Publication
Research Article
International Journal of MS Care
Effect of Group Counseling Plus Tailored Exercise on Mobility Function in Multiple Sclerosis
Author(s):
Abstract
Background:
Multiple sclerosis (MS) impairs muscular function and limits individuals’ ability to perform everyday activities requiring mobility. People with MS frequently exhibit mobility problems (ie, slower walking speed, shorter strides). General exercise training (eg, resistance, aerobic) provides modest physiological and walking mobility benefits. However, researchers suggest tailoring of interventions to address mobility specifically. We conducted a phase 2a pre-post intervention development study (Obesity-Related Behavioral Intervention Trials [ORBIT] intervention development model) of mobility exercise plus cognitive behavioral counseling to improve function and social cognitions known to encourage exercise.
Methods:
The intervention was conducted twice per week for 8 weeks followed by 1 month of self-managed mobility exercise. Participants (N = 29; mean ± SD age = 52.24 ± 11.36 years, mean time since MS diagnosis ≥11 years) were assessed at baseline and after follow-up for mobility function, social cognitions, and intervention fidelity indicators.
Results:
Results indicated significant improvements in a variety of valid measures of mobility function (eg, 400-m walk), self-regulatory efficacy for mobility exercise and symptom control, and fidelity measures with small to medium effect sizes.
Conclusions:
Positive findings suggest that the intervention seems to merit testing as a randomized pilot study following the ORBIT model.
Multiple sclerosis (MS) is a chronic disabling disease of the central nervous system characterized by spasticity, weakness of the musculature, and fatigue. One of the most common features of MS is impairment of everyday walking. Limitations in individuals’ dynamic mobility affect their activities of daily living, independent engagement with their community, and related quality of life.1
Not surprisingly, people with MS are more inactive than those without the disease.2 3 Inactivity due to MS coupled with aging with the disease lead to subsequent deconditioning in a variety of physiological systems such that mobility problems are one observable effect.4 For example, MS affects various aspects of gait kinematics, such as slower walking, shorter stride, and greater support time of both legs.5
A meta-analytic review of the effect of exercise training on mobility in persons with MS supports the view that exercise improves walking mobility.6 The accumulated evidence indicated small effects in favor of exercise training having a positive effect on walking mobility. Would effects have been greater if exercise training had more specifically focused on improving various aspects of dynamic mobility? Past authors4 have suggested that focusing on exercise programs/interventions is needed to address the specific needs of participants with MS.
For people with MS to benefit from exercise, they must adhere.4 However, exercise adherence is problematic among both asymptomatic7 and symptomatic2 3 individuals, particularly when they are left to self-manage their exercise.8
A review9 recommended that theory-based exercise interventions for MS address poor exercise adherence. One promising intervention intended to reduce the decline in adherence after supervised intervention is group-mediated cognitive behavioral (GMCB) counseling.10 11 This group-based style of intervention is based on the agency aspect of social cognitive theory12 and the group dynamics literature.11,13–15 Using the group as an agent of change, self-regulation skills such as goal-setting and self-monitoring are taught and practiced. See the study by Brawley et al15 for a summary of positive effects in different chronic disease populations. The review9 suggested that exercise behavior is positively affected by such aspects of social cognitive theory as goal-setting and self-efficacy.
One approach to address the functional limitations of people with MS is to engage individuals in a program focused on improving their dynamic mobility and gait. A second aspect would be to encourage participants’ postintervention confidence in adhering to the mobility exercise such that gains in functional performance are sustained. In the GMCB intervention approach, exercise and self-regulation counseling are coupled together to encourage and sustain behavior change.11 15
The present study represents an early stage of behavioral treatment development as characterized by the Obesity-Related Behavioral Intervention Trials [ORBIT] framework. The framework defines a refinement process leading to efficacy of prevention/management treatments for an array of chronic diseases.16 When an intervention protocol is used with a new population (MS) and different behavioral outcomes (ie, estimates of dynamic mobility), the type of testing suggested by the ORBIT model (ie, called phase 2a) is through preliminary proof-of-concept studies. Such studies precede larger studies to determine whether the treatment can achieve a benefit before larger-sample, randomized pilot testing is undertaken.16
The primary objective of the study was to determine whether various aspects of dynamic mobility and related social cognitions could be improved for individuals with MS enrolled in a dynamic mobility intervention. We hypothesized that comparing baseline with postintervention follow-up, participation would improve indices of 1) dynamic mobility and 2) social cognitions related to mobility exercise.
We also wanted to examine the fidelity of major systematically delivered components of the intervention: self-efficacy for skills learned, the group as a focus, and counseling facilitator–participant collaboration. Favorable evaluation of these fidelity indicants would confirm that participants perceived successful delivery of intervention components.
Methods
Participants and Study Design
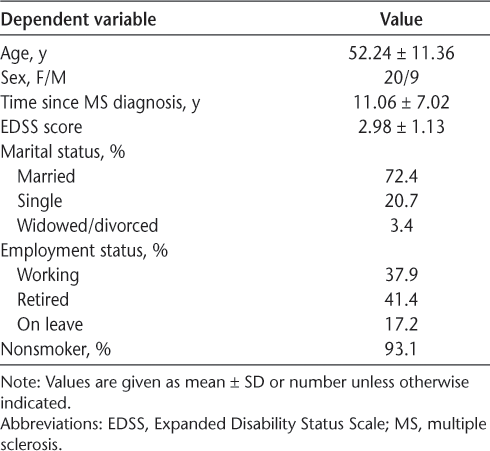
Ethics approval for the study was obtained from the University of Saskatchewan research ethics board, and all participants provided informed consent. Volunteers (n = 34) were adults recruited from community fitness centers offering programs for MS and a hospital clinic specializing in MS treatment. Exclusion screening criteria that would mitigate against safe participation in a community-based exercise program were additional health conditions (cardiovascular, respiratory, orthopedic, vision, and hearing). Participants were required to be sufficiently mobile that continuous assistance from another person or a walking aid were not needed (eg, wheelchair-dependency and inability to safely retrieve an object from the floor were exclusion criteria). All participants had relapsing-remitting MS and were assessed using the Expanded Disability Status Scale (EDSS) by the director and chief physiatrist (K.K.) at a municipal hospital MS clinic. The overall EDSS score ranges from 0 (normal, no impairment) to 10 (death due to MS). Participants’ scores ranged from 1.5 to 6.
This study used a treatment-only, pre-post design. The intervention duration was 12 weeks, with the intensive phase (ie, exercise coupled with counseling) being 8 weeks followed by a 4-week follow-up phase.
Intervention Protocol
Dynamic Mobility and Gait Training
The mobility training was designed to progressively improve dynamic mobility by challenging the various dimensions of mobility.17 For example, distance, attentional demands, and environment changes such as navigating stairs and ramps and adjusting to other people while walking all interact to influence mobility. Thus, training incorporated progressively more challenging mobility tasks (ie, walking against the flow of traffic, braking while walking down ramps) as the exercise aspect of the intervention progressed. This training included mobility task complexity consistent with the Walking InCHIANTI Toolkit (WIT).18 The toolkit provides a battery of complex walking tasks that reflect the multidimensional and complex nature of being mobile in the community. As the tasks become more complex, walking speed slows.19
The exercise training portion of the intervention was conducted twice weekly for 60 minutes per session for 8 weeks. The mobility exercise was based on the principle of progressive overload and was introduced over the course of the intervention based on known difficulty of the tasks: walking over unstable surfaces, over and around obstacles, at varying speeds, over steps and ramps, carrying weights, and while wearing sunglasses to dim ambient light. Known difficulty of tasks was informed by baseline performance and by difficulty of tasks drawn from the WIT.
GMCB Counseling
Counseling was based on the GMCB intervention model.15 Briefly, the intensive phase of the study eased participants into mobility exercise goals to provide early mastery experiences to build task self-efficacy. Self-regulatory skills were fostered through weekly goal-setting assignments, and group bonds were established through weekly discussions by group members of their successes, failures, and methods of overcoming barriers. The first week of the 4-week follow-up phase allowed participants to discuss the use of their cognitive behavioral strategies with their facilitator via an individual scripted call. This call focused on transition away from staff- and group peer-supported self-regulation to personal responsibility for self-regulation. A detailed description of the rationale and design of this GMCB approach can be found elsewhere.15 Table S1, which is published in the online version of this article at ijmsc.org, provides a summary of the progression of exercise and counseling over the weeks of intervention and follow-up.
Measures
Mobility Function
Mobility function measures were conducted at baseline and at postintervention follow-up.
Function. Mobility function was assessed by a variety of mobility tasks recognized for their validity in the gait and mobility literature: 1) a selection from the battery of walking tasks from the WIT,20 2) a timed 400-m walk,21 and 3) the Timed Up and Go (TUG) test.22 Generally, walking speed is considered a robust measure that has been linked to a range of outcomes, including response to rehabilitation, and is predictive of mobility disability.23
The WIT includes a variety of 7-m walks. The four selected tasks from the WIT were a normal-pace 7-m walk; a brisk-pace 7-m walk; a 7-m walk stepping over two obstacles at a brisk pace in reduced light; and a 7-m normal-pace walk interrupted three times by stopping to bend down and pick up small light objects (eg, a spoon). Acceleration and deceleration phases of the walk occur outside assessed performance. These were sensor-timed and performed on the platinum version of the GAITRite portable gait analysis system (CIR Systems Inc).24 Objective performance on each of these tasks is expressed as velocity by the gait analysis system. Increases in velocity are one indication of gait improvement23 in the aforementioned tasks.
A timed 400-m walk21 was performed in an open indoor facility where the distance was marked in a four-sided square where one loop is 40 m. Participants must complete ten loops for the 400-m distance. In middle-age to older women, walk time has been significantly related to balance,25 and in healthy older adults to extent of disability.26
Finally, the TUG test22 was performed individually. The TUG test was performed twice, and the best time was used in the analyses.
Physical Activity Volume. Moderate-to-vigorous physical activity volume was assessed to examine baseline activity. Moderate-to-vigorous physical activity was measured in total minutes per week excluding light-intensity activity due to difficulty with recall.27 At baseline, moderate-to-vigorous physical activity performed during the past week was assessed using a 7-day Physical Activity Recall interview. This measure has been validated against objective measures such as accelerometry.28
Social Cognitions
Social cognitions were measured at baseline and at the end of the follow-up period.
Task Efficacy and Self-regulatory Efficacy. We examined both task efficacy and self-regulatory efficacy (SRE) for two reasons. First, measurement of changes in task self-efficacy was important to determine efficacy belief change due to mastery in performing specific functional mobility tasks. Second, the GMCB counseling aspect of the intervention focused on providing participants with mastery experiences of self-regulatory strategies known to promote adherence to regular physical activity. Efficacy measures are described later herein.
Self-efficacy for MS Symptom Control. A nine-item task efficacy scale assessing confidence to manage MS symptoms and continue with most daily activities was used. Responses are scaled from 10 (very uncertain) to 100 (very certain). Reliability of the scale was α = 0.92. A validation study reported α = 0.90.29
Multiple Sclerosis Self-efficacy Scale. A nine-item task self-efficacy scale assessing confidence in completing aspects of daily functioning, the Multiple Sclerosis Self-efficacy (MSSE) Scale, was used with responses scaled from 10 (very uncertain) to 100 (very certain). Reliability of the scale was α = 0.82. A validation study reported α = 0.86.29
SRE for Mobility Exercise in the Face of Barriers. A 14-item scale assessed confidence to continue with balance and mobility activity in the face of commonly experienced barriers to adherence in the next 6 weeks.30 Responses are scaled from 0 (not at all confident) to 10 (completely confident). Reliability of the scale was α = 0.88.
Self-efficacy for Community Mobility. A nine-item task efficacy scale assessed confidence to navigate stairs, crosswalks, crowded malls, and other community mobility challenges.30 31 Responses are scaled from 0 (not at all confident) to 10 (completely confident). Reliability of the scale was α = 0.94.
SRE to Self-regulate Independent Mobility Activity. A nine-item scale assessed confidence to self-regulate mobility activity in the coming 6 weeks. Responses are scaled from 0 (not at all confident) to 10 (completely confident). Reliability of the scale was α = 0.93. The items in this measure have been used previously in a published investigation32 examining SRE for engaging in independent physical activity. Reported reliability was α = 0.78.
Intervention Fidelity: Self-regulatory Skills, Cohesion, Collaboration
A description of fidelity measures can be found in Appendix S1. Results are reported in Table S2.
Procedures
Study recruitment was ongoing and conducted in waves where individuals participated in small groups. Seven waves were required to complete the study. Recruitment was achieved via newspaper advertisements, visits to local MS exercise programs, face-to-face by a physiatrist at an MS hospital clinic, and local television news coverage of the free offering of a unique mobility intervention for individuals with MS. Screening phone calls of interested participants were conducted to determine eligibility. Preintervention baseline assessments were conducted the week before the intervention. Postintervention assessments were conducted at week 12 (ie, 4 weeks after the intervention concluded).
Group size (ie, wave size) ranged from three to six people. A minimum of three people per group was required for the planned group processes of group identification, interaction, and discussion to occur.13 An upper limit of six people ensured manageable group size33 and attention to individual differences in mobility to ensure participant safety and modification of training.
Data Analysis
Data were screened for missing data, outliers, and normality. Repeated-measures multivariate analyses of variance (MANOVAs) were conducted to examine preintervention and postintervention differences in all variables (ie, primary and fidelity). Bonferroni adjustments were made for multiple test comparisons for each group of the primary measurement constructs. These primary comparisons were used to evaluate effects of the intervention. Effect sizes are reported as Cohen’s d.
Results
Participants
Five individuals dropped out of the study (one each due to work demands, relapse of MS, and feeling that the program was too difficult and two for unexplained reasons), leaving 29 participants. The final sample had more women (n = 20) than men (n = 9). Participants’ mean ± SD age was 52.24 ± 11.36 years. See Table 1 for further demographic information. Mean ± SD weekly moderate-to-vigorous physical activity was 192.67 ± 181.49 minutes.
Baseline demographic characteristics of the 29 study participants
Mobility Function
Recall that there were six measures of mobility function. A MANOVA including the four WIT measures was conducted separately from a MANOVA including the TUG test and the 400-m walk. The overall MANOVA for the WIT measures was significant (F 4,24 = 8.694, Pillai’s trace = 0.592, P < .001, η2 partial = 0.592). Based on Bonferroni adjustment, significance of the four WIT measures was set at P < .0133 (.05 ÷ 4). Followup ANOVAs revealed that the sample significantly improved in the normal-pace walk (F 1,27 = 22.805, P < .001, η2 partial = 0.458), brisk-pace walk (F 1,27 = 10.490, P = .003, η2 partial = 0.280), and walk over two obstacles (F 1,27 = 8.319, P = .008, η2 partial = 0.236). See Table 2 for means and effect sizes.
Function difference
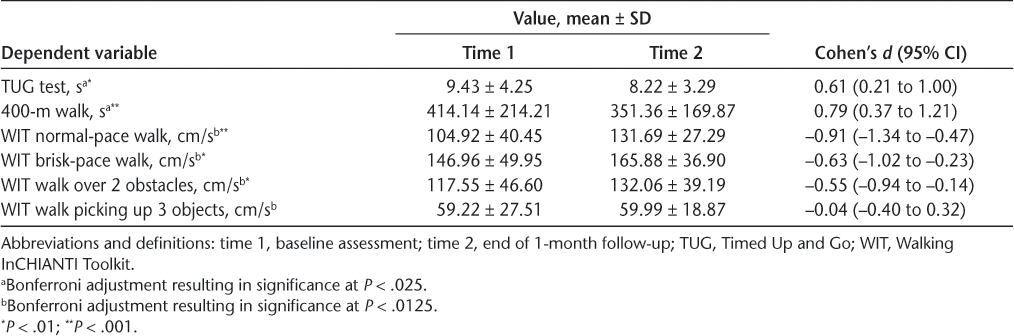
The overall MANOVA for the TUG test and the 400-m walk was significant (F 2,27 = 8.909, Pillai’s trace = 0.398, P = .001, η2 partial = 0.398). Significance was set at P = .025 according to a Bonferroni adjustment. The TUG test (F 1,28 = 10.785, P = .003, η2 partial = 0.278) and the 400-m walk (F 1,28 = 18.239, P < .001, η2 partial = 0.394) also significantly improved. See Table 2 for means and effect sizes.
Self-Efficacy: Task and Self-regulatory
The overall MANOVA for the task self-efficacy variables of the MSSE Scale and community mobility was significant (F 2,27 = 4.670, Pillai’s trace = 0.257, P = .018, η2 partial = 0.257). For these measures, significance was set at P < .025 (Bonferroni adjusted: .05 ÷ 2). The MSSE scores significantly improved (F 1,28 = 8.559, P = .007, η2 partial = 0.234), and community mobility demonstrated a small improvement effect (F 1,28 = 5.289, P = .029, η2 partial = 0.159). For means and effect sizes, see Table 3.
Task efficacy and SRE differences
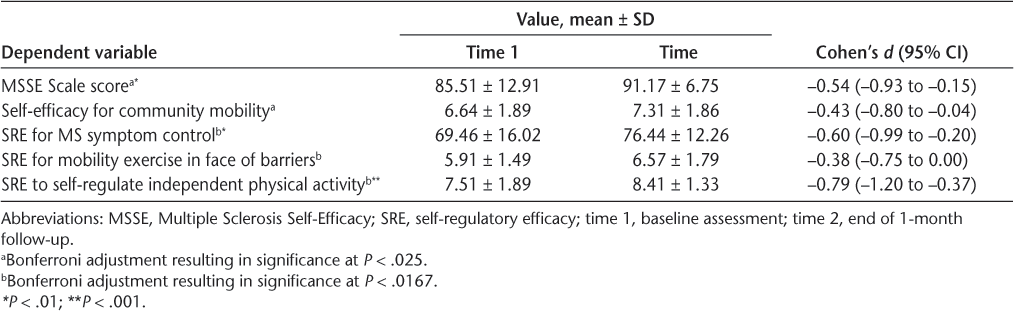
The overall MANOVA for the three SRE measures was significant (F 3,26 = 8.975, Pillai’s trace = 0.509, P < .001, η2 partial = 0.509). Significance of the three SRE measures was set at P < .0167 (.05 ÷ 3). There was significant improvement in SRE for symptom control (F 1,28 = 10.361, P = .003, η2 partial = 0.270) and for independent mobility activity in the next 6 weeks (F 1,28 = 18.044, P < .001, η2 partial = 0.392). For mobility exercise in the face of barriers (F 1,28 = 4.115, P = .052, η2 partial = 0.128), SRE was nonsignificant. See Table 3 for means and effect sizes.
Fidelity Measure Results
The results reflect participants’ responses to measures that served as a check on participants’ perceptions of the content and facilitator-member interaction systematically delivered during the intervention. See Table S2 for means and effect sizes.
Discussion
The primary objective of this study was to make a preliminary determination of whether the intervention of mobility exercise and GMCB counseling encouraged changes in indices of 1) dynamic mobility and 2) social cognitions related to adherence to mobility exercise. We also examined changes in the indicants of planned intervention content as an evaluation of intervention fidelity. Positive changes after the intervention were observed for the primary objective, and fidelity results indicated that the intervention was delivered as planned.
Of the six functional measures used to examine mobility function, five improved significantly after the intervention (ie, three WIT measures, TUG test, 400-m walk). Collectively, aspects of mobility function improved over time as a function of the intervention training, and this change was retained 1 month after the intervention was discontinued. These findings align with those of a meta-analysis that exercise training is associated with improvement in walking mobility in individuals with MS.6 Effects in the present study seem to be larger than those reported in the meta-analysis (Cohen’s d) possibly because of our focus on mobility-related exercise rather than mixed forms of training (eg, general aerobic plus resistance) and differences in mobility-related measures used (eg, present study multiple measures vs single measure).
Regarding social cognitive changes, both task efficacy measures improved between baseline assessment and the end of follow-up. Participants were more confident in their ability to perform aspects of daily functioning, despite their confidence in this area being fairly high at baseline (MSSE29). As well, participants’ efficacy to navigate stairs, crosswalks, and crowded malls (self-efficacy for community mobility) improved significantly.
Two aspects of participant SRE—for symptom control and for independent mobility activity in the next 6 weeks—significantly increased. Participants increased the confidence that they could exercise and cope with the symptoms of their MS and the confidence that they could schedule and plan for weekly mobility exercise in the 6 weeks after follow-up assessment. This increased confidence to regulate mobility exercise independent of staff and group peer support suggests that participants were motivated to maintain their mobility-specific exercises. As well, their increased confidence to be able to perform daily activities despite MS symptoms is encouraging given that the sample was already confident in this regard (ie, change from high-60% to mid-70% confidence). Thus, even among individuals with MS who were already receptive to mobility exercise, improvement in self-efficacy as a function of the intervention was observed.
Returning to the theoretical perspective that was the intervention foundation, Bandura12 would suggest that self-efficacy and participants’ self-reflection on improving functional outcomes likely operate in concert over time. Social cognitive theory suggests that, together, along with group influences, participants’ efficacy helps guide the persistent actions necessary to generate sustained outcomes and improved mobility. Collective factors can contribute to self-efficacy, such as direct experience with various aspects of mobility exercise, social support from peers in the small group, vicarious experience by observing others with MS perform exercise, and discussions of how they try to self-regulate their mobility exercise. Thus, change in efficacy in the present MS participants is likely a product of several of these systematically delivered intervention determinants for which self-efficacy is a marker. The improvements in both function and social cognitions in the present study are similar to those observed in other studies of symptomatic populations11,14,15,34 and address suggestions made by Motl and colleagues9 regarding the use of theory-driven content in MS physical activity interventions.
Relative to the intervention fidelity, the significant changes to confidence in learning self-regulatory strategies, cohesion, and facilitator collaboration indicate a mainly positive participant response to what participants experienced during their intervention. Participant adherence to the study at follow-up was 85%, suggesting that participants accepted the intervention.
Some study limitations and strengths should be noted. As characterized by the ORBIT model of behavioral intervention development,16 the present study was a phase 2a within-subjects, pre-post, treatment-only investigation. It was designed to determine whether the treatment led to change. Nine of 29 participants (31%) had mobility disability (ie, EDSS score ≥4).35 This phase 2a intervention development study concerned indicants of change while recognizing that there are limitations that go hand-in-hand with this level of study.16 These limitations entailed convenience sampling and lacking control comparisons. The strengths of the intervention study are 1) its theoretical basis (ie, group dynamics; agency aspect of social cognitive theory; also see the study by Motl et al9), 2) the empirical basis drawn from the success of the GMCB plus exercise type of intervention with other symptomatic populations,15,36,37 and 3) use of empirically supported, valid measures of function and social cognitions specific to the exercise context and relevant to this chronic disease. Interestingly, the focus of the intervention to encourage improvement in indicants of dynamic mobility seems to have caused positive change despite participants being somewhat active. This finding seems to support the suggestion that exercise needs to be focused to address the specific needs of individuals with MS.4
Three preliminary implications for future research are evident. First, dynamic walking mobility can be improved through tailored mobility exercise. Second, this mobility exercise, once learned in a structured program, has the potential to be sustained outside the program by individuals with MS who have also learned cognitive behavioral strategies to assist their mobility exercise self-regulation. This potential is suggested by the various aspects of mobility function that improved over time as a function of our intervention training and by this change being retained a month after our intervention was discontinued. Further support for the potential from this type of intervention is apparent from evidence of this type drawn from other symptomatic populations.15,36,37 Third, based on the ORBIT model’s suggestions for what follows this phase 2a study, this study provides preliminary evidence supporting future planning of a randomized pilot study (phase 2b of the ORBIT intervention development process). Such a future study would, as in this study, require that the level of mobility disability does not preclude continuous walking without assistance. Also consistent with the present study, inclusion of those with some level of mobility disability (EDSS score ≥4)35 would be advised based on the favorable mobility outcomes reported herein and the desire to target individuals who have the most to gain in terms of mobility.
PRACTICE POINTS
Tailored mobility exercise has the potential to improve mobility for individuals with MS who are minimally mobile but able to walk unassisted.
This sample overall met/exceeded physical activity guidelines and yet made improvements in their walking function after engaging in tailored mobility exercise. This finding suggests that even those engaging in high levels of traditional physical activity may benefit from tailored mobility exercise.
Acknowledgments
The authors gratefully acknowledge Dr Madelaine Gierc, for her help delivering some sessions of the intervention.
References
Motl RW, Gosney JL. Effects of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008; 14: 129– 135.
Kinnett-Hopkins D, Adamson B, Rougeau K, Motl RW. People with MS are less physically active than healthy controls but as active as those with other chronic diseases: an updated meta-analysis. Mult Scler Relat Disord. 2017; 13: 38– 43.
Klaren, RE, Motl, RW, Dlugonski, D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013; 94: 2342– 2348.
Ploughman M, Deshpande N, Latimer-Cheung AE, Finlayson M. Drawing on related knowledge to advance multiple sclerosis falls-prevention research. Int J MS Care. 2014; 16: 163– 170.
Martin CL, Phillips BA, Kilpatrick TJ, . Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006; 12: 620– 628.
Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. 2009; 23: 108– 116.
Blackwell DL, Clarke TC. State variation in meeting the 2008 federal guidelines for both aerobic and muscle-strengthening activities through leisure-time physical activity among adults aged 18–64: United States, 2010–2015. Natl Health Stat Report. 2018; 112: 1– 22.
Muller-Riemenschneider F, Reinhold T, Nocon M, Willich SN. Long-term effectiveness of interventions promoting physical activity: a systematic review. Prev Med. 2008; 47: 354– 368.
Motl RW, Pekmezi D, Wingo BC. Promotion of physical activity and exercise in multiple sclerosis: importance of behavioral science and theory. Mult Scler J Exp Transl Clin. 2018; 4: 2055217318786745.
Brawley LR, Rejeski WJ, Lutes L. A group-mediated cognitive-behavioral intervention for increasing adherence to physical activity in older adults. J Appl Biobehav Res. 2000; 5: 47– 65.
Rejeski WJ, Brawley LR, Ambrosius WT, . Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003; 22: 414– 423.
Bandura A. Self-efficacy: The Exercise of Control. Freeman; 1997.
Borek AJ, Abraham C. How do small groups promote behaviour change? an integrative conceptual review of explanatory mechanisms. Appl Psychol Health Well Being. 2018; 10: 30– 61.
Brawley LR, Rejeski WJ, Gaukstern JE, Ambrosius WT. Social cognitive changes following weight loss and physical activity interventions in obese, older adults in poor cardiovascular health. Ann Behav Med. 2012; 44: 353– 364.
Brawley LR, Flora PK, Locke SR, Gierc MSH. Efficacy of the group-mediated cognitive behavioural intervention: a decade of research. In: Eys M, Beauchamp M, eds. Group Dynamics in Exercise and Sport Psychology. 2nd ed. Routledge/Psychology Press; 2014; 183– 202.
Czajkowski, SM, Powell LH, Adler N, . From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015; 34: 971– 982.
Patla A, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Act. 1999; 7: 7– 19.
Bandinelli S, Pozzi M, Lauretani F, . Adding challenge to performance-based tests of walking: the Walking InCHIANTI Toolkit (WIT). Am J Phys Med Rehabil. 2006; 85: 986– 991.
Shumway-Cook A, Guralnik JM, Phillips CL, . Age-associated declines in complex walking task performance: the Walking InCHIANTI Toolkit. J Am Geriatr Soc. 2007; 55: 58– 65.
Ferrucci L, Bandinelli S, Benvenuti E, . Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000; 48: 1618– 1625.
Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001; 49: 1544– 1548.
Podsiadlo D, Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39: 142– 148.
Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015; 23: 314– 322.
Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003; 17: 68– 74.
Pettee Gabriel KK, Rankin RL, Lee C, Charlton ME, Swan PD, Ainsworth BE. Test-retest reliability and validity of the 400-meter walk test in healthy, middle-aged women. J Phys Act Health. 2010; 7: 649– 657.
Newman AB, Simonsick EM, Naydeck BL, . Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006; 295: 2018– 2026.
Matthews CE, Ainsworth BE, Hanby C, . Development and testing of a short physical activity recall questionnaire. Med Sci Sports Exerc. 2005; 37: 986– 994.
Hayden-Wade HA, Coleman KJ, Sallis JF, Armstron C. Validation of the telephone and in-person interview versions of the 7-day PAR. Med Sci Sports Exerc. 2003; 35: 801– 809.
Schwartz CE, Coulthard-Morris L, Zeng Q, Retzlaff P. Measuring self-efficacy in people with multiple sclerosis: a validation study. Arch Phys Med Rehabil. 1996; 77: 394– 398.
Rejeski WJ, Brawley LR. Functional health: innovations in research on physical activity with older adults. Med Sci Sports Exerc. 2006; 38: 93– 99.
Sessford JD, Jung M, Brawley LR, Forbes JL. Do older adults’ beliefs about their community mobility predict walking performance? J Aging Phys Act. 2014; 23: 272– 278.
Cramp AG, Brawley LR. Moms in motion: a group-mediated cognitivebehavioral physical activity intervention. Int J Behav Nutr Phys Act. 2006; 22: 23.
Carron AV, Brawley LR, Widmeyer WN. The impact of group size in an exercise setting. J Sport Exerc Psychol. 1990; 12: 376– 387.
Rejeski WJ, Spring B, Domanchuk K, . A group-mediated, home-based physical activity intervention for patients with peripheral artery disease: effects on social and psychological function. J Transl Med. 2014; 12: 1– 8.
Marrie RA, Rudick R, Horwitz R, . Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010; 74: 1041– 1047.
Brawley LR, Arbour-Nicitopoulos KP, Martin Ginis KA. Developing physical activity interventions for adults with spinal cord injury, part 3: a pilot feasibility study of an intervention to increase self-managed physical activity. Rehabil Psychol. 2013; 58: 316– 321.
Rejeski WJ, Brubaker PH, Goff DC Jr, . Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011; 171: 880– 886.







