Publication
Research Article
International Journal of MS Care
Successful Management of a Neurology Infusion Practice
The increasing use of infusible biologic therapies, including the novel monoclonal antibody natalizumab for the treatment of relapsing forms of multiple sclerosis, has elicited much interest among neurologists in the provision of in-office infusions for their patients. An in-office infusion center may offer neurologists a means to provide integrated care for their patients in a familiar and supportive environment. This setting is especially convenient for chronically ill patients, allowing them to receive high-quality care under the direct supervision of their neurologist and facility staff. By administering infusible treatments in a neurology practice rather than referring patients to a hospital or oncology/hematology-based infusion center, the primary neurologist can more closely monitor clinical outcomes, treatment adherence, and the occurrence of adverse effects. In addition, there is greater opportunity for patient interaction and education, which can strengthen relationships with clinical caregivers. This model is also applicable to multispecialty or hospital-based neurology groups desiring to integrate neurology infusion services. In this article, we discuss overall management strategies; staffing and scheduling issues; general coding, billing, and reimbursement methodologies; and additional financial considerations.
Multiple sclerosis (MS) is an often disabling, demyelinating disorder of the central nervous system characterized by intermittent periods of disease relapse and remission. The introduction of several disease-modifying therapies over the last 2 decades has had a significant impact on the management of MS.1–3 The US Food and Drug Administration (FDA) has approved six disease-modifying therapies for relapsing forms of MS: two interferon beta-1a (IFN - 1a) formulations,4 5 IFN -1b,6 glatiramer acetate,7 mitoxantrone, 8 and natalizumab.9 Randomized, controlled trials and extensive clinical experience support the longterm safety of self-injectable, immunomodulatory therapies (ie, IFN and glatiramer acetate) for the first-line treatment of relapsing forms of MS.3 These treatments have been shown to delay the progression of MS by reducing relapses; however, they are only partially effective (reducing the annual relapse rate by approximately 30%) and do not prevent recurring symptoms.3 10–15 In addition, their long-term effect on the prevention of disease progression and permanent disability is unclear.1 3
New infusible treatments for patients with MS continue to emerge. The targeted monoclonal antibody natalizumab (Tysabri, Biogen Idec, Inc, and Elan Pharmaceuticals, Inc) received final FDA approval in 2006 as monotherapy (administered as a 1-hour intravenous [IV] infusion once every 4 weeks) for the treatment of patients with relapsing forms of MS.9 In clinical studies, natalizumab has demonstrated significant efficacy in the treatment of MS.16 17 Together with real-world experience, results from these studies suggest that natalizumab is effective for patients with relapsing disease that is unresponsive to conventional therapies. The purchase of natalizumab is managed exclusively under the TOUCH Prescribing Program, a restricted distribution program meant to ensure appropriate use of natalizumab and close monitoring of patients for signs and symptoms of progressive multifocal leukoencephalopathy (PML) during treatment.
Mitoxantrone is an immunosuppressant and antineoplastic drug administered via IV infusion for the treatment of active relapsing and secondary progressive forms of MS. Although mitoxantrone significantly reduces disease progression and relapse rates,18 its toxicity is significant.10 15 19 20
There are currently several other targeted monoclonal antibodies—alemtuzumab, rituximab, ocrelizumab, and daclizumab—in clinical development for the treatment of MS.3 21 If ongoing studies demonstrate clinical benefit, the potential necessity for IV administration of these novel biologic agents is likely to have a significant impact on the management of this disease.22 The availability of a variety of more effective and more complex infusible agents for the treatment of MS, as well as increased demand for more well-established agents, such as methylprednisolone and IV immunoglobulins (IVIG), has elicited significant interest among neurologists in an in-office integrated infusion center model. We have extensive experience managing large, highly integrated infusion centers in our institutions and have been actively involved in developing standard operating procedures and protocols for infusible agents such as natalizumab.12 23 In this article, we discuss overall management and operational strategies; staffing and scheduling issues; coding, billing, and reimbursement methodologies; options for obtaining medications; and anticipated start-up costs and additional financial considerations.
Infusion Settings for Patients
In-Office Neurology Practice Setting
Historically, infusion services were typically offered in a hospital or hospital outpatient setting. As more infusible agents were developed for cancer treatment, oncology practices found more tightly integrated infusion services to be beneficial for improving quality of care and reducing costs and began bringing their services into the practice setting. Information regarding the location at which an infusion is given is most readily available for natalizumab, given the requirement for infusion center registration. Here we find 55% of infusions occurring in physician offices, 39% in hospital-based settings, and 6% in freestanding ambulatory centers (data on file, Biogen Idec, Inc). When given in a hospital, the service is often quite remote from the hospital-based neurology practice. In a multispecialty group practice, the infusion center is often remote from the neurology group clinical practice and is generally dominated by oncology priorities and patients, many of whom have very different needs than the long-term neurology population. In-office infusions given at a neurology practice offer patients high-quality care in a comfortable and familiar setting, allow direct drug side-effect surveillance and supervision by their neurologist, and optimize follow-up care. In-office infusions also allow for specific nursing and patient education regarding each drug infused.24 In this setting, the neurologist (and neurology practice staff) can also monitor patient adherence and clinical outcomes more closely than in situations where the patient is referred to a remote infusion center.
The provision of in-office infusions may also offer several social and psychological benefits for patients. For example, the addition of an in-office infusion center to a neurology practice fosters an environment in which patients can share information, discuss the burdens of their disease, and offer support and hope to one another. 25 The convenience of an in-office infusion center can, in turn, lead to increased patient adherence with therapy, 25 and may help to build strong relationships with health-care providers and facility staff members. For these reasons, we believe that the optimal care model is that of integrated clinical care with highly aligned infusion services. At the present time, this is not common practice.
Hospital-Affiliated Setting
Infusions offered in the hospital outpatient setting may have some benefits compared with other settings, including the use of existing infrastructure, the presence of substantial nursing and physician staff, access to necessary equipment and laboratory services, and on-site pharmacy services. Potential disadvantages of a hospital-affiliated setting include the possibility that not all of the desired infusible treatments are included on the hospital formulary. Additional challenges and considerations include the necessity for appropriate coding for reimbursement, alternative acquisition options for drugs through the hospital pharmacy, and the potential for a broad patient demographic and subsequent infusion service needs. Most importantly, communication systems must be in place to rapidly communicate infusion reaction complications or a change in clinical status back to the referring physician. In the United States, direct physician employment by hospitals is becoming more common. In this setting, there remain many advantages to operating neurology clinical services in physical continuity with neurology infusion services.
Freestanding Oncology/Hematology Infusion Center Setting
Patients requiring infusional therapy of any kind are frequently referred to freestanding oncology/hematology infusion centers because of their expertise in administering infusion-based drugs; however, the prescribing neurologist may want to examine and consider the benefit of integrating infusion therapy. The patients will experience greater continuity of care with the coordination of clinical vigilance and follow-up, centralized patient tracking, convenience, less administrative time with staff who are already familiar with patients' insurance requirements, and an already strong connection with the treating neurologist and staff. Lastly, many patients enjoy interacting with other patients with MS, an advantage that is not available in a general infusion setting.
Important Considerations Regarding an In-Office Neurology Infusion Center
By offering in-office infusions, neurologists have an opportunity to provide the best standard of patient care and optimize treatment adherence, while at the same time enhancing their practice through increased patient recruitment, improved potential for participation in clinical trials, and expanded services.25–27 However, a number of important factors should be carefully considered by an existing practice to ensure the successful establishment and management of an in-office infusion center.
Strategic Assessment
When establishing a center, it is critical to first assess the purpose(s) of providing outpatient services and to identify various opportunities and potential challenges. Neurology practices may have unique goals, such as the addition of new patients to the practice, increased recognition in the field, inclusion in clinical trials, and/or acquisition of research grants. At the same time, each practice may face different challenges ranging from availability of funds, to staffing needs and space requirements for expansion of the practice, to the existence of “competitor” infusion centers that may hinder optimal utilization. Practices should consider initiating infusion services on a limited basis with as little as one infusion chair to allow time to gain the necessary experience prior to expansion.
The total number of available patients and those who qualify for treatment can be assessed and quantified based on physician criteria. When available, best practice recommendations, such as those recently published by Coyle and colleagues23 for natalizumab therapy, should be used to select appropriate patients. Patient demand can be determined through review of satisfaction surveys, practice management databases for MS and other neurologic disorders, direct screening of patients during office visits, and retrospective review of charts for preselected qualifying criteria. Finally, an overall assessment of the market can help to establish the range of treatments to be offered.
Getting Started
Space Requirements
The available space and overall size of an existing practice will determine requirements for expansion to accommodate patients for infusion services. Maximization of existing space (eg, through dual use of examination rooms or conversion of other office space) may be adequate in the initial development stages, but with a larger patient population, a more global reassessment of practice space requirements may be needed.28
Staffing Needs
Establishing an in-office infusion center may necessitate hiring additional staff and/or changing the responsibilities of current staff members. The size of the required staff depends on several factors, including the existing size of the practice, the complexity of treatments to be administered, the number of available infusion chairs, and the potential number and clinical condition of eligible and newly recruited patients, all of which can vary widely.
In-office infusions can easily be administered in a small practice setting. A typical approach to staffing is to start with a single infusion room, 1 or 2 chairs serving 10 to 15 patients per month, and a minimal nursing staff hired on a part-time basis. Nursing staff can generally handle drug storage and reconstitution of infusible agents, with the exception of chemotherapy agents, which require special training. Regular pharmacy in-services can often be of help as well. The existing administrative staff can take on the responsibility of benefits verification and obtaining prior authorization and reimbursement for treatments. This approach allows for early clinical, billing, and coding expertise to develop and, importantly, is flexible and allows scalability once the fundamental expertise is acquired.
An experienced part-time nurse could be expected to assess up to three patients receiving the same agent in a 1-hour session. Factors associated with administering multiple agents of varying complexity (ie, tolerability profiles, infusion times, and patient monitoring requirements) can significantly affect the nurse–patient ratio and must be carefully considered. Nurse staffing requirements may also vary according to the design and flow of the space. For example, a semiprivate room with multiple infusion chairs may facilitate the assessment and management of more patients compared with several independent, closed-door rooms.
Larger-scale in-office infusion centers may require additional staff members to handle increased numbers of patients with a potentially broad demographic. In addition to relying on current on-site physicians for direct supervision of an IV-therapy program, it may be necessary to hire a dedicated infusion nurse with prior hospital, critical care, or oncology experience and IV and/or chemotherapy certification (if possible) and additional part-time or per diem nursing staff. Use of an electronic medical record (EMR) and infusion templates helps to standardize nursing assessments and reduces the amount of time required for administrative tasks, allowing more time for patient care.29 An EMR also allows for crossgroup comparison of efficacy of an agent and side-effect surveillance, as well as facilitating billing.
Regardless of the overall size of a neurology practice, a successfully managed in-office infusion center will require each staff member to carry out a specific role or roles. In general, physicians should be responsible for the identification of candidate patients and prescribing and monitoring response to therapy. Appropriately qualified nurses should be responsible for the actual administration of infusions/injections, education of patients, and recording of services and patient responses.30 With large patient volumes, a dedicated infusion clinic manager is usually required to obtain prior authorization, review insurance explanations of benefits, and organize patient scheduling. Finally, additional administrative staff should handle overall policies and procedures, the acquisition of drugs and supplies, and inventory management. Organizational strategies that include appropriate supportive staff with requisite training can ensure ease of patient access to treatment, better understanding of financial obligations prior to service, billing of payers and patients at the time of service, and receipt of reimbursements in a timely manner.31
The implementation of an in-office IV-therapy program requires the upfront education of new and existing staff and clear communication of any changing responsibilities. 28 By actively tracking and addressing issues as they evolve, the physicians, nurses, and office staff can work together to maintain a primary focus on patient care and convenience.28 We have found that regular meetings of the clinical and infusion staff greatly facilitate optimal integration.
Scheduling Considerations
Scheduling should be carefully considered during the start-up phase of a new in-office infusion center.27 The complexity of treatment should be considered when scheduling multiple patients for the same session. To optimize the use of infusion chairs and staff, patients receiving treatment that requires a longer infusion time or more intensive monitoring should be coordinated with patients who require less attention during treatment. Regardless of the number of infusion chairs available at start-up, a “walk-in” policy for patients in need of assistance with intramuscular IFN -1a injections, flu shots, treatment for infusion- or injection-related skin reactions, or pre-infusion blood work should be instituted, if possible.
In scheduling the appropriate number of hours per infusion chair, it is important to allow adequate time for patient education, discharge activities (eg, discontinuation of the infusion and cleanup before subsequent infusions), and any potential emergencies that may arise. Thus, patients should not be scheduled for the maximum daily available hours per chair. Instead, infusion start times should be staggered so as to not overwhelm the nursing staff at any point in the day and to prevent patients from having to wait for extended periods of time to receive their treatment.31
Obtaining Medications and Ensuring Their Availability for Patients
Options for obtaining medications should be carefully researched and a drug vendor chosen that can offer competitive pricing, termination of agreement without penalty, and guaranteed availability of drugs.27 Because of cost and storage issues, most practices will pursue a “just in time” drug flow from the vendor. This requires a good relationship with the vendor, as well as a comprehensive understanding of delivery times and payment deadlines.
Neurology practices should also be aware of the options available to each patient based on their individual payer and policy guidelines. For example, infusible therapies administered in the physician's office have traditionally been covered under a patient's general medical insurance policy. However, a growing trend in managed care is the shift of medication-associated costs (including those associated with infusible drugs) to a patient's prescription drug benefit policy, thereby allowing the insurer more opportunities to contain costs and control patient access to treatment. This often has major cost implications for the patient, as insurers frequently create specialty drug tiers (under the pharmacy benefit) that require higher patient cost-sharing.32
Buy and Bill
In general, two plans for drug acquisition are available to physicians and are determined by the benefit structure. 28 Under the patient's medical benefit, the drug can be acquired through the “Buy and Bill” plan. The physician's office first establishes an account with a wholesaler of medical products and specialty medications to purchase the drug, which involves the determination of pricing, supplies to be included in the agreement, and terms for payment, which can be extended from net 30 to net 60 or 90 days, depending on the arrangement. Drugs can be ordered on a daily basis and are usually shipped to the physician's office the next day, thus ensuring availability for patients. Based on the established contract, physicians can purchase drugs from the pharmacy, distributor, or wholesaler. The cost of drugs can be based on wholesaler acquisition cost (WAC) or average wholesale price (AWP) less a percentage, which is not constant and is determined by many variables (such as the range and volume of drugs to be purchased, and geographic region). By negotiating locally, physicians can obtain substantially reduced pricing for the purchase of drugs and at the same time be reimbursed by managed-care payers for direct costs (Table 1).
Sample prices of common infusible MS therapies
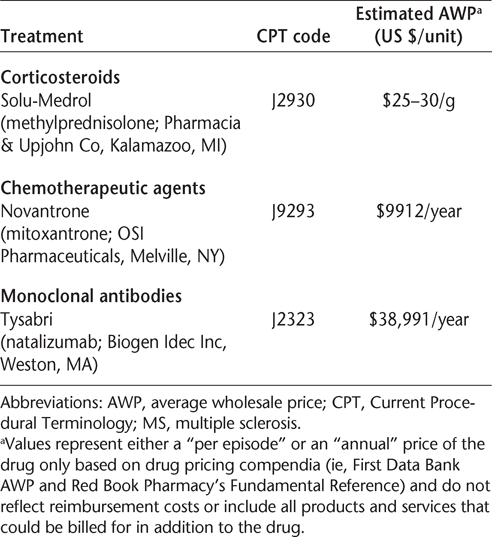
Patient benefits must be verified and prior authorization obtained before the drug is purchased (in the case of agents administered within 1–2 days) or infused to ensure reimbursement for drug costs and associated services by the third-party payer. Patients should be aware of any copayments required by the payer and must submit the appropriate payment to the physician's office prior to treatment.33 Copayments may be as much as 20% of the cost for the drug and service (in some cases as high as $200–400 per treatment).
Assignment of Benefits
A physician's office may elect to obtain medications using an “Assignment of Benefits” plan, which is typically covered by the patient's prescription drug benefit policy. This arrangement requires patient authorization for health benefit payments to be made directly from the payer to a designated specialty pharmacy. After prescriptions are forwarded to the specialty pharmacy, the drugs are shipped directly to the physician's office33 in patientspecific doses, which can help to coordinate treatment schedules and simplify inventory management. Under this arrangement, the specialty pharmacy verifies patient insurance information and bills the patient for the copayment and the payer for reimbursement of drug costs, whereas the physician's office bills the payer for any services associated with drug administration. “Specialty pharmacy”–acquired medications will typically make up some portion of the practice infusion mix and may help to stabilize financial risk. Hospital-based neurology practices can sometimes participate with the hospital pharmacy buying group to obtain additional discounts on medication.
Inventory Management
Successful management of drug inventory (regardless of the acquisition option used) is easily achieved through careful review of the patient schedule for the coming week. Medications with a long half-life (eg, methylprednisolone) can be ordered in bulk to maximize savings; however, weekly ordering for a just-in-time inventory system is recommended for drugs with a shorter half-life or premixed medications.27 28 Each dose used should be trackable by the serial number and lot to a given patient. The specific dose sent by specialty pharmacies should be used by the patient it was intended for without switching among inventory. Drugs should be received in a centralized location by a designated staff member and should be stored by serial and lot number in a dedicated, secured refrigerator with a temperature-monitoring system. In the case of a power outage, a backup generator or alarm system could save valuable inventory. In addition, an insurance policy should be in place to cover the cost of lost supplies.
Policies and Procedures for New Services
Standard operating procedures need to be developed for the introduction of outpatient infusion services to a neurology practice.28 Specifically, infusion protocols should encompass patient assessment and monitoring criteria, procedures for medication admixture, guidelines for adverse event management, and postinfusion practice policies. The infusion protocol should also outline patient follow-up procedures and include a template for infusion documentation that can be used to record key patient and clinical information (eg, ordering and supervising physician, administering nurse, drug name, drug lot and serial numbers, drug strength, adverse events, infusion start and stop time, IV catheter size, IV site, vital signs, and any patient education provided during the visit). Additionally, reasonable suggestions from patient satisfaction surveys should be considered for incorporation into standard procedures.
Successful management of an in-office infusion center requires particular attention to rapidly changing payer regulations and reimbursement policies, an understanding of key strategies for negotiating with private payers, and a proactive approach to insurance verification and preauthorization.28 Some authorizations may be straightforward, whereas others may require the failure of previous platform therapies. Once treatment is authorized, adherence to the payer guidelines is paramount. For example, infusion within a 28-day period could result in denial of payment (for all infusions) if the required schedule for a given drug is every 30 days. With larger volumes, a dedicated infusion manager is generally recommended.
Most drug manufacturers provide specialized customer service programs to assist patients and physicians with their product-related reimbursement questions. Program staff members closely monitor changing payer policies and can provide current information on coding and billing, assistance with claims and related appeals, and insurance investigation services to offer a “thumbnail sketch” of patient benefits and to help patients understand what portion of the drug cost is their responsibility based on their particular coverage. This is especially important for smaller physician practices that do not have a dedicated authorization coordinator. In addition, regional and national website portals are available to physicians (eg, WNYhealthenet Partners at http://www.wnyhealthenet.org/indexIE.jsp and NaviNet at http://www.navinet.net/, respectively) that can provide up-to-the-instant verification of insurance status. Insurance verification services for federal payers (eg, Medicare, Medicaid) may require a small fee.
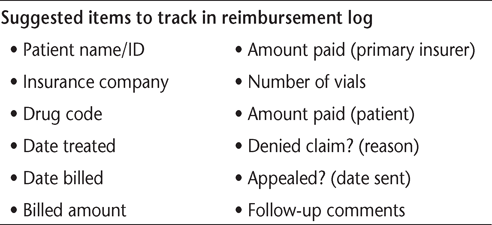
It is also highly recommended that payers be continually monitored for contract compliance. A reimbursement tracking log (Table 2) may be helpful in auditing the payers' explanations of benefits against contracted fee schedules and in evaluating and documenting payers' responsiveness in addressing reimbursement issues. A reference sheet for each payer, including contact information for the prior authorization department, should be maintained in the patient's file along with a copy of their insurance plan and current insurance card. Many practices use spreadsheets to sort by payer or other variable. Procedures for addressing claims denials and appropriate processes for submitting a subsequent appeal should be established to ensure proper handling and prompt reconciliation of claims appeal responses.34–36
Sample reimbursement tracking log
Coding and Billing
To properly optimize reimbursement for in-office infusions, it is imperative to accurately select Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes to report all infusion drugs used, their quantities, and associated services.27 Assigned staff members should be aware of code changes, the addition of new categories for drug administration/infusions, and which services are included in billing of the drug (and therefore not separately billable). Accurate infusion nursing documentation with total infusion time and complications is essential for appropriate billing.
Sample coding for infusion services is outlined in Table 3. The reportable time used to determine appropriate billing and add-on codes refers to the actual infusion time only. Preparation time, patient assessment time, time waiting for delivery of the drug from the pharmacy, time flushing the port between subsequent infusions, postservice monitoring, and patient time in the waiting room cannot be counted toward billable infusion time. Thus, protocols for documenting the time involved in each phase of the infusion process are particularly valuable to allow billing for associated services and time, and may ensure higher overall reimbursements. In addition, prior to billing services to the physician's provider number, special attention should be paid to a third-party payer's guidelines for “incident-to” billing in a case where services were performed by nonphysician staff (eg, physician assistants, nurse practitioners, or clinical nurse specialists).
Sample coding and national average allowable payment rate for infusion services
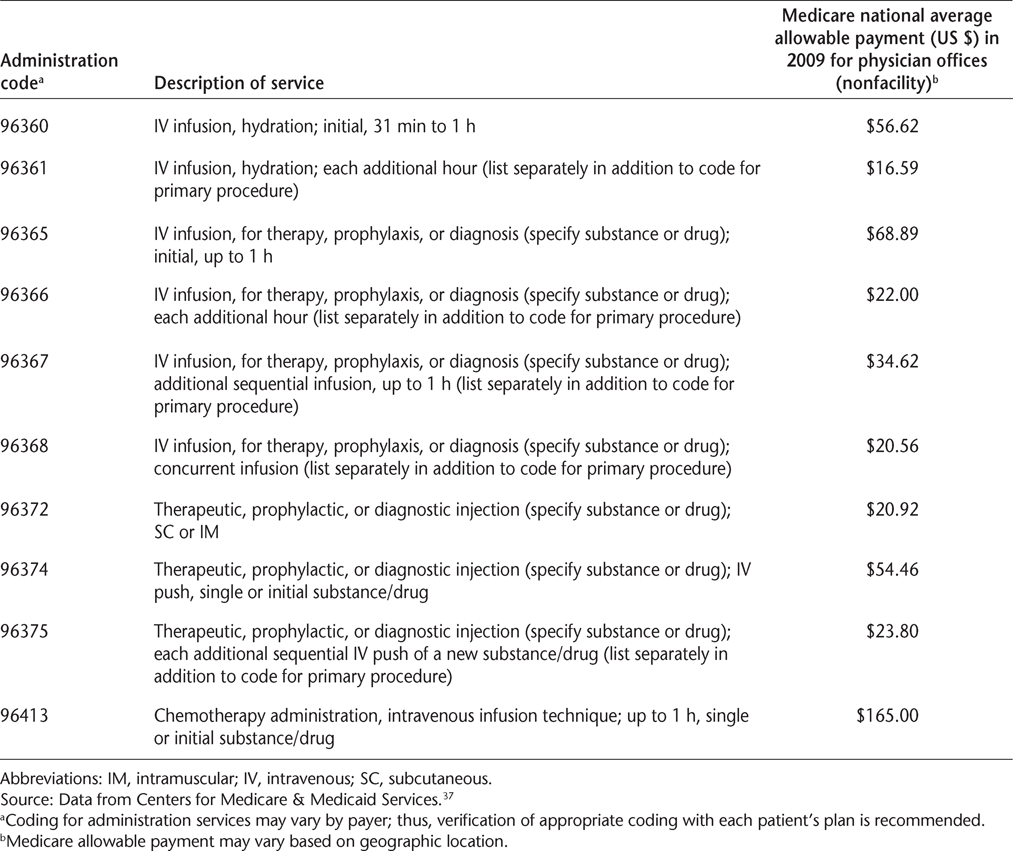
Hydration codes (Table 3) should be used specifically for the reimbursement of hydration services, although it is important to note that this type of service may not be offered in all practices.27 This is because hydration services are considered low risk and, thus, may be performed by staff with less advanced training and require minimal patient monitoring.27 Certain codes may not be used for incidental hydration services or for reimbursement of services in which saline or sterile water was used as a diluent for the infusion of another drug; however, the saline solution itself can be billed.27
Infusion charges should be reviewed and billed within 2 days. During the start-up phase, communication with individual insurers and review of contracts is essential. Previously discussed metrics for reimbursement (AWP, WAC) are generally determined by the insurer.
Practice Management Costs and Revenues
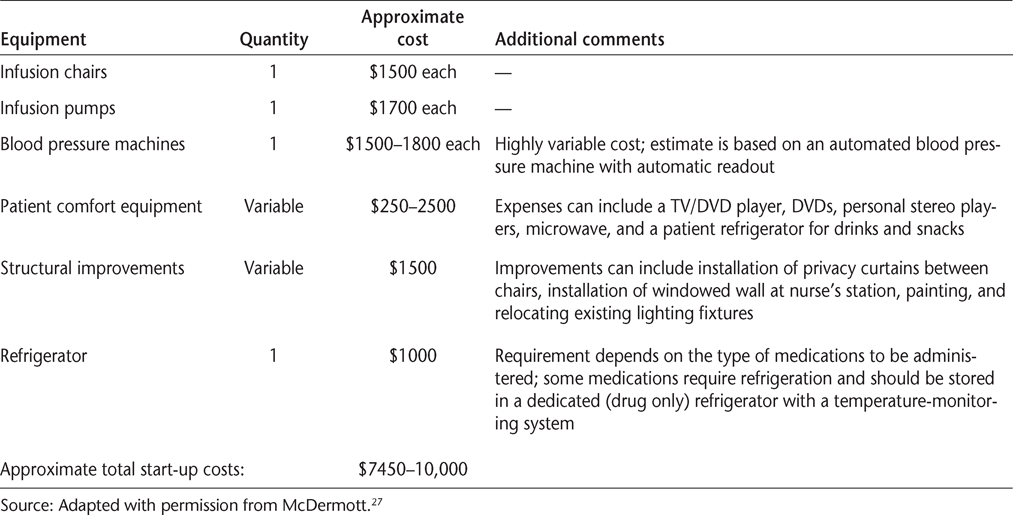
In addition to fixed costs, which may include space rental, equipment leases, staff salaries and benefits, utilities, and taxes, patient-related costs will also be incurred and make up the majority of practice management expenses.27 28 A summary of start-up costs related to patient comfort, medical supplies, and related equipment is shown in Table 4. It is important to recognize that these costs can easily be scaled up or down as necessary. For example, anticipated start-up costs for a small, in-office infusion center, employing dual use of existing office space to accommodate a single infusion chair with 10 to 15 patients per week, may range from as low as $7450 to $10,000 (Table 4).27 Expected start-up costs for a larger-scale center providing multiple infusion chairs could range from $17,400 to more than $30,000, not including expenses for medications.27
Sample in-office infusion center start-up costs
To help offset these initial costs, physicians may wish to consider increasing revenues through nonpatient sources. For instance, funding for patient education programs, physician education programs, and other workshops (on related health, legal, or financial issues) sponsored by the infusion center can be obtained from local hospitals and the pharmaceutical industry. Other infusion center staff needs and activities that may be funded through grants or philanthropic entities (such as health-care foundations, major donors, or MS Society chapters) include the hiring of a case manager, social worker, or dietitian, and the provision of respite care.
Conclusion
As more infusible biologic agents continue to emerge and demonstrate promising efficacy, greater infusion complexity, and the need for regular surveillance, the neurologic community is beginning to move to more highly aligned infusion practice. In addition to offering a convenient and supportive environment in which patients can receive treatment, this approach may also increase the potential for clinical trials participation, optimize treatment adherence, improve clinical vigilance in the identification of adverse effects, and create new revenue to help defray the high cost of caring for MS patients.
Despite the numerous factors that must be considered, potential challenges to establishing a successful integrated infusion center are not insurmountable, even in a smaller neurology practice setting. We believe it is also a better model for hospital-based outpatient neurologists, as well as multispecialty group practices. Integrating infusion by proximity and a neurology-centric clinical care process model clearly improves patient care, as well as streamlining the process and making it much easier to respond to infusion reactions and manage longterm side effect surveillance.
PracticePoints
New infusible treatment options for people with MS continue to emerge and are likely to have a significant impact on the clinical management of this disease. The availability of these agents has elicited considerable interest among neurologists in the establishment of integrated in-office infusion centers.
Several important factors should be carefully considered by an existing practice to ensure the successful establishment and management of an in-office infusion center.
By offering in-office infusions, neurologists can provide the highest level of integrated patient care and optimize treatment adherence, while at the same time enhancing their practice through increased patient recruitment, improved potential for participation in clinical trials, and expanded services.
Acknowledgments
Editorial support for the writing of this manuscript was provided by Jamie L. Kistler, PhD, of MedErgy (Yardley, PA) and funded by Biogen Idec, Inc. Susan H. Parker, RN, CMPE, and Aileen Soper of Xcenda (Charlotte, NC) provided an editorial review of the manuscript, which was funded by Biogen Idec, Inc.
References
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000; 343: 938–952.
Goodin DS, Frohman EM, Garmany GP Jr, . Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002; 58: 169–178.
Wingerchuk DM. Current evidence and therapeutic strategies for multiple sclerosis. Semin Neurol. 2008; 28: 56–68.
Avonex (interferon beta-1a) IM injection [package insert]. Cambridge, MA: Biogen Idec, Inc; 2006.
Rebif (interferon beta-1a) SC injection [package insert]. Rockland, MA: EMD Serono, Inc; 2008.
Betaseron (interferon beta-1b) for SC injection [package insert]. Montville, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2008.
Copaxone (glatiramer acetate injection) [package insert]. Kansas City, MO: Teva Neuroscience, Inc; 2007.
Novantrone (mitoxantrone) for injection concentrate [package insert]. Rockland, MA: EMD Serono, Inc; 2007.
Tysabri (natalizumab) injection for intravenous use [package insert]. South San Francisco, CA: Elan Pharmaceuticals, Inc; 2008.
Compston A, Coles A. Multiple sclerosis. Lancet. 2002; 359: 1221–1231.
Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008; 71: 766–773.
Rose JW, Foley J, Carlson N. Monoclonal antibody treatments for multiple sclerosis. Curr Neurol Neurosci Rep. 2008; 8: 419–426.
Jacobs LD, Cookfair DL, Rudick RA, . Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996; 39: 285–294.
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, part I: clinical results of a multi-center, randomized, double-blind, placebo-controlled trial. Neurology. 1993; 43: 655–661.
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998; 352: 1498–1504.
Polman CH, O'Connor PW, Havrdova E, . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006; 354: 899–910.
Rudick RA, Stuart WH, Calabresi PA, . Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006; 354: 911–923.
Hartung HP, Gonsette R, Konig N, . Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002; 360: 2018–2025.
Jacobs LD, Beck RW, Simon JH, . Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000; 343: 898–904.
Neuhaus O, Kieseier BC, Hartung HP. Immunosuppressive agents in multiple sclerosis. Neurotherapeutics. 2007; 4: 654–660.
Lutterotti A, Martin R. Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol. 2008; 7: 538–547.
Buttmann M, Rieckmann P. Treating multiple sclerosis with monoclonal antibodies. Expert Rev Neurother. 2008; 8: 433–455.
Coyle PK, Foley JF, Fox EJ, Jeffery DR, Munschauer FEI, Tornatore C. Best practice recommendations for the selection and management of patients with multiple sclerosis receiving natalizumab therapy. Mult Scler. 2009; 15: S26–S36.
Johns Hopkins Neurology and Neurosurgery. This infusion center's in an IV league of its own. Brainwaves. 2004;16.
Rossman HS, Lawson S. Setting up a neurology-based infusion center: rationale and guidelines. Appl Neurol. 2005; 1: D1–D8.
Versel N. Build your own infusion clinic. Biotechnology Healthcare. 2005:35–40.
McDermott M. Infusion Billing Update: 2006: opportunities to ensure proper reimbursement and program success. 2006:1–10. http://www.aan.com/globals/axon/assets/2342.pdf. Accessed May 19, 2011.
Debusk K, Parker S. Utilization of Biologics in the Neurology Clinic: Operational and Reimbursement Considerations: A CME/CE Webcast for Neurologists and Neurology Clinic Nurses. Presented by the Johns Hopkins Multiple Sclerosis Center and the Lash Group. http://www.jhasim.com/multimedia/pmw/PMW_FINAL.SWF. Accessed May 19, 2011.
Weimar C. Going all-digital is easier said than done. Physician Exec. 2009; 35: 20–22.
McQuilkin DJ. Risk Management and Profitability Through Practice Staffing with Licensed Clinical Personnel. Englewood, CO: American College of Medical Practice Executives; 2007.
Healthcare Financial Management Association (HFMA). Consumerism in Health Care. Westchester, IL: HFMA; 2006.
Cohen M, Morrow T, Penna P. Managing the expanded use of biologics across therapeutic areas: an example from b-cell targeted therapies. Am J Manag Care. 2006; 12: S24–S37.
Silverman E. The first generation of biodrug management: to what results? Biotechnology Healthcare. 2006:30–40.
American Medical Association. Is your practice losing revenue through inappropriate health plan adjustments? www.ama-assn.org/ama/pub/category/18013.html. Accessed November 4, 2009.
Avitzur O. Managed care games: how to say no to payer abuse. Neurology Today. 2004; 4: 55–56.
Jones CL, Mills TL Jr. Negotiating a contract with a health plan. Fam Pract Manag. 2006; 13: 49–55.
Centers for Medicare & Medicaid Services. Physician fee schedule, 2009. http://www.cms.hhs.gov/PhysicianFeeSched/. Accessed November 4, 2009.
Financial Disclosures: Dr. Foley has sat on the scientific advisory board for Biogen Idec, Inc, since 2008. He has been a participant in speakers' bureaus for both Biogen Idec, Inc, and Teva Pharmaceuticals since 1995. Ms. Dunne has no conflicts of interest to disclose.
Funding/Support: This project was supported by Biogen Idec, Inc.







