Publication
Research Article
International Journal of MS Care
Stabilization Without Rituximab After Disease Activation in an Alemtuzumab-Treated Patient with Multiple Sclerosis and a Literature Overview
Abstract
There has been an increasing number of reports describing B-cell–associated disease activation in patients with multiple sclerosis after alemtuzumab treatment. Herein, 4.5 months after a first alemtuzumab infusion, a 33-year-old female patient had altered gait and vision loss associated with gadolinium enhancement of the optic nerves and chiasm on brain magnetic resonance imaging. The patient's blood showed normal B-cell counts concurrent with abnormally low T-cell counts. The patient stabilized after receiving steroids and prolonged plasma exchange without the use of rituximab. This case report adds to the growing body of literature of B-cell–associated disease activation after alemtuzumab infusion and provides a therapeutic strategy to stabilize patients when rituximab is not readily available or if contraindications to its use exist.
Alemtuzumab is a humanized anti-CD52 monoclonal antibody that has been shown in several clinical trials to reduce clinical relapses and brain volume loss in people with multiple sclerosis (MS).1 2 Dosing requires at least two intravenous courses at 12-month intervals.1 2 After each dose there is depletion of both T and B lymphocytes, yet data indicate that B cells recover more quickly than T cells, putting patients at risk for other autoimmune disorders, such as thyroid disease and immune thrombocytopenia.3 Recently, there is a growing body of literature suggesting increased disease activity after alemtuzumab induction associated with B-cell recovery.4–8 Of the 16 cases report to date, most were treated with fingolimod before alemtuzumab treatment.4–6 8 Of those treated for increased disease activity, most received rituximab.4 5 7 We describe an adult patient who presented with vision loss, cognitive decline, and altered gait who was stabilized with only steroid and extended plasma exchange therapy, without concomitant use of rituximab. The patient consented to the publication of the material contained herein.
Case Presentation
A 33-year-old woman was diagnosed at age 16 years as having relapsing-remitting MS based on clinical and magnetic resonance imaging (MRI) criteria. She had been taking several different disease-modifying therapies, including interferon beta-1a, interferon beta-1b, and natalizumab. Natalizumab use was discontinued due to a positive JC virus titer (JCV index, 0.73). Four months later she was admitted to the hospital for a multifocal relapse involving dysarthria, declining mobility, and cognitive impairment for several weeks. Examination was notable for trouble with following commands, word-finding difficulty, dysarthria, and paraparesis. The MRI performed before alemtuzumab use showed several new T2 lesions and areas of gadolinium enhancement (Figure 1A and Figure S1 [A1 and A2], which is published in the online version of this article at ijmsc.org). She received her first alemtuzumab infusion during the hospitalization. The preinfusion lymphocyte count was 1.24 × 109/L (reference range, 1.50–7.50 × 109/L). Approximately 6 weeks after hospitalization she was referred to our clinic, where her examination findings returned to baseline levels (visual acuity: 20/30 OD and 20/20 OS, no dysarthria or word-finding difficulty, and ambulatory without assistance). Follow-up brain MRI 3 months later showed no new lesions and resolution of the gadolinium-enhancing lesions (Figure 1B and Figure S1 [B1 and B2]). Laboratory investigations showed an elevated thyrotropin level (5.28 mIU/L) and a lymphocyte count of 0.87 × 109/L.
Serial T1-weighted brain magnetic resonance images of the patient after gadolinium administration
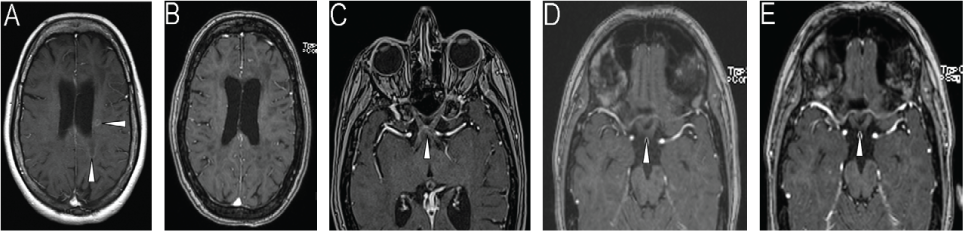
One month later the patient presented with bilateral vision loss and altered gait (ataxia and began using a walker). Examination showed a visual acuity of 20/100 – 1 at 1 ft OD and finger counting (one of three responses correct) OS along with worsening paraparesis. Urgent MRI showed multiple new gadolinium-enhancing lesions, including in the optic chiasm and nerves (Figure 1C and Figure S1 [C1–C4]), deep white matter, posterior fossa, and upper cervical spinal cord. Treatment was initiated with 5 days of intravenous methylprednisolone (Solu-Medrol; Pfizer, New York, NY) (1000 mg/d), followed by a 14-day prednisone taper. Her lymphocyte count was 0.77 × 109/L, and flow cytometry showed a normal number of B cells (0.33 × 109/L; reference range, 0.079–0.36 × 109/L) and a decreased absolute T-cell count (0.089 × 109/L; reference range, 0.53–2.19 × 109/L). Seven days later her gait improved (narrowed stance and ability to walk a few steps without assistance), as did her visual acuity (20/80 OD and 20/160 OS). Repeated brain MRI 7 weeks later showed reduced gadolinium enhancement of the optic chiasm and fewer gadolinium-enhancing lesions (not shown). Shortly after MRI, the patient began to develop worsening cognitive decline and vision. She was treated with 5 days of oral prednisone (1250 mg/d) followed by hospitalization with 3 days of intravenous Solu-Medrol (1000 mg/d). Plasma exchange was initiated 4 weeks later because of incomplete improvement of neurologic status. The patient received nine plasma exchanges over 6 weeks. Prednisone therapy was maintained at 80 mg/d during plasma exchange. After five exchanges, her visual acuity improved to 20/50 OD and 20/40 OS, and her cognition and gait returned to baseline status (narrow-based gait, walking without assistance). Prednisone use was tapered to 30 mg/d, and brain MRI approximately 2 months (Figure 1D and Figure S1 [D1–D5]) and 3 months (Figure 1E and Figure S1 [E1–E5]) later showed resolution of all gadolinium-enhancing lesions and no new lesions. Five weeks later, her visual acuity was 20/25 OD and 20/40 OS.
Discussion
At the time of manuscript submission, there were 16 reported cases of alemtuzumab-associated disease activation (Table 1).4–8 Disease activation, including clinical and radiologic activity, occurred within 4 to 9 months of alemtuzumab infusion. Some studies suggested that a common feature of these patients is exposure to fingolimod before alemtuzumab therapy.6 Specifically, it is hypothesized that alemtuzumab may not be able to target existing lymphocytes sequestered in lymph organs by fingolomid.6 Other studies suggest that after alemtuzumab infusion, the recovery of B cells relative to T cells initiates a B-cell–mediated autoimmune response.3–8 This may result in either autoimmune targeting of systemic organs (ie, thyroid, platelets, or kidney) or the central nervous system (MS disease activation).3–8
Summary of case reports in the literature
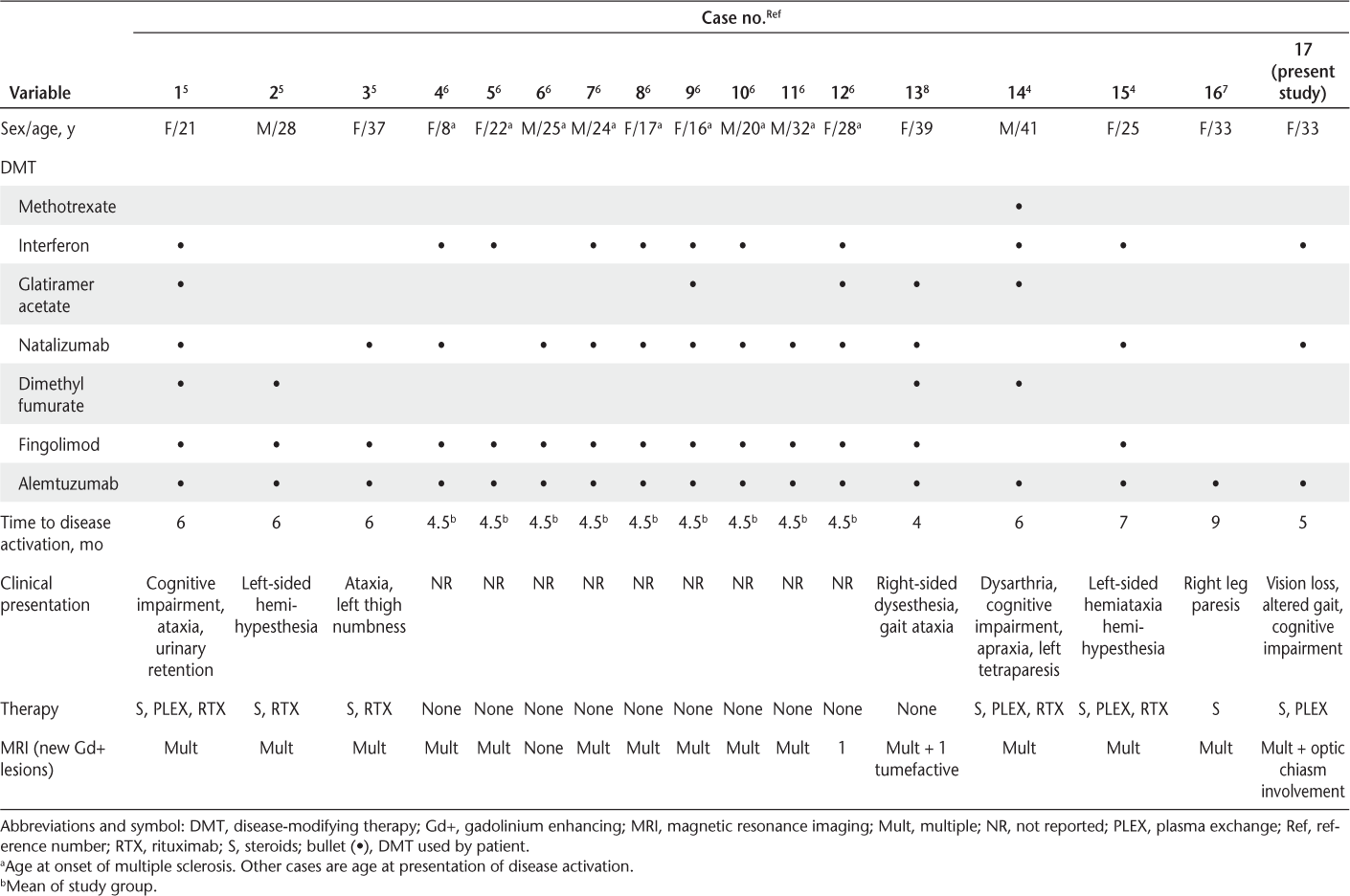
Similar to the other cases of alemtuzumab-associated MS disease activation, the present adult patient relapsed within 5 months of alemtuzumab infusion. In addition, there was relative B-cell to T-cell reconstitution at the time of disease activation. Yet, there are some interesting and unique aspects to this case. First, this patient presented with optic chiasm and optic nerve involvement, which, from our review of the published literature, has not been reported with alemtuzumab-associated disease activation. Second, this patient, as did two others in the literature,4 7 never received fingolimod. Of those two patients, one stabilized after treatment with methylprednisolone and the other after receiving methylprednisolone, plasma exchange, and rituximab.4 7 Because rituximab was not readily available at the time to treat MS, we needed to use other strategies to stabilize the patient. In addition to steroid therapy, we chose extended plasma exchange. In MS, a typical plasma exchange protocol involves five to seven exchanges over 2 weeks.9 We chose to continue plasma exchange without concomitant use of rituximab for 6 weeks, which stabilized the patient both clinically (improved visual acuity and gait) and by MRI (no new lesions). A mechanism by which plasma exchange improves MS is unknown, but both immunoglobulins and soluble substances such as cytokines are altered.10–12 For example, plasma exchange has been shown to remove antibodies and circulating immune complexes and reduce tumor necrosis factor alpha levels.10–12 A recent study showed a dramatic response to plasma exchange in patients with MS with biopsy-proven antibody-mediated demyelination (pattern 2 lesions).10 In this case presentation, we cannot determine the patient's pattern type or an exact mechanism, but either B-cell–associated cytokines and immunoglobulins may be involved because both are associated with MS disease pathogenesis.10 13
In summary, this case report adds to the growing body of literature of disease activation in the setting of normal B-cell counts and abnormally low T-cell counts after alemtuzumab infusion and provides a therapeutic strategy to stabilize these patients when rituximab is not readily available or if contraindications to its use exist.
PRACTICE POINTS
This case emphasizes the importance of recognizing the clinical, imaging, and laboratory presentation of disease activation (relapse, new gadolinium-enhancing lesions on magnetic resonance images, and normal B-cell counts concurrent with low T-cell counts) after alemtuzumab therapy for MS.
Although rituximab may be used during these episodes, it may not be readily available; therefore, steroids and plasma exchange alone may be used to stabilize patients with MS.
Financial Disclosures
The authors declare no conflicts of interest.
References
Cohen JA, Coles AJ, Arnold DL, et al; Care-MS I Investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828.
Coles AJ, Twyman CL, Arnold DL, et al; Care-MS II Investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839.
Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74:961–969.
Haghikia A, Dendrou CA, Schneider R, et al. Severe B-cell–mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol. 2017;16:104–106.
Wehrum T, Beume LA, Stich O, et al. Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology. 2018;90:e601–e605.
Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e320.
Schwenkenbecher P, Deppe J, Hummert MW, et al. Management of MS-relapse during alemtuzumab therapy: is it really B-cell–mediated? Mult Scler Relat Disord. 2018;19:6–7.
Barton J, Hardy TA, Riminton S, et al. Tumefactive demyelination following treatment for relapsing multiple sclerosis with alemtuzumab. Neurology. 2017;88:1004–1006.
Weinshenker BG, O'Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–886.
Stork L, Ellenberger D, Beissbarth T, et al. Differences in the reponses to apheresis therapy of patients with 3 histopathologically classified immunopathological patterns of multiple sclerosis. JAMA Neurol. 2018;75:428–435.
Goto H, Matsuo H, Nakane S, et al. Plasmapheresis affects T helper type-1/T helper type-2 balance of circulating peripheral lymphocytes. Ther Apher. 2001;5:494–496.
Shariatmadar S, Nassiri M, Vincek V. Effect of plasma exchange on cytokines measured by multianalyte bead array in thrombotic thrombocytopenic purpura. Am J Hematol. 2005;79:83–88.
Li R, Rezk A, Miyazaki Y, et al; Canadian B Cells in MS Team. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7:310ra166.







