Publication
Research Article
International Journal of MS Care
Impact of Switching to Fingolimod Versus Injectable Disease-Modifying Therapy Cycling on Risk of Multiple Sclerosis–Related Relapses
Abstract
Background:
Clinical and real-world studies have shown significant reductions in multiple sclerosis (MS) relapses with fingolimod versus injectable disease-modifying therapies (DMTs). Multiple sclerosis relapse rate and incidence were compared in patients switching from an injectable DMT to fingolimod and those cycling from one injectable DMT to another or remaining on their original injectable DMT.
Methods:
Retrospective analysis was performed using Commercial and Medicare Supplemental claims data (July 1, 2010, to June 30, 2016) of adults with MS receiving ≥1 injectable DMT. Relapses were identified from MS-related hospitalization, outpatient emergency department or office visit, and corticosteroid administration. Annualized relapse rate ratio was estimated.
Results:
Of 16,352 patients, 1110 were switchers to fingolimod, 908 were injectable DMT cyclers, and 14,334 were nonswitchers. At baseline, rate and incidence of MS relapses were higher in switchers and injectable DMT cyclers versus nonswitchers (P < .001); mean ± SD relapse rates declined from 0.4 ± 0.7, 0.4 ± 0.7, and 0.2 ± 0.5 at baseline to 0.2 ± 0.5, 0.3 ± 0.6, and 0.1 ± 0.4 after follow-up in switchers, injectable DMT cyclers, and nonswitchers, respectively. Relapse incidence declined in each cohort. The highest reductions in relapse rate and incidence were in switchers to fingolimod, where relapse risk was significantly reduced versus injectable DMT cyclers (22%, P = .0433) and nonswitchers (47%, P < .001).
Conclusions:
This study provides evidence that patients switching from an injectable DMT to fingolimod have the highest reductions in annualized rate and incidence of MS relapses and significantly reduced risk of relapse versus injectable DMT cyclers and nonswitchers.
Multiple sclerosis (MS) is a chronic, progressive, immune-mediated disease of the central nervous system.1 It is one of the most prevalent disabling neurologic conditions, estimated to affect more than 900,000 individuals in the United States.2 Currently, there is no cure for MS, and available treatments aim to prevent relapses and/or delay disability accumulation. Injectable disease-modifying therapies (DMTs), including interferon beta-1a (IFNβ-1a) (subcutaneous and intramuscular administrations), pegylated IFNβ-1a, IFNβ-1b, and glatiramer acetate, have commonly been used as first-line therapy for MS. A recent summary of meta-analyses evaluated the efficacy of a range of DMTs, including injectable therapies, and found that injectable DMTs improve short-term disability progression; however, there is little evidence of efficacy over longer periods.3 Furthermore, treatment adherence remains an issue. Adherence rate, which comprises acceptance, persistence, and compliance according to the World Health Organization,4 is lower and discontinuation rates higher with injectable DMTs than with oral DMTs such as fingolimod.5–8 The most common reasons for discontinuation of injectable DMTs include flulike symptoms, injection-site reactions, difficulty in self-administering injections, needle phobia, suboptimal efficacy and tolerability, and patient perception of medication risk and benefit, as well as additional financial, emotional, and physical burdens associated with the treatment.9–11 Adherence to DMTs has been associated with a significant reduction in MS-related hospitalizations,12 resource utilization,9 and relapse rates13 and improvement in health-related quality of life,4 which collectively reduce the total cost of MS therapy.14
Fingolimod was the first oral DMT approved by the US Food and Drug Administration for relapsing forms of MS.15 In clinical trials, fingolimod has shown significant reductions in the annualized relapse rate (ARR) compared with placebo and intramuscular IFNβ-1a.16 17 In addition, switching from IFNβ-1a to fingolimod showed reduction in the 12-month relapse rate in patients with relapsing-remitting MS.18 The present study aimed to evaluate and compare the annualized rate and incidence of MS relapses in patients switching from any injectable DMT to fingolimod with those in patients switching between injectable DMTs (injectable DMT cyclers) and those who remained on the same injectable DMT (injectable DMT nonswitchers) in a real-world setting.
Methods
Study Design and Data Sources
A retrospective longitudinal analysis was conducted using the US pharmacy claims data from the MarketScan Commercial Claims and Encounters database (Truven Health Analytics) and the Medicare Supplemental database from July 1, 2010, to June 30, 2016. These databases contain data from managed-care organizations, large employers, hospitals, and Medicare and Medicaid programs. Both databases consist of adjudicated medical and pharmacy claims from 2001 onward with longitudinal follow-up. Data were deidentified according to the US Health Insurance Portability and Accountability Act of 1996. The study did not involve collection, use, or transmission of individually identifiable data, and, thus, no institutional review board approval was required.
Patient Population
Patients aged 18 years and older at the index date (treatment switch date or injectable DMT start date for nonswitchers) with a confirmed diagnosis of MS (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 340; ICD-10-CM [revision] diagnosis code G35) were included. The 12-month period before the index date was considered as the baseline period, and the 12-month period after the index date was considered as the follow-up period. Patients who were continuously enrolled in the respective health plan for at least 1 year before and 1 year after the index date and had at least one claim for the injectable DMT of interest (interferon or glatiramer acetate) during the baseline and 1-year follow-up periods were included in the analysis. Patients with claims for any other DMTs besides those of interest during the baseline and follow-up periods were excluded. Data were included for each patient only for 12 months before (baseline period) and 12 months after (follow-up period) their single index date; any additional switches by patients were not included.
Based on the pattern of therapy switch, patients with MS included in the analysis were classified into three mutually exclusive cohorts: patients who switched from injectable DMTs to fingolimod (switchers to fingolimod), patients who switched from one injectable DMT to another (injectable DMT cyclers), and patients who were treated with an injectable DMT and remained on the same injectable DMT (injectable DMT nonswitchers).
Study Outcomes
Baseline demographic characteristics (age, sex, US geographic region, and health insurance plan type) were recorded for all three cohorts. The Charlson Comorbidity Index for the baseline period was calculated as the weighted sum of 17 clinical conditions (eg, cancer, diabetes, liver disease, peptic ulcer), with higher total index scores indicative of a greater comorbidity burden in these patients.19 The rate and incidence of MS relapses and MS-related symptoms were recorded for the baseline and follow-up periods. Relapses of MS were assessed based on a published claims-based algorithm shown to have a 67% positive predictive value when originally tested in 300 patients with MS.20 21 According to this algorithm, patients who had either an inpatient claim with a diagnosis of MS at the primary position or an outpatient emergency department or office visit with a diagnosis of MS at any position followed by a claim for oral or intravenous corticosteroid administration within 7 days of the outpatient claim were considered. Multiple relapses within a 30-day window were treated as a single relapse event, and the first available service date was considered the relapse date.
Statistical Analysis
Descriptive analysis was used for continuous and categorical variables. Mean ± SD values were computed for the continuous variables, and counts and proportions were calculated for the categorical variables. Relapse rates, defined as the total number of relapses among patients in each cohort, were computed for each treatment setting (hospital, emergency department, or physician’s office) and overall in the baseline and follow-up periods. Similarly, relapse incidences, defined as the number of patients that experienced relapse among all patients in each group, were computed for each treatment setting (hospital, emergency department, or physician’s office) and overall in the baseline and follow-up periods.
Preliminary analyses revealed that the patient populations varied to such an extent that propensity score matching or other risk-adjustment methods would not appropriately control covariates to estimate the treatment effects. Therefore, difference-in-difference analyses were performed to estimate the treatment outcomes, comparing cohort differences in the preindex and postindex periods.22 This method enables the comparison of differences in outcomes between groups, both before and after an intervention, while controlling for bias from unobserved variables that remain fixed over time.23 First, data on the incidence and rate of MS relapses were visualized over the baseline and follow-up periods using the trend analysis for all three cohorts. This visual analysis was performed to rule out the possibility that any differences observed were the result of regression to the mean. Second, crude difference-indifference estimates were used to quantify the magnitude of change in the rate of MS relapses from preindex to postindex (formulas 1 and 2).23
Formula 1 applies to switchers to fingolimod or another injectable DMT versus injectable DMT nonswitchers. Difference-in-difference mean relapse rate = ([mean rate of patients in switch cohort in postindex period – mean rate of patients in switch cohort in preindex period] – [mean rate of patients in injectable DMT nonswitch cohort in postindex period – mean rate of patients in injectable DMT nonswitch cohort in preindex period]).
Formula 2 applies to switchers to fingolimod versus switchers to another injectable DMT (injectable DMT cyclers). Difference-in-difference mean relapse rate = ([mean rate of patients in switcher cohort in postindex period – mean rate of patients in switcher cohort in preindex period] – [mean rate of patients in injectable DMT cycler cohort in postindex period – mean rate of patients in injectable DMT cycler cohort in preindex period]).
Finally, a negative binomial generalized linear regression model was fitted to the repeated measures data to estimate the annualized relapse rate ratio over 1 year between the following groups: switchers to fingolimod and injectable DMT cyclers, switchers to fingolimod and injectable DMT nonswitchers, and injectable DMT cyclers and injectable DMT nonswitchers.23 For each comparison, the response variable (dependent variable) was the number of relapses recorded per patient while receiving therapy, and it was assumed to follow a negative binomial distribution. The number of relapses recorded while receiving therapy (count of number of relapses for each patient) was the dependent variable. The analysis was conducted using the factors of time period (baseline vs follow-up) and treatment cohort to evaluate the effects of these variables and any interactions between them on the number of relapses. All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Of 73,021 patients treated with one of the injectable DMTs of interest, 16,352 met the inclusion criteria. Of the included patients, 1110 switched from an injectable DMT to fingolimod (switchers to fingolimod), 908 switched from one injectable DMT to another (injectable DMT cyclers), and 14,334 continued to be treated with the same injectable DMT (injectable DMT nonswitchers) during follow-up (Table S1, published in the online version of this article at ijmsc.org). The mean ± SD age of injectable DMT nonswitchers (50.3 ± 10.4 years) was significantly higher than that of injectable DMT cyclers (45.9 ± 11.6 years, P < .001) and switchers to fingolimod (45.1 ± 10.8 years, P < .001). The largest group of patients included in the analysis were females aged 45 to 54 years from the south region who had a fee-for-service insurance plan (Table S2). During the baseline period, the mean Charlson Comorbidity Index was significantly lower in injectable DMT nonswitchers compared with injectable DMT cyclers (0.4 vs 0.5, P < .001). Common comorbidities reported in all three cohorts included chronic pulmonary disease, diabetes without chronic complications, cerebrovascular disease, and hemiplegia or paraplegia. The injectable DMT cyclers reported a higher rate of MS-related symptoms, followed by switchers to fingolimod and injectable DMT nonswitchers (Table S2).
Relapse Rates
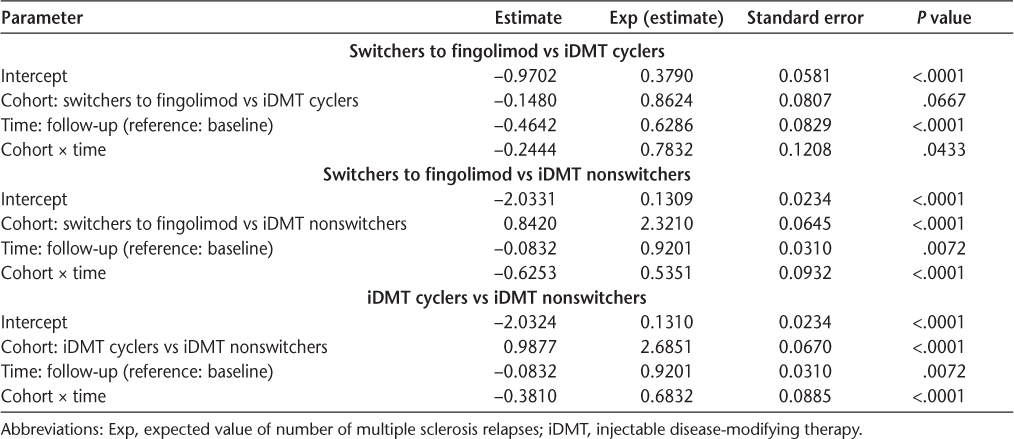
During the baseline period, the mean ± SD ARRs were identical in switchers to fingolimod and injectable DMT cyclers (0.4 ± 0.7). In contrast, the mean ± SD relapse rate was significantly lower in injectable DMT nonswitchers (0.2 ± 0.5, P < .001). The mean ± SD relapse rates reduced in the follow-up period in all three cohorts, with the highest rate of reduction in switchers to fingolimod (0.2 ± 0.5) (Table S3). Relapses of MS were more commonly identified from outpatient office visits than from outpatient emergency department visits or hospitalizations in both periods (Table S3). In switchers to fingolimod and injectable DMT cyclers, the rate of MS relapses increased before the treatment switch and subsequently reduced after the treatment switch. In switchers to fingolimod, the MS relapse rate increased from 0.06 (−3 quarter [Q]) to 0.13 (−1Q), reduced sharply to 0.05 in 1Q, and remained stable between 0.035 and 0.048 during the entire follow-up period (Figure S1). In injectable DMT cyclers, the MS relapse rate increased from 0.08 (−3Q) to 0.14 (−1Q), dropped to 0.07 in 1Q, and remained between 0.08 and 0.06 during the entire follow-up period (Figure S1). The MS relapse rate remained stable during baseline (0.038–0.035) and follow-up (0.038-0.036) in nonswitchers. Switchers to fingolimod experienced the highest reduction in MS relapse rate ratio compared with injectable DMT cyclers (22%; relapse rate ratio = 0.78, P = .0433) and injectable DMT nonswitchers (47%; relapse rate ratio = 0.54, P < .0001) (Table 1). The injectable DMT cyclers experienced a 32% reduction in MS relapse rate ratio compared with injectable DMT nonswitchers (relapse rate ratio = 0.68, P < .0001) (Table 1).
Risk of multiple sclerosis relapses: negative binomial with repeated measures
Relapse Incidence
During the baseline period, the incidence of MS relapses was the highest in injectable DMT cyclers (31.4%), followed by switchers to fingolimod (27.6%). In contrast, the incidence was the lowest in injectable DMT nonswitchers (12.4%) (Table S3). During the follow-up period, the incidence of MS relapses reduced to 20.5%, 14.3%, and 11.2% in injectable DMT cyclers, switchers to fingolimod, and injectable DMT nonswitchers, respectively, with the highest reduction observed in switchers to fingolimod (Table S3). In switchers to fingolimod and injectable DMT cyclers, the incidence of MS relapses increased before the treatment switch and subsequently reduced after the treatment switch (Figure S2). During the baseline period, the incidence of MS relapses increased from 6% in −4Q to 12% in −1Q in switchers to fingolimod. After the treatment switch, the incidence of MS relapse dropped to 5% in 1Q and remained stable between 3.4% and 4.5% during the entire follow-up period. A similar trend in the incidence of relapses was observed in the injectable DMT cyclers. During the baseline period, the incidence of relapses in injectable DMT cyclers was found to increase from 7% in −4Q to 14% in −1Q, before the treatment switch. After the treatment switch, the incidence of relapses declined steeply to 7% in 1Q and remained between 5% and 7% during the entire follow-up period (Figure S2). The incidence of relapses remained stable during the baseline (3%–4%) and follow-up (3%–3.7%) periods in nonswitchers (Figure S2).
Discussion
This real-world study compared the annualized rates and incidence of MS relapses among patients who switched from an injectable DMT to fingolimod or to another injectable DMT. The rates and incidence of MS relapses increased before treatment switch in both switchers to fingolimod and injectable DMT cyclers but remained stable in injectable DMT nonswitchers throughout the observation period. Although the lowest relapse rate was achieved in the injectable DMT nonswitchers, which was the largest group of patients, both the rates and incidence of MS relapses, as well as the Charlson Comorbidity Index, were higher in injectable DMT cyclers than in switchers to fingolimod. The association of comorbidities with the injectable DMT cyclers may be due to these patients being more commonly prescribed injectable DMTs, or may reflect a suboptimal state of overall health. In addition, the increase in MS relapse rates and incidence during the baseline period in both switchers to fingolimod and injectable DMT cyclers may reflect a lack of efficacy or the occurrence of adverse events leading to the treatment switch, as has been observed in clinical practice.24
Patients with MS who switched from an injectable DMT to fingolimod demonstrated greater reduction in MS relapse rates than those who switched to another injectable DMT during follow-up. The incidence of MS relapses followed a similar trend as the relapse rates. The risk of MS relapse reduced significantly by 47% in patients who switched to fingolimod compared with nonswitchers and by 32% in injectable DMT cyclers compared with nonswitchers. These results are consistent with previous findings from clinical trials and real-world studies16,18,25–27; in the 12-month phase 3 TRANSFORMS (Trial Assessing Injectable Interferon vs Fingolimod Oral in Relapsing-Remitting Multiple Sclerosis), fingolimod showed better efficacy in terms of ARR compared with intramuscular IFNβ-1a,16 and switching to fingolimod reduced the ARR by 50% compared with IFNβ-1a.26 In the TRANSFORMS extension study, switching to fingolimod showed a 30% to 36% reduction in the ARR.18 Patients treated with fingolimod for 2 years showed a sustained treatment effect with improved clinical and magnetic resonance imaging (MRI) outcomes compared with patients who switched from IFNβ-1a to fingolimod.16 18 A US claims database analysis reported a 61% lower ARR after 1-year follow-up in patients who switched from interferon to fingolimod compared with those who switched to glatiramer acetate. The probability of relapse was 59% lower in patients with MS who switched to fingolimod compared with those who switched to glatiramer acetate.25 Furthermore, a matched retrospective analysis of data collected prospectively from the MSBase cohort showed that switching from injectable immunomodulators to fingolimod is associated with fewer relapses, more favorable disability outcomes, and greater treatment persistence compared with switching to another injectable preparation.27
This study analyzed the US pharmacy claims data from the MarketScan Commercial Claims and Encounters database and the Medicare Supplemental database. The MarketScan Commercial Claims and Encounters database is a large US administrative health plan database containing inpatient, outpatient, and pharmacy data from multiple payers, accounting for approximately 40 million members across the Unites States. Thus, it is considered to be representative of the commercially insured US population. The Medicare Supplemental database includes data from employer-sponsored Medicare supplemental plans, accounting for approximately 3 million people in the United States 65 years and older and those younger than 65 years who are eligible for Medicare coverage. Thus, it is representative of the US Medicare population with supplemental insurance.
As with most retrospective studies using pharmacy claims data, the present study has several limitations. Because the data include only commercially insured patients or those with commercial insurance supplementing their Medicare insurance, results may not be generalizable across different patient populations. It is also possible that there may have been coding errors within the diagnostic and procedure codes used for reimbursement purposes with administrative claims data, although such errors would likely have occurred randomly and irrespective of treatment cohort. Notably, this analysis was retrospective in nature and relied on existing coding in the claims data, which did not collect approximate MS relapses as a discrete event. Therefore an algorithm based on a combination of different proxies, as previously published, was used instead.20 This algorithm was shown to have a 67% positive predictive value when tested in 300 patients with MS, and although it may be a less sensitive way to detect MS relapses, the ARRs reported using this technique were similar to those reported in clinical trials.18 26 In addition, due to the lack of MS relapse-specific codes, patients with mild relapses who did not require, or could not take, corticosteroids will not have been included in the study.
The results of the present study should be interpreted with caution because the analysis was hypothesis generating and no adjustments were made for multiple comparisons. Clinical measures, such as MS severity and MRI data, were not examined due to unavailability in the claims database. Hence, we could not account for the degree of MRI activity and/or the severity of the relapse that may have influenced the clinical decision to switch to fingolimod or to cycle injectable DMT. Furthermore, the precise nature of different types of treatment switch was not captured. It should be recognized that transitioning from one type of IFNβ to another, or between products with varying mechanisms of action, such as from an interferon to glatiramer acetate, may result in different outcomes. However, previous studies have shown that these differences are likely to be minimal28 and analysis of these types of switches was beyond the scope of this study. We also recognize that the retrospective identification of relapses is a potential weakness, although use of the validated algorithm may have mitigated this factor to some extent. Indication bias was also possible in that patients with more severe breakthrough disease might have been transitioned to fingolimod due to its perceived greater efficacy.
Given that we examined data from only the 12-month period after the index date of each patient, analysis of the longer-term effects of treatment switch on rate and incidence of MS relapses was beyond the scope of the present study. Future studies may benefit from an extended postindex follow-up period that would enable longitudinal analyses to be undertaken. In addition, statistical comparisons between switchers to fingolimod and injectable DMT cyclers would provide greater insight into the impact of each specific treatment switch on MS relapses.
In conclusion, this is a large real-world study comparing MS relapses in US patients who switched from an injectable DMT to either fingolimod or another injectable DMT. The greatest reduction in relapse rates and incidence was observed in those who switched from an injectable DMT to fingolimod, compared with those who switched to another injectable DMT or did not switch. Patients who switched from an injectable DMT to fingolimod also had a significantly reduced risk of MS relapse compared with injectable DMT cyclers and nonswitchers. This study provides real-world evidence to support fingolimod as an alternative treatment for patients receiving injectable DMTs when a change in therapy is clinically indicated. Considering the limitations of retrospective analysis, these findings should be generalized with caution.
PRACTICE POINTS
This study adds to the evidence that patients with MS experiencing a lack of efficacy can effectively switch disease-modifying therapy (DMT).
Patients who switched from an injectable DMT to fingolimod had the highest reductions in the annualized rate and incidence of MS relapses, as well as a significant reduction in relapse risk compared with patients switching to other injectable DMTs and nonswitchers.
This large real-world study supports the use of fingolimod as an alternative treatment option for patients receiving injectable DMTs.
Acknowledgments
The authors thank Mrs Vijayalakshmi Vasanthaprasad and Lohit Badgujar (Novartis Healthcare Pvt Ltd, Hyderabad, India) for providing medical writing support and editorial support. Roshani Shah, MPH, of Novartis Pharmaceuticals Corp and Lauren Chessum, DPhil, formerly of Fishawack Communications are also thanked for their editorial support after submission.
References
Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013; 19( suppl): S15– S20.
Wallin MT, Culpepper WJ, Campbell JD, . The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019; 92: e1029– e1040.
Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol. 2019; 9: 1150.
Devonshire V, Lapierre Y, Macdonell R, . The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011; 18: 69– 77.
Agashivala N, Wu N, Abouzaid S, . Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013; 13: 138.
Bergvall N, Petrilla AA, Karkare SU, . Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014; 17: 696– 707.
Portaccio E, Amato MP. Improving compliance with interferon-beta therapy in patients with multiple sclerosis. CNS Drugs. 2009; 23: 453– 462.
Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016; 354: i3518.
Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010; 30: 89– 100.
Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care. 2013; 15: 36– 45.
Bruce JM, Hancock LM, Arnett P, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med. 2010; 33: 219– 227.
Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011; 28: 51– 61.
Oleen-Burkey MA, Dor A, Castelli-Haley J, Lage MJ. The relationship between alternative medication possession ratio thresholds and outcomes: evidence from the use of glatiramer acetate. J Med Econ. 2011; 14: 739– 747.
Yermakov S, Davis M, Calnan M, . Impact of increasing adherence to disease-modifying therapies on healthcare resource utilization and direct medical and indirect work loss costs for patients with multiple sclerosis. J Med Econ. 2015; 18: 711– 720.
Weinstock-Guttman B. An update on new and emerging therapies for relapsing-remitting multiple sclerosis. Am J Manag Care. 2013; 19( suppl): s343– s354.
Cohen JA, Barkhof F, Comi G, . Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010; 362: 402– 415.
Kappos L, Radue EW, O’Connor P, . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010; 362: 387– 401.
Khatri B, Barkhof F, Comi G, . Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011; 10: 520– 529.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373– 383.
Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010; 13: 618– 625.
Ollendorf DA, Jilinskaia E, Oleen-Burkey M. Clinical and economic impact of glatiramer acetate versus beta interferon therapy among patients with multiple sclerosis in a managed care population. J Manag Care Pharm. 2002; 8: 469– 476.
UCLA Institute for Digital Research and Education. Negative binomial regression: SAS data analysis examples. Accessed December 19, 2019. https://stats.idre.ucla.edu/sas/dae/negative-binomial-regression
Zhou H, Taber C, Arcona S, Li Y. Difference-in-differences method in comparative effectiveness research: utility with unbalanced groups. Appl Health Econ Health Policy. 2016; 14: 419– 429.
Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. 2015; 3: 545– 555.
Bergvall N, Makin C, Lahoz R, . Relapse rates in patients with multiple sclerosis switching from interferon to fingolimod or glatiramer acetate: a US claims database study. PLoS One. 2014; 9: e88472.
Cohen JA, Barkhof F, Comi G, . Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol. 2013; 260: 2023– 2032.
He A, Spelman T, Jokubaitis V, . Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol. 2015; 72: 405– 413.
Melendez-Torres GJ, Armoiry X, Court R, . Comparative effectiveness of beta-interferons and glatiramer acetate for relapsing-remitting multiple sclerosis: systematic review and network meta-analysis of trials including recommended dosages. BMC Neurol. 2018; 18: 162.







