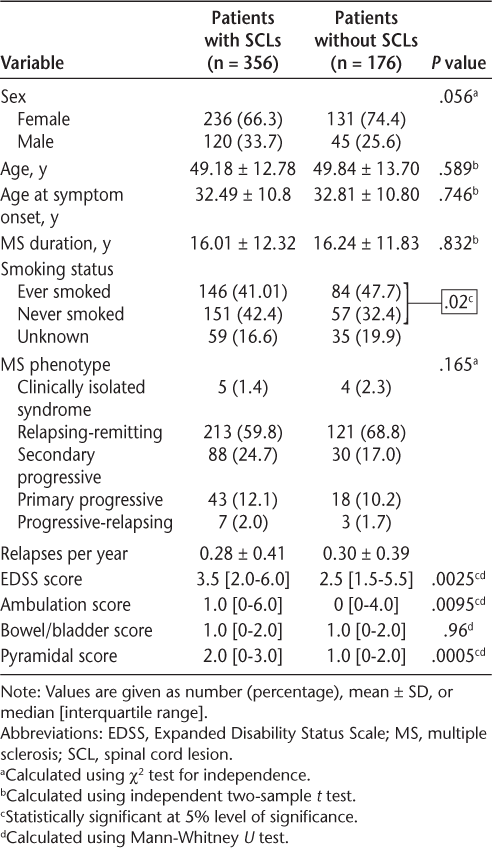
Of the 532 study patients, 356 (66.9%) had SCLs; of these, 150 (42.1%) had both cervical and thoracic cord lesions, 180 (50.6%) had only cervical cord lesions, and 26 (7.3%) had only thoracic cord lesions. Table 3 shows the differences between the clinicodemographic profiles of patients with SCLs and patients without SCLs. The median EDSS score of patients with SCLs was higher than that of patients without SCLs (3.5 vs 2.5, P = .0025). In terms of functional scores, the median ambulation and pyramidal scores of patients with SCLs were statistically significantly higher than those of patients without SCLs. Table S1, which is published in the online version of this article at ijmsc.org, compares the clinicodemographic profiles of patients with and without SCLs with EDSS scores of at least 6. The patients with SCL with an EDSS score of at least 6 were younger, with a mean ± SD age of 57.09 ± 11.15 years, than patients without SCLs with EDSS scores of at least 6 (mean ± SD age, 61.41 ± 9.18 years).
Clinicodemographic profile of patients with SCLs and patients without SCLs
Burden and Distribution of SCLs in Subset of Study Population
Of 68 patients diagnosed as having MS between January 1, 2017, and October 22, 2018, 50 had SCLs (mean ± SD of 2.8 ± 1.9 lesions). High cervical spine lesions (C1–C4) were the most commonly found lesions (28% of the subpopulation). Figure S1 illustrates statistically significant differences in the baseline EDSS and ambulation scores found between patients with high cervical spine lesions and those with a combination of high and low cervical spine lesions and low thoracic spine lesions (P = .046, P = .043), with the latter having higher scores (baseline median EDSS score of 4 vs 1; baseline median ambulation score of 1 vs 0).
The distribution of baseline bowel/bladder scores was different between patients with cervical spine involvement only and those with both cervical and thoracic spine involvement (P = .039), with the latter positively skewed for worse disability scores. A statistically significant difference in the baseline pyramidal scores was found between patients with one SCL and those with greater than or equal to four SCLs (P = .040), with the latter having higher scores (median baseline pyramidal scores of 1.5 vs 0). Baseline EDSS, ambulation, bowel/bladder, and pyramidal scores correlated with total SCL number on the baseline MRI (rs = 0.43, P = .002; rs = 0.36, P = .010; rs = 0.38, P = .007; and rs = 0.42, P = .002, respectively).
Association of SCLs with DMT Use and Smoking Status
Patients with SCLs were 55% less likely to have been on continuous DMT since diagnosis than patients without SCLs (adjusted OR = 0.45, 95% CI = 0.25–0.81, P = .008). Patients with SCLs who were never on DMT were older (mean ± SD age = 56.6 ± 13.6 years), had a longer mean ± SD disease duration (19.4 ± 14.7 years) with progressive disease (61.2% having secondary progressive MS or primary progressive MS), had a lower mean ± SD number of relapses per year (0.13 ± 0.23), and had higher disability (median EDSS score = 5.5 and median ambulation score = 4) than patients with SCLs who were on continuous DMT since diagnosis. No statistically significant association was found between presence of SCLs and interrupted DMT use (P = .60). Patients with SCLs were 39% less likely to have ever smoked than patients without SCLs (adjusted OR = 0.61, 95% CI = 0.41–0.93, P = .02).
Discussion
The present results show that having SCLs was associated with higher EDSS, ambulation, and pyramidal scores in patients with MS. Also, the baseline EDSS, ambulation, bowel/bladder, and pyramidal scores were found to be correlated with total SCL number. These findings are consistent with those of previous studies, where SCLs were found to be correlated with clinical findings. A cross-sectional study of Hispanic patients with MS reported that the group with scattered SCLs had a higher median EDSS score of 3 compared with the median score of 1.75 in the group without SCLs.12 In another study conducted to assess the relationship between spinal cord T2 hyperintense lesions and clinical status in patients with MS, pyramidal functional system scores were found to be correlated with thoracic T2 lesion number (rs = 0.46, P = .01) and total number of SCLs (rs = 0.37, P = .04).13 The present results also support the association of SCLs with disability and emphasize the potential significance of SCLs in patients with MS from a real-world clinic setting.
The role of spinal MRI as a surrogate marker of treatment response and disease progression is not widely consolidated. Spinal cord MRIs are not routinely performed on all patients with MS in the absence of spinal symptoms, and imaging guidelines tend to focus on spinal cord imaging primarily for diagnostic purposes. According to the 2018 Consortium of Multiple Sclerosis Centers (CMSC) MRI protocol, “a spinal cord MRI is recommended if the brain MRI is non-diagnostic or if the presenting symptoms are referable to the spinal cord.”14 For the baseline examination in patients with CIS or suspected MS, the CMSC protocol recommends “spinal cord MRI if myelitis, insufficient features on brain MRI to support diagnosis, or age>40 with non-specific brain MRI findings.”14 Similarly, the Magnetic Resonance Imaging in MS (MAGNIMS) consensus guidelines indicated that “at symptom onset, spinal cord imaging is recommended in patients with clinical features localized to the spinal cord to rule out alternative cord pathology (e.g., compression, spinal cord tumor, neuromyelitis optica (NMO), vasculitides) and in those with non-spinal CIS not fulfilling brain MRI for dissemination in space (DIS).”15(p296) The Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring state that “patients with a new typical CIS should receive a diagnostic brain and/or spinal cord MRI within a week of their first presentation.”16(p164) The neurologist members of the panel recommended to include information about the “presence and appearance of any spinal cord lesions”16(p166) in an effective MRI report. However, routine spinal cord MRI is not unequivocally recommended for monitoring in any guidelines.
Spinal cord disease activity is not always coupled with brain disease activity, and, therefore, brain imaging alone may underestimate inflammatory activity. A study evaluating the significance of asymptomatic spinal MRI lesions in patients with MS found that 25.2% of patients with RRMS developed at least one new asymptomatic SCL over median follow-up of 17 months.17 This percentage was lower than that in patients who developed at least one new asymptomatic brain lesion (43.7%). However, the study reported that adding spinal MRI in the follow-up of stable patients with RRMS reveals approximately 10% of disease activity that could have been otherwise neglected. The present study highlights the importance of SCLs in patients with MS, but further research is needed to evaluate the logistics and cost of adding spinal cord MRI to the routine monitoring of patients with MS.
The burden and distribution of SCLs may also have clinical relevance. A subanalysis conducted in the present study showed that high cervical spine lesions (C1–C4) were the most commonly found lesions. This is in line with a retrospective study that also reported the highest amount of lesions in upper cervical levels (C2, 10.2%; C3, 12.4%; C4, 9.8%).18 The present study also demonstrated a statistically significant difference in the baseline pyramidal scores between patients with one SCL and those with four or more SCLs. The association of higher baseline lesions with higher levels of disability in patients with MS was also reported in a recently published study.19 In this study, one or more SCLs at baseline were found to be associated with worse EDSS scores at 15 years of follow-up (β = 1.53, P < .01). The prognostic value of SCLs, however, should be considered in the context of other prognostic factors.
Despite a large proportion of the study sample having RRMS (62.8%), we found that only 41.5% of the research-consented cohort of Saskatoon MS Clinic patients were on an MS DMT. This percentage supports a recent study that found that only 37% of patients with MS in Saskatchewan had ever been prescribed an MS DMT.8 This is low compared with the United States and Europe, where studies have reported that 66% and 57% of patients, respectively, received a DMT.20 21 However, given that the Saskatoon MS Clinic had a rehabilitative focus in the past, it could have a comparatively higher proportion of people with disability or progressive MS who either may not benefit from DMTs or may not qualify for provincial funding for DMTs in Saskatchewan. Disease-modifying therapies have shown efficacy in preventing clinical relapses and new MRI lesions.22 The present results demonstrate that patients with SCLs were 55% less likely to have been on continuous DMT since diagnosis than patients without SCLs. A limitation of this analysis is a treatment selection bias wherein those with lower disability scores are more likely to access treatment.
Smoking is a known independent risk factor for MS susceptibility, and smokers have a greater chance of developing progressive disease.23 A meta-analysis of six studies had earlier shown that smoking increases the risk of MS, with an OR of approximately 1.5.24 We found in this study that patients without SCLs were more likely to have ever smoked than those with SCLs. This was an unexpected finding given the results of the previous meta-analysis and the biological negative effects of smoking. This finding is likely a statistical artifact given that this was a small study in which smoking status was unknown for 17.7% of the sample, and we did not explore other potential confounders. The bulk of published research has shown a detrimental effect of smoking in RRMS. The exact biological pathway through which smoking acts to increase the risk of MS is not yet characterized. Possible mechanisms could include neural injury and immunomodulation.
The prevalence and pattern of SCLs, as described in this study, were comparable with the existing literature. Previous studies have shown SCLs in 68% to 92% of patients with predominant cervical spine involvement.4–6 12 Although this study is limited by the single-clinic cross-sectional design and to research-consented participants, the real-world clinical data still support the potential prognostic value and clinical significance of SCLs. The relatively low rate of use of highly effective DMTs limits data generalizability to current and future MS cohorts. We also did not have quantitative data on spinal cord atrophy/volume loss, which could have provided additional information. A further limitation of this study is that MS phenotype description was restricted to the traditional categories of relapsing, secondary progressive, and progressive course. Further descriptors of disease course in terms of active disease or progression were not captured. Our site is registered with the MSBase international registry, which currently does not routinely use these later descriptors. Future studies could be designed to establish the prognostic effect of SCLs in patients with active disease versus progression. This area of research could improve the understanding of the underlying mechanisms of clinical disability and progression, leading to better prognostication and treatment decisions.
In conclusion, the present study reported SCLs in 67% of the study population (N = 532), with predominant involvement of the cervical spine, consistent with the existing literature. Median EDSS, ambulation, and pyramidal scores of patients with SCLs were higher than those of patients without SCLs. Patients with SCLs with EDSS scores of at least 6 were younger and had higher mean pyramidal scores than patients without SCLs with EDSS scores of at least 6. Patients with SCLs were 55% less likely to have been on continuous DMT since diagnosis than patients without SCLs. Future research examining the effect of smoking should consider factors such as complete data ascertainment, confounding variables, and plausible biological mechanisms.25–27
PRACTICE POINTS
Patients with MS with spinal cord lesions (SCLs) had higher Expanded Disability Status Scale (EDSS), ambulation, and pyramidal scores.
Patients with MS with SCLs attained an EDSS score of 6 or greater at a younger age.
Patients with SCLs were 55% less likely to have been on continuous disease-modifying therapy since diagnosis than patients without these lesions.
Acknowledgments
We are grateful to the patients volunteering for research, our clinical and research team at the Saskatoon MS Clinic, and the office of the Saskatchewan MS Clinical Research Chair, College of Medicine, University of Saskatchewan. We are also thankful to the CMSC for accepting our abstract based on these data and giving us an opportunity to present our results at the Poster Session of the 2019 CMSC Annual Meeting.
References
Reich D, Lucchinetti C, Calabresi P. Multiple sclerosis. N Engl J Med. 2018; 378: 169– 180.
Lukas C, Sombekke M, Bellenberg B, . Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology. 2013; 269: 542– 552.
Lassmann H. Spinal cord pathology in multiple sclerosis. Lancet Neurol. 2015; 14: 348– 349.
Mrabet S, Souissi A, Larnaout F, . Spinal cord lesions predict disability progression in relapsing multiple sclerosis. Mult Scler Relat Disord. 2018; 26: 238– 239.
Gass A, Rocca M, Agosta F, . MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol. 2015; 14: 443– 454.
Eden D, Gros C, Badji A, . Spatial distribution of multiple sclerosis lesions in the cervical spinal cord. Brain. 2019; 142: 633– 646.
Weier K, Mazraeh J, Naegelin Y, . Biplanar MRI for the assessment of the spinal cord in multiple sclerosis. Mult Scler. 2012; 18: 1560– 1569.
Al-Sakran L, Marrie R, Blackburn D, Knox K, Evans C. Establishing the incidence and prevalence of multiple sclerosis in Saskatchewan. Can J Neurol Sci. 2018; 45: 295– 303.
Hader W. Disability and survival of multiple sclerosis in Saskatoon, Saskatchewan. Can J Neurol Sci. 2010; 37: 28– 35.
Hader W, Yee I. Incidence and prevalence of multiple sclerosis in Saskatoon, Saskatchewan. Neurology. 2007; 69: 1224– 1229.
Evans C, Marrie R, Zhu F, . Adherence and persistence to drug therapies for multiple sclerosis: a population-based study. Mult Scler Relat Disord. 2016; 8: 78– 85.
Amezcua L, Lerner A, Ledezma K, . Spinal cord lesions and disability in Hispanics with multiple sclerosis. J Neurol. 2013; 260: 2770– 2776.
Stankiewicz J, Neema M, Alsop D, . Spinal cord lesions and clinical status in multiple sclerosis: a 1.5 T and 3 T MRI study. J Neurol Sci. 2009; 279: 99– 105.
2018 Revised Guidelines of the Consortium of MS Centers MRI Protocol for the Diagnosis and Follow-up of MS. Accessed July 26, 2019. https://www.mscare.org/page/MRI_protocol
Filippi M, Rocca M, Ciccarelli O, . MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016; 15: 292– 303.
Traboulsee A, Létourneau-Guillon L, Freedman M, . Canadian Expert Panel recommendations for MRI use in MS diagnosis and monitoring. Can J Neurol Sci. 2015; 42: 159– 167.
Zecca C, Disanto G, Sormani M, . Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler. 2015; 22: 782– 791.
Elsone L, Platkajis A, Karelis G, Saukans I. Frequency and localization of spinal cord demyelination in MS patients, coexistence of intervertebral disc protusion. Acta Chirurgica Latviensis. 2011. doi: 10.2478/v10163-012-0014-8.
Brownlee W, Altmann D, Prados F, . Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain. 2019; 142: 2276– 2287.
Sanchirico M, Caldwell-Tarr A, Mudumby P, Hashemi L, Dufour R. Treatment patterns, healthcare resource utilization, and costs among Medicare patients with multiple sclerosis in relation to disease-modifying therapy and corticosteroid treatment. Neurol Ther. 2018; 8: 121– 133.
Thompson A, Kobelt G, Berg J, Capsa D, Eriksson J, Miller D. New insights into the burden and costs of multiple sclerosis in Europe: results for the United Kingdom. Mult Scler. 2017; 23( suppl): 204– 216.
Rae-Grant A, Day G, Marrie R, . Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018; 90: 777– 788.
Wingerchuk D. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord. 2011; 5: 13– 22.
Hawkes C. Smoking is a risk factor for multiple sclerosis: a metanalysis. Mult Scler. 2007; 13: 610– 615.
Rom O, Avezov K, Aizenbud D, Reznick A. Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol. 2013; 187: 5– 10.
Cloos P, Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology. 2004; 5: 139– 158.
Makrygiannakis D, Hermansson M, Ulfgren A, . Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008; 67: 1488– 1492.







