Publication
Research Article
International Journal of MS Care
Effect of Eszopiclone on Sleep Disturbances and Daytime Fatigue in Multiple Sclerosis Patients
The prevalence of moderate-to-severe sleep problems is significantly higher among people with multiple sclerosis (MS) than in the general population. In 2002, we found a significant relationship between fatigue and disrupted sleep in patients with relapsing-remitting MS (RRMS). The objectives of this study were to determine whether eszopiclone (Lunesta; Sunovion Pharmaceuticals Inc, Marlborough, MA) was superior to placebo in improving sleep among patients with MS-related fatigue and sleep complaints (primary end point); and to assess the impact of improved sleep on daytime fatigue and functioning (secondary end point). This was a double-blind, placebo-controlled pilot trial lasting 7 weeks. Thirty ambulatory adults under age 65 years with RRMS, fatigue, and sleep disturbances were randomized to receive either eszopiclone or placebo. The outcome measures included subjective and objective changes in sleep-onset latency (SOL), total sleep time (TST), wakefulness after sleep onset (WASO), sleep efficiency (SE), fatigue scales, and neuropsychological measures of daytime functioning. Compared with placebo, eszopiclone was superior only in increasing TST. Fatigue improved in both groups, but there was no statistically significant correlation between increased TST and improved fatigue, and no statistically significant differences were observed between the two groups. Thus, in this study, eszopiclone did not improve sleep sufficiently to improve fatigue in MS patients. This result may be due to the multifactorial nature of sleep disturbances and fatigue in people with MS.
The prevalence of moderate-to-severe sleep problems has been found to be significantly higher among people with multiple sclerosis (MS) than in the general population (51.5% vs. 33.1%).1 Fatigue is the most frequent MS symptom, affecting 76% to 92% of patients.2 3 In 2002, we examined the relationship of fatigue to sleep disturbances in MS patients, comparing three age- and sex-matched groups: 15 MS patients with fatigue, 15 MS patients without fatigue, and 15 healthy controls. We found a significant relationship between fatigue and sleep disturbances (Fisher exact test, P = .0028).4 It stands to reason that control of sleep disturbances should improve daytime fatigue.
To our knowledge, no studies have been conducted on the impact of eszopiclone (Lunesta; Sunovion Pharmaceuticals Inc, Marlborough, MA) on fatigue either in MS patients or in the general population. The goals of the present study were to determine whether 1) eszopiclone is superior to placebo in improving sleep of individuals with MS-related fatigue and sleep complaints; 2) improving sleep of fatigued MS patients will improve their daytime fatigue; and 3) improving sleep of MS patients will improve their subjective assessments of next-day functioning.
Methods
This was a double-blind, placebo-controlled pilot trial lasting 7 weeks. The study protocol was reviewed and approved by the University of Vermont College of Medicine's Human Studies Committee. The entire study was conducted at the University of Vermont College of Medicine's Department of Neurology. The trial was registered at Clinicaltrials.gov with the identifier NCT00594087. Participants were recruited through institutional review board–approved letters sent to all patients with confirmed MS who were followed at the University of Vermont MS Center. The exclusion and inclusion criteria were applied later in deciding whether or not to enroll the patients in the trial. Because the University of Vermont is the only academic medical center in our clinical catchment area, most MS patients in the region were seen at the MS Center at some time. Recruitment ran from November 2006 through April 2008. Recruitment took longer than expected because a large number of patients who were interested in our study were already enrolled in one of the many other MS trials being conducted at the time, none of which allowed participation in more than one trial at a time.
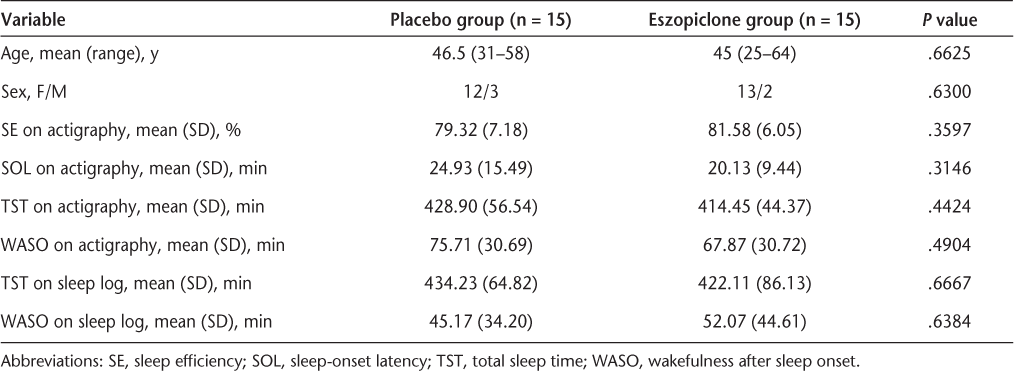
We recruited 30 consecutive ambulatory adult relapsing-remitting MS (RRMS) patients with fatigue (Fatigue Descriptive Scale [FDS] score of >5)5 who also had subjective sleep complaints of trouble falling asleep or staying asleep or had nonrefreshing sleep identified during clinic interviews. The FDS is a short scale with a score ranging from 0 to 17, with 5 being the cutoff between fatigued and nonfatigued. It is specifically designed for use in individuals with MS and has been well validated. It includes questions on modality (type of fatigue, such as with physical activity or at rest), frequency, and severity of fatigue and impact of heat on fatigue.5 Participants were randomized 1:1 according to a randomization chart to receive either placebo or a sedative-hypnotic agent. The goal was to determine whether the patients' sleep disturbances improved and, if so, whether there were any associated improvements in fatigue. We chose eszopiclone as the study sedative-hypnotic agent because it is a new nonbenzodiazepine γ-aminobutyric acid (GABA[A]) receptor modulator that has been approved by the US Food and Drug Administration (FDA) for long-term treatment of insomnia. The data available show persistent benefit in both shortening sleep-onset latency (SOL) and increasing sleep efficiency (SE) for a period of 6 months in patients with insomnia without any escalation of dose.6 All participants had a Kurtzke's Expanded Disability Status Scale (EDSS) score7 of less than 5 because we did not want to subject participants with ambulation problems to the risk of falls. All 30 participants provided informed consent.
We arrived at the number of 30 participants by the following method. We entered the confidence interval (CI) 0.9 ± 0.05, power of 90%, and effect size of 1.00 in the nQuery Advisor software (Statistical Solutions, Saugus, MA) to calculate the minimum number to treat. Given that the prevalence of sleep disturbances in a fatigued MS population was 80% in our previous study and the number of MS patients seen in our MS clinic is 600 per year, the nQuery Advisor software calculated the minimum number to be 15 per arm.
Patients were screened during their first visit (day 1) and underwent a comprehensive sleep history to rule out symptoms of sleep-related breathing disorders and sleep-related movement disorders such as restless legs syndrome (RLS). A Mini-Mental State Examination (MMSE) was administered to exclude individuals with severe cognitive impairment (MMSE of ≤26) because the impact of eszopiclone is not well studied in people with cognitive dysfunction and we did not want to subject participants to the risk of nighttime falls. A Center for Epidemiologic Studies Depression Scale (CESD)8 was administered to exclude people with severe depression (CESD score of ≥22), as depression can affect fatigue perception. The patients then completed an Epworth Sleepiness Scale (ESS),9 an FDS, and a Modified Fatigue Impact Scale (MFIS).10
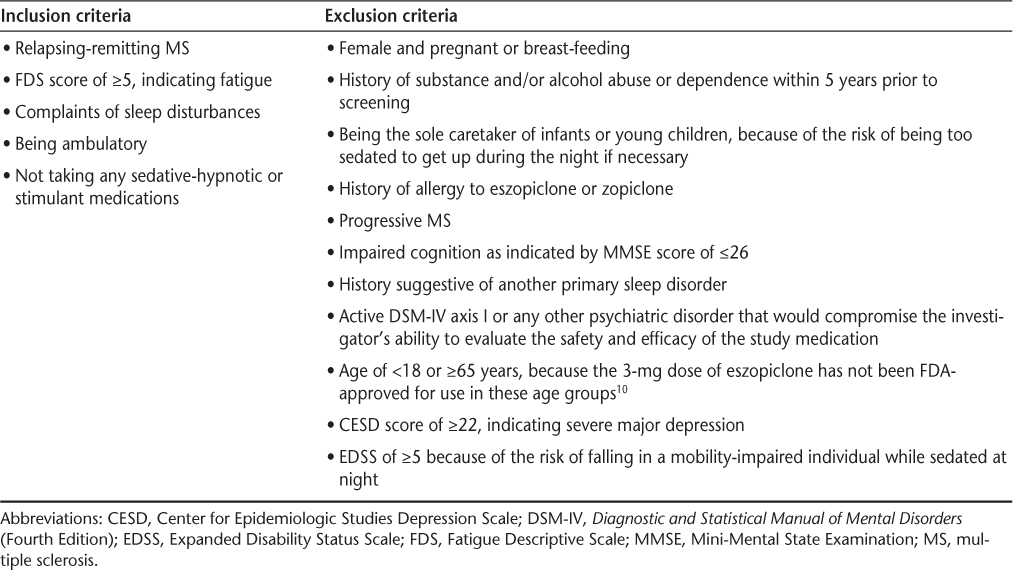
Patients eligible for inclusion in the study were adults under the age of 65 years with RRMS who had fatigue and subjective sleep disturbances (we did not qualify or quantify these disturbances), had no history suggestive of RLS or sleep-related breathing disorder, were not depressed, and were ambulatory. Although history alone does not rule out sleep-related breathing disorder, patients who were strongly suspected of having one during the initial visit underwent polysomnography to rule it out. We also excluded patients who were currently receiving sedative-hypnotic agents such as zolpidem, eszopiclone, zaleplon, benzodiazepines, trazodone, and sedating antidepressants taken at night for sleep, as well as patients currently taking stimulants, amantadine, and modafinil. The full inclusion and exclusion criteria are listed in Table 1. All patients screened underwent recent laboratory work, including complete blood count (CBC) and a complete metabolic panel (CMP), that was reviewed, and female subjects were referred for pregnancy testing if they were not using contraception.
Demographics and initial sleep variables
At the conclusion of the first visit, participants were given an actigraph to wear on their nondominant wrist for 2 weeks and sleep logs to maintain. Mini Mitter/Respironics actigraphs and their well-validated sleep algorithm were used. Before their second visit (day 14), participants were randomized by the pharmacist using a randomization table to either 2 mg of eszopiclone or matching placebo in a double-blind fashion. On day 14, participants underwent a series of neuropsychological tests to establish a baseline. These included the Controlled Oral Word Association (COWA)11 for verbal fluency; the Stroop Test (ST) for executive cognitive function; the Digit Cancellation test (d2) for attention and concentration; the Lafayette Grooved Pegboard Test (GPT)12 for psychomotor skill; the Rey Auditory Verbal Learning Test (RAVLT)11 for immediate and delayed recognition and recall; the Symbol Digit Modalities Test (SDMT)11 for complex scanning, visual tracking, and motor skills; and the Profiles of Mood States (POMS)13 for mood. Neuropsychological assessments were performed to determine whether eszopiclone would affect the participants' cognitive abilities during the day and to establish a baseline in MS patients. At the conclusion of the visit on day 14, participants were given 1 week's worth of either active drug or placebo and instructed to continue actigraphy and maintenance of sleep logs.
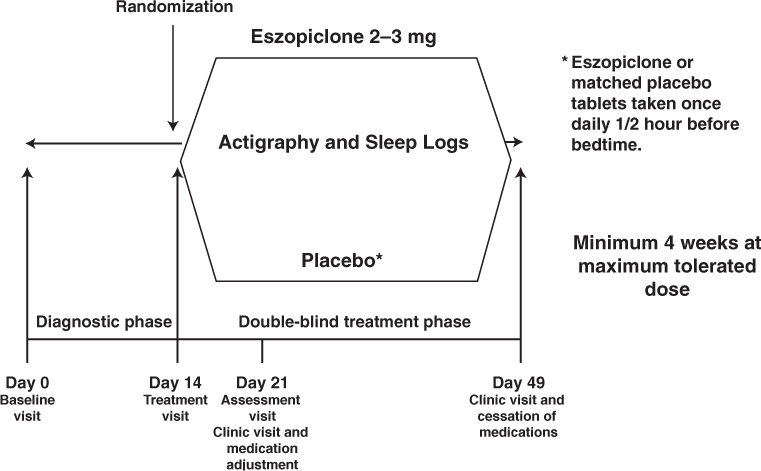
During the third visit (day 21), participants were asked about their sleep patterns and possible side effects of the medication and also completed another FDS. If participants experienced mild side effects or felt that their sleep had improved significantly, they were kept on the same dose of the medication for the next 4 weeks until the end of the study. If they reported continued sleep problems, their dose was increased to 3 mg of either eszopiclone or matching placebo. If the participant experienced significant side effects, he or she was withdrawn from the study. All those who continued with the study continued the actigraphy and sleep logs. On day 49 at the final visit, participants brought in their actigraphs and completed sleep logs as well as any leftover pills. They underwent the same neuropsychological testing as before starting the medication and completed an ESS, an FDS, and an MFIS. Figure 1 illustrates the chronology of the study.
Study design
Data Analysis
The sleep logs and the actigraphs were scored by a blinded principal investigator, and the results were tabulated both in Excel (Microsoft Corp, Redmond, WA) and in SPSS (SPSS, Chicago, IL). The neuropsychological testing was administered and scored by a licensed clinical psychologist. Trained research assistants doublescored and entered all neuropsychological test data and other measures into SPSS for Windows. Analysis of variance (ANOVA) was performed using analysis of covariance (ANCOVA) by a biostatistician; all tests were two-tailed with a 5% level of significance.
Statistical analysis included two parts. The first part was a descriptive summary of all baseline variables—such as demographics (age, gender) and characteristics (sleep variables on actigraph and diary, fatigue, and cognition) —by treatment (eszopiclone vs. placebo). Comparisons on these baseline variables between groups were conducted using an ANOVA model with treatment as a fixed effect for continuous variables, and using the Cochran-Mantel-Haenszel (CMH) test for categorical variables. We considered all the participants on eszopiclone as one group regardless of whether they were taking 2 or 3 mg; therefore, the two different doses did not affect our power calculation.
The second part of the analysis was the ANCOVA model. The rationale for ANCOVA in this study was as follows:
1) The primary analysis was comparison of the primary end point, which is change from baseline in total sleep time (TST) measured via actigraphy over the entire study period, between the eszopiclone treatment and placebo groups. This end point was calculated as the mean change in TST from baseline to the end of the study.
2) As described for the primary end point, all secondary end points were calculated as the mean change from baseline to the end of the study.
3) Because sleep-onset latency (SOL) and wakefulness after sleep onset (WASO) were known to be not normally distributed and their log transformation was known to be normally distributed, they were log-transformed before the analysis. Thus, all primary and secondary end points were normally distributed before the analysis. Sleep-onset latency on actigraphy was calculated based on what time the participants reported going to bed on their sleep logs and when onset of sleep occurred according to the actigraph.
4) The baseline value of an end point is a measure of uncontrolled variation and is irrelevant to the definition of the treatment effect. If the treatment effect varies substantially across the baseline, the end point needs to be adjusted for the variation in the baseline. Therefore, an ANCOVA model with the baseline as the covariate was expected to have the lowest variation.
5) Considering that the treatment effect may differ depending on the baseline value, we used an ANCOVA model with the interaction term between the treatment and the baseline covariate to examine whether there were equal slopes for treatment groups.
Results
A total of 38 patients were screened. Of these 38 patients, 3 were excluded because their CESD score surpassed the cutoff for depression, 2 were excluded because of a suspicion of obstructive sleep apnea (OSA), which was later confirmed by polysomnography, 2 were excluded because they were not willing to discontinue their prestudy sedative-hypnotic medications, and 1 was excluded because she was unable to maintain a regular sleep schedule. Three additional patients underwent polysomnography on the suspicion of OSA but stayed in the study after OSA was ruled out. All the patients scored either 29 or 30 on the MMSE, and none were excluded because of substance abuse.
Of the 30 participants enrolled in the study, 29 completed the study and 1 dropped out because of severe adverse effects; 3 others reported very mild adverse effects but remained in the study. All four of the participants with adverse effects were in the eszopiclone arm. The severe adverse effects were severe grogginess and light-headedness in the morning. These symptoms resolved after the drug was stopped. In two participants, mild grogginess and headache in the morning resolved after 2 days. The fourth participant experienced a persistent bad taste but did not stop taking the drug. No other adverse events were reported.
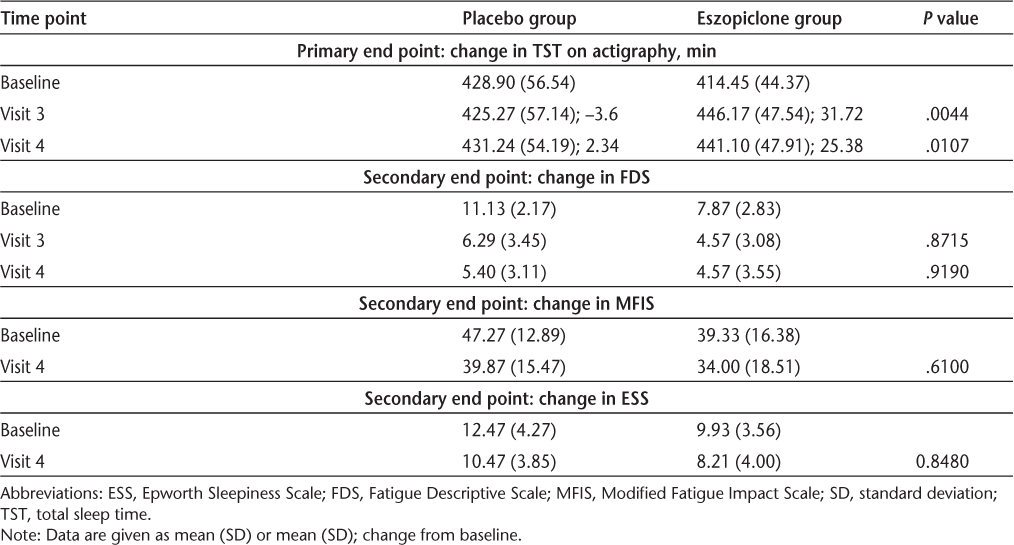
There was a significant, albeit modest, difference in change from baseline to Visit 3 and Visit 4 in TST as measured by actigraphy between the treatment and placebo groups, regardless of whether the dose of eszopiclone was increased to 3 mg or kept at 2 mg. All other sleep variables—including objective (actigraphy) SE (P = .7550), SOL (P = .2686), WASO (P = .5464), number of naps (P = .4399), and mean duration of naps (P = .4230); and subjective (sleep log) TST (P = .5078), WASO (P = .4658), number of naps (P = .5993), and mean duration of naps (P = .8443)—showed no significant change from baseline in either group. The FDS scores at baseline were slightly higher in the participants in the treatment group, but the change from baseline in the fatigue measures was not significant. The neuropsychological measures were within the normal ranges at day 14 and were not significantly changed in any subjects at day 49. There were no significant differences in change from baseline in the neuropsychological measures between the two groups. The results for primary and secondary end points are given in Tables 2 and 3.
Demographics and initial sleep variables
Results for primary and secondary end points
Discussion
To our knowledge, this was the first study of the effect of eszopiclone on sleep disturbances and correlation with measures of fatigue in people with MS. Although eszopiclone modestly improved objective TST in the study population, it did not improve SOL, SE, or WASO. Despite the modest improvement in objective TST, there were no statistically significant differences between the placebo and treatment groups in measures of fatigue before or after treatment.
The original trials testing the efficacy of eszopiclone involved participants with more severe insomnia than our participants had, with a TST of 6.5 hours or less, SOL of more than 20 minutes, and WASO of more than 20 minutes.14 Therefore, the magnitude of improvement in the participants' sleep was greater than in our study. Not all of our participants had a TST of less than 7 hours, although the mean and the median were just under that value, and not all of them had a SOL of more than 20 minutes, although the mean and the median were close to that value. We recruited our participants on the basis of subjective sleep complaints rather than objective measures of sleep disturbance. Also, because we wanted to use the smallest effective dose to prevent serious adverse effects, we maintained the dose at 2 mg in 11 participants, even though the standard adult dose is usually 3 mg.14 We found no statistically significant difference in the response to 3 mg compared with 2 mg in the active treatment arm.
If we had chosen participants with MS and primary insomnia or at least sleep variables consistent with the original study and had used 3 mg of eszopiclone in all participants, the results might have been different. On the other hand, sleep disturbances and complaints in MS patients could be secondary to other issues, and a medication designed to treat primary insomnia may not work for them. We did not a priori differentiate between primary and secondary sleep disturbances.
A number of sleep disorders have been reported to be more prevalent in MS patients than in the general population, including RLS.15 Another common disorder among MS patients is periodic limb movement disorder (PLMD).16 We screened for RLS but not for PLMD, although the three participants who underwent polysomnography before enrolling in the trial did not have high indices of PLM. Narcolepsy or primary hypersomnias have also been described in MS patients; both of these conditions can disrupt nighttime sleep.17–19 Our participants did have abnormally high mean and median ESS scores of 11 (normal is ≤10), but no cataplexy, sleep paralysis, or hypnic hallucinations were reported, and individuals with narcolepsy generally score around 17.20 Depression certainly can cause disrupted sleep,21 but we also screened out participants with CESD scores of 22 or higher. Pain, nocturia, spasms, and decreased mobility, together with certain lesion sites, can also cause sleep disturbances, making sleep complaints in MS multifactorial. 22 23 We postulate that this was the case with our participants, possibly explaining why they did not respond as robustly to eszopiclone as individuals with primary insomnia would have. In addition, we treated our participants for only 5 weeks. The question may be raised whether longer treatment would have resulted in greater improvement in sleep variables, although in the original eszopiclone studies response was apparent from day 1 and was maximal within 15 days for most individuals.5
In our study, no statistically significant difference was noted in change in fatigue measures between the placebo and treatment groups despite the significant objective increase in TST in the treatment group. This may be explained by the strong placebo effect: 40% of the participants receiving placebo improved from “fatigued” to “nonfatigued” on the FDS and had a drop of 10 or more points on the MFIS, compared with 50% of participants receiving eszopiclone. Other studies have also shown a strong response to placebo for fatigue in MS patients.24 25
We believe that the most likely explanation for the results of this study is that central fatigue in MS is multifactorial, with sleep disturbances playing only a modest role in its pathophysiology. Some studies have shown that specific basal ganglia lesions as well as global brain atrophy and other neuroanatomical changes correlate with fatigue.26–28 Endocrine abnormalities29 as well as changes in metabolism30 have also been found to correlate with the presence and severity of fatigue in MS; moreover, the presence of pain also affects fatigue.31 Although both our initial study and later studies have shown sleep disturbances to be prevalent in MS patients,4 32 one recent study that examined six individuals with a benign form of MS did not find any sleep disturbances.30
Limitations of our study include small sample size, recruitment of participants with subjective sleep complaints, and the use of actigraphy rather than the more accurate polysomnography for objective measurement (although polysomnography would have been less practical for long-term assessment of sleep/wake patterns), and the fact that participants continued to take immunomodulators and other medications that were neither stimulants/antifatigue medications nor sedativehypnotic agents. The receipt of other medications may have affected the sleep patterns of our participants, as has been recently shown.33
Conclusion
Sleep disturbances in MS are prevalent and appear to be multifactorial. They do respond at least partially to eszopiclone, suggesting that they are due at least in part to a decrease in central GABA activity, as has been shown in individuals with primary insomnia.34 The partial improvement in sleep patterns with eszopiclone did not result in significant improvement in daytime fatigue. The explanation may be either that response to eszopiclone was not robust enough to improve fatigue or that factors other than sleep disturbances are primarily responsible for MS-related fatigue.
PracticePoints
Sleep disturbances are very common among people with MS.
MS patients with sleep disturbances respond at least partially to eszopiclone.
The fact that treatment of sleep disturbances does not fully eliminate fatigue among MS patients indicates that factors other than sleep disturbances are involved in MS-related fatigue.
References
Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008; 14: 1127–1130.
Krupp LB, Coyle PK, Doscher C, . Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995; 45: 1956–1961.
Reder AT, Antel JP. Clinical spectrum of multiple sclerosis. Neurol Clin. 1983; 1: 573–599.
Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch Neurol. 2004; 61: 525–528.
Iriarte J, Katsamakis G, de Castro P. The Fatigue Descriptive Scale (FDS): a useful tool to evaluate fatigue in multiple sclerosis. Mult Scler. 1999; 5: 10–16.
Krystal AD, Walsh JK, Laska E, . Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003; 26: 793–799.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1: 385–401.
Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994; 17: 703–710.
Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler. 2005; 11: 198–202.
Valentino P, Cerasa A, Chiriaco C, . Cognitive deficits in multiple sclerosis patients with cerebellar symptoms. Mult Scler. 2009; 15: 854–859.
Bryden PJ, Roy EA. A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn. 2005; 58: 258–268.
Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the Profile of Mood States (POMS): two partial replications. J Clin Psychol. 1984; 40: 1270–1277.
Najib J. Eszopiclone, a nonbenzodiazepine sedative-hypnotic agent for the treatment of transient and chronic insomnia. Clin Ther. 2006; 28: 491–516.
Manconi M, Ferini-Strambi L, Filippi M, . Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. 2008; 31: 944–952.
Ferini-Strambi L, Filippi M, Martinelli V, . Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci. 1994; 125: 194–197.
Younger DS, Pedley TA, Thorpy MJ. Multiple sclerosis and narcolepsy: possible similar genetic susceptibility. Neurology. 1991; 41: 447–448.
Wang CY, Kawashima H, Takami T, . [A case of multiple sclerosis with initial symptoms of narcolepsy]. No To Hattatsu. 1998; 30: 300–306.
Autret A, Lucas B, Henry-Lebras F, de Toffol B. Symptomatic narcolepsies. Sleep. 1994;17(8 suppl):S21–24.
Sangal RB, Mitler MM, Sangal JM. Subjective sleepiness ratings (Epworth sleepiness scale) do not reflect the same parameter of sleepiness as objective sleepiness (maintenance of wakefulness test) in patients with narcolepsy. Clin Neurophysiol. 1999; 110: 2131–2135.
Leo G, Rao M, Bernardin L. Sleep disturbances in multiple sclerosis. Neurology. 1991; 41(suppl 1):320.
Tachibana N, Howard RS, Hirsch NP, Miller DH, Moseley IF, Fish D. Sleep problems in multiple sclerosis. Eur Neurol. 1994; 34: 320–323.
Clark CM, Fleming JA, Li D, Oger J, Klonoff H, Paty D. Sleep disturbance, depression, and lesion site in patients with multiple sclerosis. Arch Neurol. 1992; 49: 641–643.
Kos D, Duportail M, D'Hooghe M, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler. 2007; 13: 996–1003.
Stankoff B, Waubant E, Confavreux C, . Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005; 64: 1139–1143.
Sepulcre J, Masdeu JC, Goni J, . Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler. 2009; 15: 337–344.
Tellez N, Alonso J, Rio J, . The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology. 2008; 50: 17–23.
Tedeschi G, Dinacci D, Lavorgna L, . Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci. 2007; 263: 15–19.
Tellez N, Comabella M, Julia E, . Fatigue in progressive multiple sclerosis is associated with low levels of dehydroepiandrosterone. Mult Scler. 2006; 12: 487–494.
Vetrugno R, Stecchi S, Scandellari C, . Sleep-wake and body core temperature rhythms in multiple sclerosis with fatigue. Clin Neurophysiol. 2007; 118: 228–234.
Patrick E, Christodoulou C, Krupp LB. Longitudinal correlates of fatigue in multiple sclerosis. Mult Scler. 2009; 15: 258–261.
Kaynak H, Altintas A, Kaynak D, . Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol. 2006; 13: 1333–1339.
Mendozzi L, Tronci F, Garegnani M, Pugnetti L. Sleep disturbance and fatigue in mild relapsing remitting multiple sclerosis patients on chronic immunomodulant therapy: an actigraphic study. Mult Scler. 2010; 16: 238–247.
Winkelman JW, Buxton OM, Jensen JE, . Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008; 31: 1499–1506.
Financial Disclosures: Dr. Wang is employed by Sunovion Pharmaceuticals Inc, which manufactures eszopiclone (Lunesta). The other authors have no conflicts of interest to disclose.
Funding/Support: This study was funded by an investigator-initiated grant from Sunovion Pharmaceuticals Inc.







