Publication
Research Article
International Journal of MS Care
Cognition and Physical Disability in Predicting Health-Related Quality of Life in Multiple Sclerosis
Author(s):
Many studies have shown that multiple sclerosis (MS) has a significant impact on patient health-related quality of life (HRQOL), but the relative contributions of physical versus cognitive disability are not well established. Most studies have relied on HRQOL outcomes that depend largely on patient mood, life satisfaction, and personal happiness. The Sickness Impact Profile (SIP) is a measure of HRQOL known for its relatively strong emphasis on task completion and activities of daily living. As such, the SIP may be less influenced by depression. We sought to determine the relative influence of physical disability and cognition, above and beyond demographic and disease variables, in predicting HRQOL. Patients (n = 132) and healthy controls (n = 26) underwent complete neuropsychological evaluation using the Minimal Assessment of Cognitive Function in MS (MACFIMS) battery and a series of self-report measures assessing depression, fatigue, and HRQOL. The SIP was also administered. Correlation analysis and group comparisons revealed significant associations between cognition and HRQOL outcomes. Logistic regression models comparing the Expanded Disability Status Scale (EDSS) and cognitive tests in predicting poor physical HRQOL retained both EDSS and Symbol Digit Modalities Test (SDMT) performance, while models predicting poor psychosocial and poor overall HRQOL retained only the SDMT. These findings support cognition as a significant predictor of overall HRQOL, psychosocial HRQOL, and, interestingly, physical HRQOL.
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system with a variable and broad range of physical, psychological, and cognitive symptoms. It is widely recognized that MS can have a significant impact on a patient's health-related quality of life (HRQOL)1—that is, their happiness or satisfaction in meaningful daily activity despite the disease.2 However, research in this area has often failed to account for the numerous disease and clinical features of MS, leading to a lack of consensus as to the most important predictors of reduced HRQOL in this population.3 4 This problem is of great interest in the comprehensive care of MS patients, particularly as HRQOL and adjustment to illness may help predict disease progression4 (eg, through influencing susceptibility to relapses, engagement in positive health behaviors, use of active coping strategies, and so on). Previous research in MS has shown associations between poor HRQOL and progressive disease course,5–9 greater physical disability,10–15 disease duration,10 fatigue,11 12 16 17 and depression.3 6 17–21 Of these, physical and cognitive capacity are measured by performance-based reliable measures, and it is not clear which ability domain is most critical for poor HRQOL in MS patients. Many studies have shown cognitive impairment to be a predictor of reduced HRQOL,10 22–25 while others have not supported this association.26
Given the increasing attention to HRQOL across medical and psychological illnesses, an abundance of HRQOL measures are available, both generic and disease-specific. One of the most popular generic HRQOL measures, the 36-item Short Form Health Status Survey (SF-36),27 generates physical and mental component scores based on the patient's perception of his or her health status. One of the most commonly employed MS-specific HRQOL measures, the Multiple Sclerosis Quality of Life–54 (MSQOL-54),28 adds 18 MS-specific items to the original SF-36. While allowing for greater sensitivity in within-disease comparison,29 the MSQOL-54 is again based on the patient's subjective report of life satisfaction. For example, patients are asked to rate how much time they have felt “discouraged” (item 38) and “weighed down” (item 41) by their illness, and item 33 presents sad and happy facial expressions along a 10-point scale and directs patients to rate their overall “quality of life.”
In contrast, although it is a self-report survey, the Sickness Impact Profile (SIP)30 31 is based largely on judgments of the frequency of instrumental behaviors and the capacity to complete a diverse range of activities of daily living. Without the emphasis on subjective satisfaction, the SIP may be less likely to be influenced by depression. In emphasizing the capacity to engage in activities, the SIP allows a more concrete appraisal of activity. Patients are asked to read a series of statements and indicate those that are applicable to them. Examples of items from each SIP subscale are presented in Table 1. The SIP items range from mild (“I go up and down stairs more slowly”) to more severe (“I do not walk at all”) disability and yield physical and psychosocial dimension scores, in addition to a total score representing overall disability.
Sickness Impact Profile: item examples
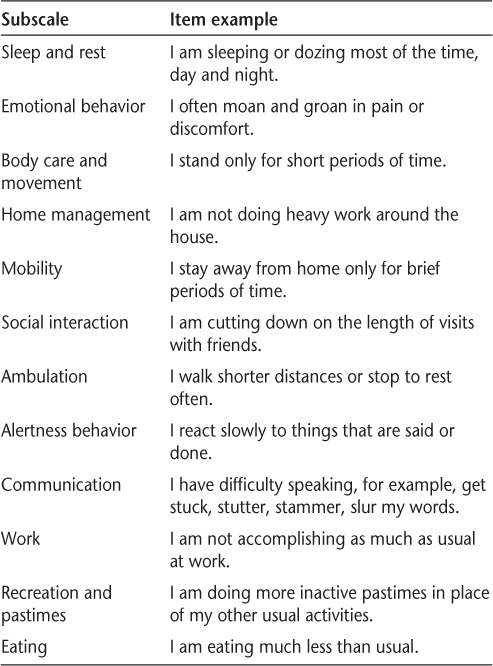
The SIP is widely used and well validated in a wide range of disease populations31 and has been used in several studies in MS as a behaviorally based measure of HRQOL.15 21 32–36 In one study employing both the SIP and the SF-36, the SIP was shown to have stronger associations with the Multiple Sclerosis Functional Composite (MSFC) and EDSS than the SF-36.34 Using the SIP, our objective was to determine the relative influence of physical disability and cognitive impairment in predicting HRQOL in a large sample of MS patients. Controlling for demographic and disease characteristics, including depression and fatigue, our outcomes were physical, psychosocial, and overall HRQOL.
Methods
Participants
The study sample included 132 MS patients and 26 healthy controls recruited at an MS care center in the eastern United States. All participants provided informed consent to be included in the study as per institutional review board requirements. Exclusion criteria for MS patients were 1) current or past medical or psychiatric disorder other than MS that could affect cognitive function, 2) current substance abuse, 3) neurological impairment that might interfere with psychometric testing, and 4) MS relapse or corticosteroid pulse within 6 weeks of neuropsychological testing.
For the MS group, the mean (SD) age was 46.4 (10.3) years and the mean amount of education was 14.4 (2.1) years. The majority of the sample (73%) was female and white (90%). The mean disease duration was 11.7 (8.3) years. Diagnosis and MS course were based on established guidelines for research protocols in MS37 (relapsing-remitting [RR] = 94; secondary progressive [SP] = 38). The median EDSS38 score was 3.5 (range = 0–6.5), obtained within 6 months of neuropsychological testing for all patients. Controls had a mean age of 43.6 (11.5) years and a mean amount of education of 15.0 (2.0) years. The majority (58%) were female and white (89%).
Tests and Study Procedures
All patients and controls underwent complete neuropsychological evaluation using the Minimal Assessment of Cognitive Function in MS (MACFIMS) battery39 40 and a series of self-report measures. The Controlled Oral Word Association Test (COWAT)41 assessed verbal fluency and consisted of three 60-second trials in which the participant generated as many words as possible beginning with a designated letter of the alphabet. The Judgment of Line Orientation (JLO) test42 measured visual/spatial processing and required participants to accurately match the position and direction of two unlabeled lines to two lines on a labeled model. The California Verbal Learning Test, second edition (CVLT2)43 measured verbal learning and memory. During the learning phase a word list was presented and participants recalled the list five times consecutively and following a delay period. The Brief Visuospatial Memory Test–Revised (BVMTR)44 assessed visual/spatial learning and memory. During the learning phase a display containing abstract geometric figures was presented. Participants reproduced as many figures as possible on each of three learning trials and following a 25-minute delay period. Rao's adaptations45 of the Symbol Digit Modalities Test (SDMT)46 and Paced Auditory Serial Addition Test (PASAT 3.0 and 2.0)47 were used to measure mental processing speed and working memory. The SDMT required participants to voice the number associated with a random array of target symbols defined by a key at the top of the page. The PASAT presented a series of single-digit numbers and required participants to add each new digit to the one immediately preceding it; digits were presented at a rate of one every 3 seconds on the first trial and every 2 seconds on the second trial. Finally, the Delis-Kaplan Executive Function System (DKEFS)48 Sorting Test was employed to measure executive function. Participants were given six cards and asked to sort the cards into two groups and to verbally describe the sorting principle applied.
Self-report measures included the Beck Depression Inventory–Fast Screen (BDIFS),49 Fatigue Severity Scale (FSS),50 and SIP.30 The SIP total and dimension scores range from 0 to 100, with higher scores reflecting greater dysfunction and a score of 20 indicating severe dysfunction or the need for substantial daily care.51–53
Statistical Analyses
Statistical analyses were conducted using SPSS, version 18.0 (SPSS, Chicago, IL). The MS and control groups were compared on demographic and predictor variables using univariate analysis of variance (ANOVA). Relationships between individual cognitive tests and HRQOL variables were then examined in the patient group using partial correlations controlling for age and education. Based on diagnostic criteria for the MAC-FIMS battery,39 MS patients were also broadly classified as cognitively normal or cognitively impaired (two or more cognitive test z scores of –1.5 or less), and HRQOL total, dimension, and category scores were evaluated.
As total and dimension scores on the SIP were not normally distributed, the measure was dichotomized using the above-described cutoff score of 20 to indicate poor HRQOL. Logistic regression models were then used to determine whether cognition or physical disability better predicts HRQOL. We included three models, controlling for the effects of age, education, sex, disease course, disease duration, BDIFS, and FSS. The models were designed to predict patients with poor versus good HRQOL using the SIP physical dimension, SIP psychosocial dimension, and SIP total score, while controlling for subjectively reported emotional distress and fatigue. We selected the EDSS as the measure of physical disability, and CVLT2 delayed recall, BVMTR delayed recall, SDMT, and PASAT 3.0 raw scores as potential cognitive predictors, as these tests represent the most commonly seen cognitive deficits in MS, episodic memory and processing speed, respectively.39 54 In each model, the first block consisted of demographic variables, BDIFS, and FSS. The second block consisted of EDSS and cognitive test scores using a forward stepwise selection procedure with P to enter = .05 and P to exit = .10.
Results
Group (MS vs. healthy control) differences in age and education were not statistically significant (Table 2). As expected, MS patients had lower HRQOL in the SIP physical dimension (P < .001), SIP psychosocial dimension (P < .001), and SIP total score (P < .001), as well as lower performance on all cognitive tests except the JLO. MS patients also reported greater fatigue (P < .001), and there was a trend for more depression in the MS group (P < .10).
Demographic, HRQOL, and cognitive characteristics for MS and control groups
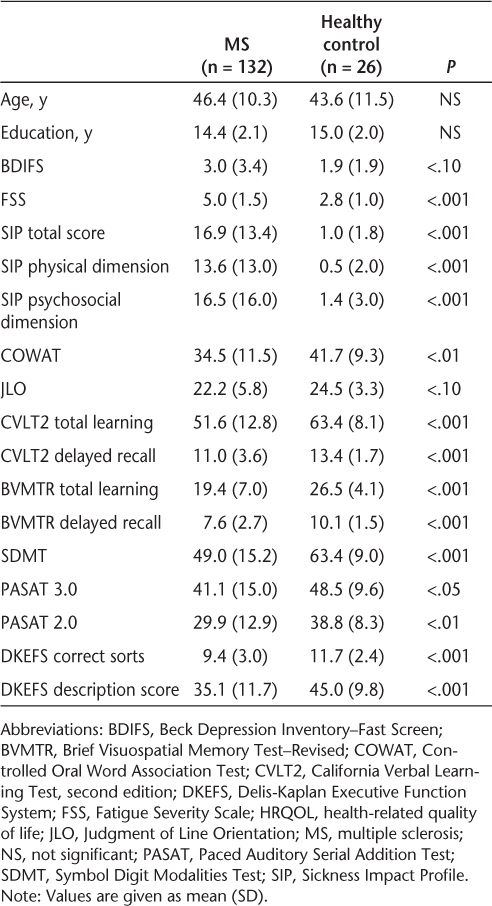
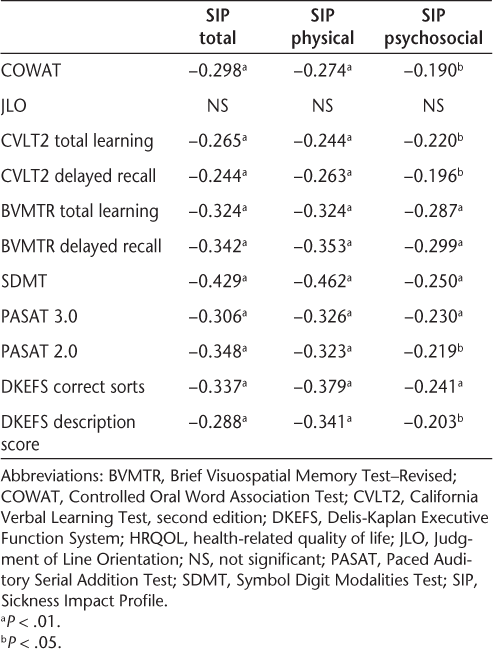
Table 3 shows partial correlations between cognitive tests and HRQOL outcomes. When controlling for the effects of age and education, lower performance on cognitive tests was associated with worse HRQOL. All cognitive tests except the JLO were significantly associated with SIP total, physical, and psychosocial domains. The data reveal small to medium correlations, with somewhat larger correlations between cognition and physical versus psychosocial HRQOL.
Partial correlations between cognitive and HRQOL measures controlling for age and education
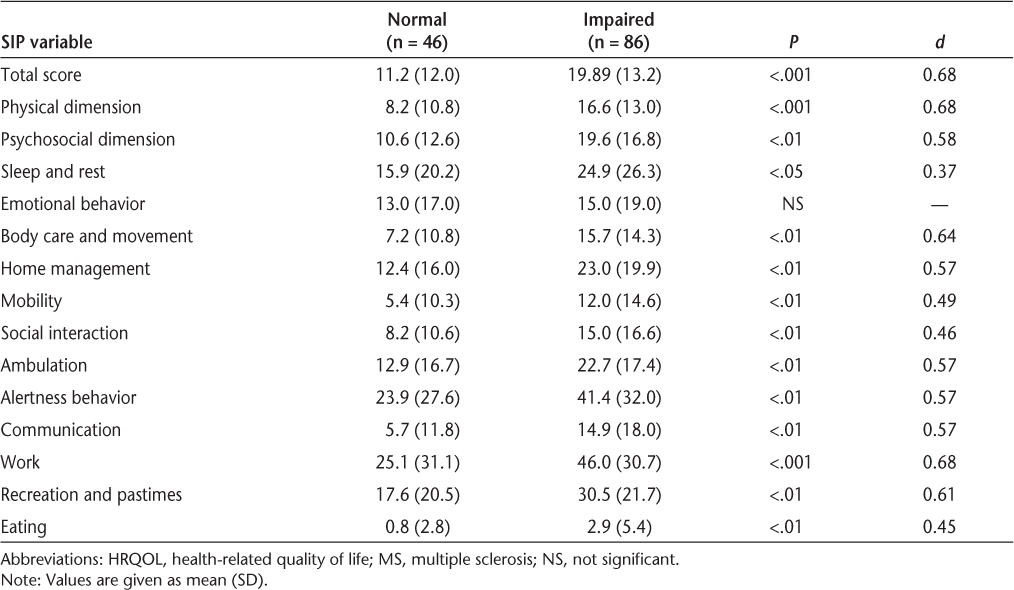
Patients were classified by overall cognitive status. Group differences in HRQOL between cognitively normal and cognitively impaired MS patients are shown in Table 4. MS patients classified as cognitively impaired reported significantly lower HRQOL in the SIP physical dimension (P < .001), SIP psychosocial dimension (P < .01), and SIP total score (P < .001), as well as lower HRQOL in all individual SIP categories except emotional behavior.
Comparison of HRQOL between cognitively normal and cognitively impaired MS patients
Logistic regression models predicting poor HRQOL in the SIP physical dimension retained both EDSS (Wald = 7.25, P < .01) and SDMT (Wald = 7.50, P < .01), correctly classifying 79% of patients. Poor HRQOL in the SIP psychosocial dimension was significantly predicted only by SDMT (Wald = 9.70, P < .001), correctly classifying 82% of patients. Finally, the model predicting poor HRQOL in the SIP total score retained only the SDMT (Wald = 13.98, P < .001), correctly classifying 81% of patients.
Discussion
The primary objective of this study was to evaluate the relative predictive value of physical and cognitive disability in HRQOL among MS patients while controlling for demographic and subjective distress variables, including depression and fatigue. We selected the SIP as our measure of HRQOL based on its emphasis on specific behaviors and abilities versus patient perception of health or disease status. Interestingly, while the EDSS was first to enter the regression model predicting poor physical HRQOL, cognitive impairment as represented by poor SDMT score was also retained in this model. Only the SDMT was retained in the models predicting psychosocial and overall HRQOL, with lower scores on the SDMT significantly predicting poor psychosocial HRQOL and poor overall HRQOL. Our data regarding the EDSS and its relationship with physical components of HRQOL are consistent with previous research,10–15 while the meaning of cognition as a predictor warrants further exploration. The findings suggest the possibility of a greater role for cognitive impairment in HRQOL among MS patients, and require replication in larger patient samples with other HRQOL outcomes.
The finding that cognition plays a role in physical HRQOL is interesting, and suggests that beyond actual physical capability, cognition may have a significant impact on one's ability to engage in meaningful physical activities. For example, MS patients who are cognitively impaired may be more likely to experience falls or may require more assistance with physical activities of daily living than patients with equal physical disability but without cognitive impairment. Although walking has customarily been understood as a largely automatic motor task, recent evidence suggests the involvement of higher-order cognitive processing and control of gait.55 56 Associations between walking speed and measures of executive function and attention have been shown in young and older adult populations,56–58 patients with traumatic brain injury,59 Parkinson's disease,60 and Alzheimer's disease.61 Further research is necessary to understand the connection seen in these data between poor physical HRQOL and cognitive impairment, including variables that might mediate that relationship, such as personality factors.
The only SIP subscale for which there was no abnormality in MS was emotional behavior. Relatively speaking, the SIP would seem to measure a person's perception of instrumental capacity more than their happiness or satisfaction with activities and lifestyle. This is not to say that the latter dimension is not important. For example, many MS patients may be happier after they come to accept that they cannot walk after 20 years with MS than when they are suffering from depression and fatigue in the beginning stages of the disease. The relative importance of the SIP versus more conventional measures of HRQOL such as the MSQOL-54 in terms of prognosis and risk for complications of the disease remains to be seen.
Table 4 reveals that with the exception of SIP summary scores, the greatest area of disability was reported to be in the area of vocation status. We have previously addressed vocational disability and its predictors.3 In that large cross-sectional study of 120 MS patients, cognitive status was a primary predictor over and above depression, subjectively reported fatigue, EDSS score, and psychiatric symptoms. More recently, we have identified degrees of deterioration on specific cognitive tests that place patients at high risk for vocational disability, such as a 4-point loss on the SDMT.62
Our findings indicate that mental processing speed may influence a wide range of daily activities, including recreational activity, social interaction, task completion, and workplace demands. In multiple dimensions of HRQOL, greater cognitive impairment is associated with worse functioning and diminished activity. This highlights the broad range of physical, emotional, psychological, and social domains affected by cognitive impairment and the importance of early detection and management of cognitive symptoms in MS. Accurate and timely characterization of cognitive impairment in MS may have implications for patient and caregiver education, potential coping/compensation strategies, and therapeutic interventions aimed at improving patient HRQOL. Cognitive rehabilitation and training efforts have shown mixed outcomes in MS,63–65 but recent evidence suggests that intensive and domain-specific cognitive training in attention, processing speed, and executive function is effective.66 Behavioral interventions may include referrals for speech therapy or occupational therapy, and pharmacological interventions are under continued investigation for efficacy in treating cognitive impairment in MS.
PRACTICEPOINTS
Multiple sclerosis (MS) is well known to have a significant impact on patient health-related quality of life (HRQOL). Yet the relative value of cognitive and physical impairment in predicting HRQOL in MS is not well established.
Surprisingly, in addition to physical disability, cognition emerges as a significant predictor of physical HRQOL, suggesting that cognition may affect the patient's ability to engage in meaningful physical activity. Only cognition significantly predicted psychosocial and overall HRQOL, with lower cognitive test scores predicting worse HRQOL.
The association between cognition and HRQOL domains in MS highlights the importance of early identification of cognitive impairment in the comprehensive care of MS patients.
References
Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology. 1999; 53: 1098–1103.
Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler. 1999; 5: 251–259.
Benedict RH, Wahlig E, Bakshi R, . Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005; 231: 29–34.
Mitchell AJ, Benito-Leon J, Gonzalez JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol. 2005; 4: 556–566.
Busche KD, Fisk JD, Murray TJ, Metz LM. Short term predictors of unemployment in multiple sclerosis patients. Can J Neurol Sci. 2003; 30: 137–142.
Patti F, Cacopardo M, Palermo F, . Health-related quality of life and depression in an Italian sample of multiple sclerosis patients. J Neurol Sci. 2003; 211: 55–62.
Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T. Quality of life in multiple sclerosis: influence of interferon-beta1 a (Avonex) treatment. Mult Scler. 2002; 8: 377–381.
Beiske AG, Naess H, Aarseth JH, . Health-related quality of life in secondary progressive multiple sclerosis. Mult Scler. 2007; 13: 386–392.
Janardhan V, Bakshi R. Quality of life and its relationship to brain lesions and atrophy on magnetic resonance images in 60 patients with multiple sclerosis. Arch Neurol. 2000; 57: 1485–1491.
Benito-Leon J, Morales JM, Rivera-Navarro J. Health-related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur J Neurol. 2002; 9: 497–502.
Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. 2002; 205: 51–58.
Merkelbach S, Sittinger H, Koenig J. Is there a differential impact of fatigue and physical disability on quality of life in multiple sclerosis? J Nerv Ment Dis. 2002;190:388–393.
Turpin KV, Carroll LJ, Cassidy JD, Hader WJ. Deterioration in the health-related quality of life of persons with multiple sclerosis: the possible warning signs. Mult Scler. 2007; 13: 1038–1045.
Hopman WM, Coo H, Edgar CM, McBride EV, Day AG, Brunet DG. Factors associated with health-related quality of life in multiple sclerosis. Can J Neurol Sci. 2007; 34: 160–166.
Miller DM, Rudick RA, Baier M, . Factors that predict health-related quality of life in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2003; 9: 1–5.
Pittion-Vouyovitch S, Debouverie M, Guillemin F, Vandenberghe N, Anxionnat R, Vespignani H. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci. 2006; 243: 39–45.
Motl RW, Suh Y, Weikert M. Symptom cluster and quality of life in multiple sclerosis. J Pain Symptom Manage. 2010; 39: 1025–1032.
Benito-Leon J, Morales JM, Rivera-Navarro J, Mitchell A. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil. 2003; 25: 1291–1303.
Lobentanz IS, Asenbaum S, Vass K, . Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand. 2004; 110: 6–13.
Glanz BI, Healy BC, Rintell DJ, Jaffin SK, Bakshi R, Weiner HL. The association between cognitive impairment and quality of life in patients with early multiple sclerosis. J Neurol Sci. 2010; 290: 75–79.
Gottberg K, Einarsson U, Fredrikson S, von Koch L, Holmqvist LW. A population-based study of depressive symptoms in multiple sclerosis in Stockholm County: association with functioning and sense of coherence. J Neurol Neurosurg Psychiatry. 2007; 78: 60–65.
Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. 2001; 58: 1602–1606.
Shawaryn MA, Schiaffino KM, LaRocca NG, Johnston MV. Determinants of health-related quality of life in multiple sclerosis: the role of illness intrusiveness. Mult Scler. 2002; 8: 310–318.
Marrie RA, Miller DM, Chelune GJ, Cohen JA. Validity and reliability of the MSQLI in cognitively impaired patients with multiple sclerosis. Mult Scler. 2003; 9: 621–626.
Cutajar R, Ferriani E, Scandellari C, . Cognitive function and quality of life in multiple sclerosis patients. J Neurovirol. 2000;6 suppl 2:S186–S190.
Barker-Collo SL. Quality of life in multiple sclerosis: does information-processing speed have an independent effect? Arch Clin Neuropsychol. 2006;21:167–174.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), part I: conceptual framework and item selection. Med Care. 1992; 30: 473–483.
Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995; 4: 187–206.
Özakbas S, Akdede BB, Kösehasanogullari G, Aksan Ö, Idiman E. Difference between generic and multiple sclerosis-specific quality of life instruments regarding the assessment of treatment efficacy. J Neurol Sci. 2007; 256: 30–34.
Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981; 19: 787–805.
Damiano AM. Sickness Impact Profile: User's Manual and Interpretation Guide. Baltimore, MD: Johns Hopkins University; 1996.
Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996; 39: 432–441.
Gianino JM, York MM, Paice JA, Shott S. Quality of life: effect of reduced spasticity from intrathecal baclofen. J Neurosci Nurs. 1998; 30: 47–54.
Miller DM, Rudick RA, Cutter G, Baier M, Fischer JS. Clinical significance of the multiple sclerosis functional composite: relationship to patient-reported quality of life. Arch Neurol. 2000; 57: 1319–1324.
Voss WD, Arnett PA, Higginson CI, Randolph JJ, Campos MD, Dyck DG. Contributing factors to depressed mood in multiple sclerosis. Arch Clin Neuropsychol. 2002; 17: 103–115.
Gottberg K, Einarsson U, Ytterberg C, . Health-related quality of life in a population-based sample of people with multiple sclerosis in Stockholm County. Mult Scler. 2006; 12: 605–612.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996; 46: 907–911.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452.
Benedict RH, Cookfair D, Gavett R, . Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2006; 12: 549–558.
Benedict RH, Fischer JS, Archibald CJ, . Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002; 16: 381–397.
Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1989.
Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. 2nd ed. New York: Oxford University Press; 1994.
Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version. 2nd ed. San Antonio, TX: Psychological Corporation; 2000.
Benedict RHB. Brief Visuospatial Memory Test—Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1997.
Rao SM. A Manual for the Brief, Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. New York, NY: National Multiple Sclerosis Society, 1990.
Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1982.
Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977; 44: 367–373.
Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001.
Beck AT, Steer RA, Brown GK. BDI–Fast Screen for Medical Patients: Manual. San Antonio, TX: Psychological Corporation; 2000.
Sandroni P, Walker C, Starr A. Fatigue in patients with multiple sclerosis. Arch Neurol. 1992; 49: 517–524.
Somerville SM, Silver R, Patrick DL. Services for disabled people; what criteria should we use to assess disability? Community Med. 1983;5:302–310.
Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009; 38: 115–123.
Damiano AM, Patrick DL, Guzman GI, . Measurement of health-related quality of life in patients with amyotrophic lateral sclerosis in clinical trials of new therapies. Med Care. 1999; 37: 15–26.
Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, part I: frequency, patterns, and prediction. Neurology. 1991; 41: 685–691.
Alexander NB, Hausdorff JM. Guest editorial: linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2008; 63: 1325–1328.
Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008; 23: 329–342.
Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM. How does explicit prioritization alter walking during dual-task performance? effects of age and sex on gait speed and variability. Phys Ther. 2010; 90: 177–186.
Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005; 164: 541–548.
Cantin JF, McFadyen BJ, Doyon J, Swaine B, Dumas D, Vallee M. Can measures of cognitive function predict locomotor behaviour in complex environments following a traumatic brain injury? Brain Inj. 2007;21:327–334.
Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22: 1248–1256.
Allali G, Assal F, Kressig RW, Dubost V, Herrmann FR, Beauchet O. Impact of impaired executive function on gait stability. Dement Geriatr Cogn Disord. 2008; 26: 364–369.
Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol. 2010; 24: 1131–1145.
Benedict RH, Shapiro A, Priore R, Miller C, Munschauer F, Jacobs L. Neuropsychological counseling improves social behavior in cognitively-impaired multiple sclerosis patients. Mult Scler. 2000; 6: 391–396.
Jonsson A, Korfitzen EM, Heltberg A, Ravnborg MH, Byskov-Ottosen E. Effects of neuropsychological treatment in patients with multiple sclerosis. Acta Neurol Scand. 1993; 88: 394–400.
Solari A, Motta A, Mendozzi L, . Computer-aided retraining of memory and attention in people with multiple sclerosis: a randomized, double-blind controlled trial. J Neurol Sci. 2004; 222: 99–104.
Mattioli F, Stampatori C, Bellomi F, Capra R, Rocca M, Filippi M. Neuropsychological rehabilitation in adult multiple sclerosis. Neurol Sci. 2010;31(suppl 2):S271–S274.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This research was supported by National Multiple Sclerosis Society grant 4060A3/1.







