Publication
Research Article
International Journal of MS Care
Short Report: Prevalence of Cognitive Impairment in Newly Diagnosed Relapsing-Remitting Multiple Sclerosis
Abstract
Background:
Cognitive impairment is common in multiple sclerosis (MS) and can manifest early in the disease process, sometimes as early as the first demyelinating event. However, the frequency of cognitive impairment in a newly diagnosed MS population has not been evaluated comprehensively in a clinical population. We sought to examine the prevalence of cognitive impairment in relapsing-remitting MS (RRMS) within a year of diagnosis in a clinic where cognitive testing at diagnosis is part of routine practice.
Methods:
A retrospective medical record review of persons with RRMS assessed in a cognitive MS clinic identified 107 patients assessed by the Minimal Assessment of Cognitive Function in Multiple Sclerosis battery within 1 year of a confirmed RRMS diagnosis.
Results:
The cohort was predominantly female (n = 82 [76.6%]) and white (n = 93 [86.9%]). Only 36 patients (33.6%) were diagnosed as having RRMS based on a second clinical event. Processing speed was the most frequently impaired domain (n = 38 [35.5%]). Only 37 patients (34.6%) were within normal limits on all cognitive domains. Regarding mood symptoms, 25 patients (23.4%) were positive for depressive symptoms; 59 (55.1%), for anxiety. Severe fatigue was correlated with a lower score on the Symbol Digit Modalities Test (SDMT) (r = −0.380, P < .001), and higher depressive scores were correlated with lower performance on the SDMT (r = −0.397, P < .001) and the Paced Auditory Serial Addition Test (r = −0.254, P = .009).
Conclusions:
Cognitive impairment, specifically processing speed, and mood symptoms are frequently present in persons with newly diagnosed RRMS.
Cognitive impairment is a common and disabling consequence of multiple sclerosis (MS). There is considerable variability in persons with MS regarding degree and type of cognitive impairment1; the notable areas of dysfunction are in processing speed, learning, and memory. Although cognitive impairment is insidious and progressive over time, studies show that it can manifest early in the disease process.2 3 Yet, the frequency of cognitive impairment in newly diagnosed persons with MS has not been comprehensively studied. Furthermore, studies in the past used older diagnostic criteria4–6 rather than the more recent and strongly supported 2010 McDonald criteria.7 Since 2012, the London MS Clinic (London, Ontario, Canada) has referred all newly diagnosed persons with MS for cognitive testing to serve as a baseline for comparison with future assessments, allowing for a comprehensive assessment of the prevalence of cognitive impairment in a newly diagnosed population. Thus, the aim of this retrospective medical record review was to examine the prevalence of cognitive impairment in persons with relapsing-remitting MS (RRMS) within a year of diagnosis.
Methods
Participants
A retrospective medical record review was conducted of persons with MS assessed at the London MS Clinic between January 1, 2012, and July 31, 2014. Included were persons with MS who had a cognitive assessment within 1 year of diagnosis of RRMS, per the 2010 McDonald diagnostic criteria.7 Participants were excluded if there was a diagnosis of a psychiatric disorder, learning disability, or neurologic condition that could cause cognitive impairment (eg, Alzheimer's disease/mild cognitive impairment, traumatic brain injury).
Procedure
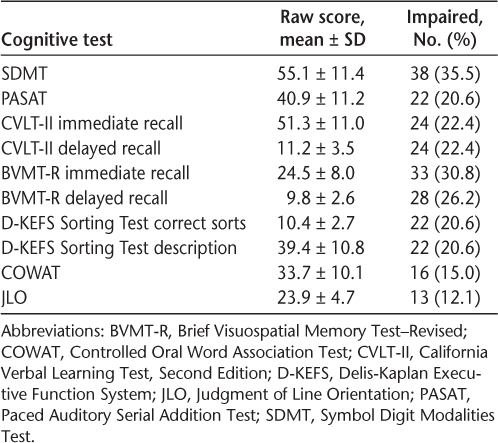
All the participants were assessed using the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) battery, a comprehensive battery for MS developed by a consensus committee and later found to be valid and reliable.8 9 The battery consists of the following seven neuropsychological tests: 1) Judgment of Line Orientation10: a measure of visuospatial perception; 2) Controlled Oral Word Association Test11: a measure of generative verbal fluency; 3) California Verbal Learning Test, Second Edition (CVLT-II)12: a measure of auditory/verbal episodic memory, with immediate recall (IR) and delayed recall (DR) components; 4) Brief Visuospatial Memory Test–Revised (BVMT-R)13: a measure of visuospatial memory, with IR and DR components; 5) Paced Auditory Serial Addition Test (PASAT) (S.M. Rao, PhD, unpublished data, 1991): a measure of speed and working memory in the auditory domain; 6) Symbol Digit Modalities Test (SDMT) (S.M. Rao, PhD, unpublished data, 1991): a measure of processing speed; and 7) Delis-Kaplan Executive Function SystemSorting Test14: a measure of higher executive function. A trained psychometrist administered this battery to each participant during baseline testing at the London MS Clinic. Using the previously reported regression-based norms of a control (non-MS) population from the seminal MACFIMS paper,8 z scores were calculated for each test in the battery.
As recommended by the MACFIMS consensus statement, measures of mood symptoms (the Hospital Anxiety and Depression Scale [HADS]15) and fatigue (the Fatigue Severity Scale [FSS]16) were also included due to the confounding nature of these two common MS symptoms. The HADS is a self-reported scale that detects anxiety (HADS-A) and depressive (HADS-D) symptoms. The HADS is composed of 14 questions, each scored from 0 (no symptoms) to 3 (severe symptoms). Composite scores for the HADS-A and HADS-D are calculated individually. The FSS, a measure of generalized fatigue, is composed of seven questions that are scored from 0 (no symptoms) to 7 (severe symptoms). The total score is divided by 7, thus the final score ranges from 0 to 7.
Statistical Analysis
Descriptive statistics were used for all demographic and cognitive test results. Pearson correlations or univariate analysis was used to determine whether any demographic or disease characteristic was associated with cognitive test performance. Owing to the multiple comparisons made, P < .01 was used to indicate a significant relationship. Where significant associations emerged, logistic regression models (forward conditional) with P to enter .05 and to exit .10 were used to evaluate the predictive value regarding impairment on the cognitive test, using Nagelkerke R 2.
All the statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp, Armonk, NY). The Health Sciences Research Ethics Board of Western University (London, Ontario, Canada) approved the study.
Results
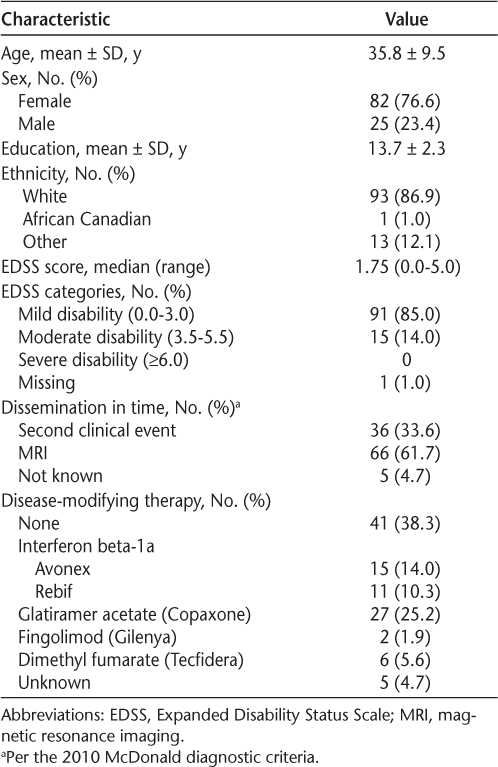
A baseline assessment was performed in 107 newly diagnosed persons with MS; demographic characteristics are presented in Table 1. The mean ± SD age of the cohort was 35.8 ± 9.5 (range, 16–58) years, and 82 participants were female (76.6%). In terms of the MS characteristics, the median Expanded Disability Status Scale score was 1.75 (range, 0.0–5.0), and only 36 participants (33.6%) were diagnosed as having MS based on a second clinical event. As expected in a newly diagnosed cohort, just more than half had started disease-modifying therapy at the time of assessment (n = 66 [61.7%]).
Characteristics of the 107 study participants
Scores on the components of the MACFIMS battery are presented in Table 2. The domain of most frequent impairment was processing speed, specifically the SDMT, with 38 participants (35.5%) in the impaired range. Only 37 patients (34.6%) were within normal limits on all the cognitive domains tested (Figure 1).
Impairment on Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) tests and domains
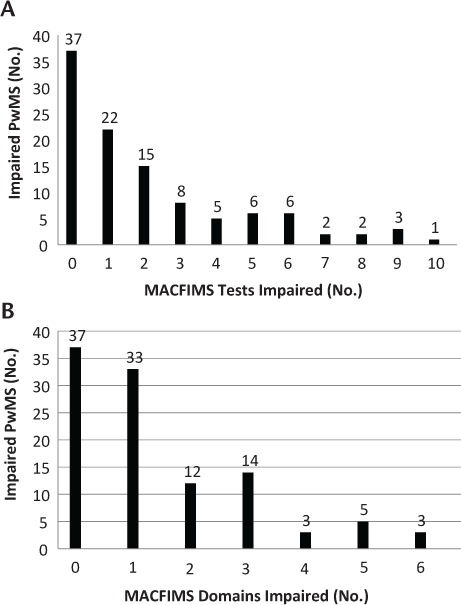
Mean raw scores on each Minimal Assessment of Cognitive Function in Multiple Sclerosis component and frequency of impairment
Fatigue, depression, and anxiety were also assessed. The mean ± SD FSS score was 3.9 ± 1.7 (scoring: normal/no fatigue, 0–3.9; moderate fatigue, 4.0–5.5; severe fatigue, 5.6–7.0); the mean ± SD raw score for HADS-A was 8.0 ± 4.3, with 59 persons with MS (55.1%) in the affected range (scoring: normal, 0–7; affected, ≥8). The mean ± SD raw score for HADS-D was 4.3 ± 3.9, with 25 patients (23.4%) in the affected range (scoring: normal, 0–7; affected, ≥9).
Significant correlations were found between age and BVMT-R-IR (r = −0.256, P = .008) and SDMT (r = −0.357, P < .001) scores, indicating that older age was associated with lower scores on both tests. Similarly, higher educational level was also correlated with better performance on the CVLT-II-IR (r = 0.314, P = .001), BVMT-R-IR (r = 0.257, P = .008), and PASAT (r = 0.302, P = .002). Male participants performed worse on both CVLT-II measures than female participants (CVLT-II-IR: males 45.4 ± 12 vs. females 53.2 ± 10.0, P = .002; CVLT-II-DR: males 9.7 ± 3.9 vs. females 11.8 ± 3.5, P = .009). No other statistically significant relationships were found.
Logistic regression analysis was performed to determine the contribution of the significant demographic variables, noted previously herein, to cognitive performance. This was done by comparing individuals who were unimpaired versus impaired on the CVLT-II-IR, CVLT-II-DR, BVMT-R-IR, and PASAT because significant relationships were identified for these tests only. For the CVLT-II-IR, sex and years of education were retained in the model, contributing 8.6% and 7.3% of the variance, respectively. For the CVLT-II-DR, again, sex and years of education were retained in the model, contributing 8.6% and 5.9% of the variance, respectively. Also, BVMT-R-IR retained the same variables in the model, but, in contrast, education had a greater contribution to the variance (6.1%), whereas age contributed 5.3% of the variance. Finally, for the PASAT, only education was retained in the model, contributing 17.1% of the variance.
Severe fatigue was correlated with a lower score on the SDMT (r = −0.380, P < .001), and higher depressive scores were correlated with lower performance on both tests of processing speed, the SDMT (r = −0.397, P < .001) and the PASAT (r = −0.254, P = .009). There were no statistically significant correlations between anxiety scores and any cognitive domains.
Discussion
This study furthers present knowledge regarding the prevalence of cognitive impairment in newly diagnosed persons with MS. This study improves on and extends past research by using a comprehensive newly diagnosed clinical population and, thus, is more generalizable because all persons with MS newly diagnosed as having RRMS underwent this testing. Furthermore, this population fit the most recent diagnostic criteria, the 2010 McDonald criteria.7
This study also addresses the prevalence of various clinical factors that may influence the decline of cognitive function, such as fatigue, depression, and anxiety. These factors have been examined in conjunction with cognitive impairment because they are common comorbidities to MS,17 with more than half of the present participants demonstrating anxiety and approximately a quarter demonstrating depressive symptoms.
Of the domains assessed using the MACFIMS battery, processing speed was most frequently impaired in this newly diagnosed RRMS population. Often, persons with MS seem to have severe difficulties with information processing and the speed at which they do so, commonly demonstrated by prolonged reaction times and slow memory scanning.18 These results are supported by a wealth of data from previous literature,19–21 which report a unified conclusion that processing speed is the most frequently affected cognitive domain in persons with MS.
The aforementioned studies, however, did not specifically examine newly diagnosed MS. The rate of cognitive impairment in the present population is lower than that reported in previous studies. Duque and colleagues22 examined baseline measures of 44 persons with MS and reported 31% as impaired, primarily on processing speed. Jønsson et al.23 examined 80 persons with MS within 1 year of diagnosis, of which 75 had RRMS based on the Poser criteria. This study found that 22.5% of participants demonstrated no cognitive impairment. Similarly, Deloire et al.24 examined 57 patients with RRMS within 6 months of confirmed diagnosis, again using the Poser criteria. In this study, 87.7% of participants had abnormal results on at least one test compared with controls. In contrast, 34.6% of the present population did not demonstrate any cognitive impairment. The lower frequency of cognitive impairment in the present study compared with previous studies may be due to use of the 2010 McDonald diagnostic criteria, which allow the diagnosis of RRMS to be made earlier, possibly creating lead time bias; thus, the present population may be more in keeping with previous studies on clinically isolated syndrome, the clinical precursor to MS. McIntosh-Michaelis et al.25 noted that cognitive impairment often occurred very early in the course of MS. In a systematic review of clinically isolated syndrome and cognitive impairment, Anhoque et al.26 also found evidence of cognitive impairment; the most frequently affected domains were visuospatial skills, executive function, and processing. Similar to the present results, this study directs attention to the levels of dysfunction that may exist early in the disease course.
The results of the present study are both consistent and inconsistent with some of the previous literature on the association between cognitive impairment and fatigue, depressive symptoms, and anxiety. Similar to previous research,27 significant differences in SDMT performance were found between fatigued and nonfatigued persons with MS. The present results differ from past literature, however, such that fewer domains were found to be associated with depressive symptoms. Recent work by Morrow et al.28 on depression and anxiety in persons with MS has shown associations with processing speed, visuospatial memory, and executive function impairment, whereas processing speed alone was the only domain to have a significant correlation with depressive symptoms in the present population. Furthermore, there was no evidence of a correlation between anxiety and cognitive impairment in the present study, which opposes the results of Morrow et al.28 that determined the relationship of anxiety with processing speed as well as memory impairments. A potential reason for this lies in the differences in the moderating variable of disease duration. There is some evidence for the increase of anxiety and depressive symptoms as the disease progresses,29 and because the present participants are newly diagnosed, these symptoms may not be developed enough to have an influence on cognitive ability as of yet. In addition, Blair et al.29 demonstrated that processing speed mediates the influence of depression on cognitive impairment in younger persons with MS, which can explain the prevalence of processing speed impairment in the present population and its mediating effect on depressive symptoms in the early stages of the disease.
There are limitations to the present study. First, it is a retrospective study based on a clinical population of only one tertiary referral MS clinic. Regarding the fatigue, anxiety, and depression scores, the data were self-reported. Furthermore, the relationship with cognitive impairment was purely correlational, and causal processes that are operating remain unclear. In addition, the data were gathered from patients with RRMS and, therefore, cannot necessarily be generalized to other MS types, such as primary progressive or secondary progressive MS. Finally, this study was based on a tertiary care MS clinic and may not be fully generalizable to other practices. Finally, since this study was completed, new diagnostic criteria have been published; it will be interesting to assess whether rates of cognitive impairment in newly diagnosed persons with MS differ using these new criteria.30
In conclusion, this study further supports that cognitive impairment can present at the time of RRMS diagnosis. Processing speed, specifically, was the most commonly impaired domain, and the clinical factors of fatigue and depression were associated with increased impairment. Future studies can extend these findings by examining different populations of people with MS and by investigating the causal processes that are operating as the underlying mechanisms behind these impairments.
PRACTICE POINTS
Cognitive impairment is present in a variety of domains within a year of diagnosis of relapsing-remitting MS.
Processing speed is the most frequently impaired domain in this time frame.
Clinical factors such as fatigue and depression are associated with increased impairment in processing speed.
Acknowledgments
The authors thank Ms. Koula Pantazopoulos, psychometrist, and Ms. Heather Rosehart, research associate, for their help with this project.
References
Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16:283–288.
Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. 2001;58:1602–1606.
Glanz BI, Healy BC, Hviid LE, Chitnis T, Weiner HL. Cognitive deterioration in patients with early multiple sclerosis: a 5-year study. J Neurol Neurosurg Psychiatry. 2012;83:38–43.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127.
Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol. 2005;58:840–846.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2006;12:549–558.
Benedict RH, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16:381–397.
Benton AL. Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press; 1994.
Benton AL, Hamsher KD, Sivan A. Multilingual Aphasia Examination. 3rd ed. Iowa City, IA: AJA Associates; 1994.
Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II California Verbal Learning Test Manual Adult Version. San Antonio, TX: The Psychological Corporation; 2000.
Benedict R. Brief Visuospatial Memory Test-Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources; 1997.
Delis DC. Manual for the Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001.
Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler. 2009;15:1518–1524.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123.
Patti F. Cognitive impairment in multiple sclerosis. Mult Scler. 2009;15:2–8.
Rogers JM, Panegyres PK. Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J Clin Neurosci. 2007;14:919–927.
Archibald CJ, Fisk JD. Information processing efficiency in patients with multiple sclerosis. J Clin Exp Neuropsychol. 2000;22:686–701.
Demaree HA, DeLuca J, Gaudino EA, Diamond BJ. Speed of information processing as a key deficit in multiple sclerosis: implications for rehabilitation. J Neurol Neurosurg Psychiatry. 1999;67:661–663.
Kail R. Speed of information processing in patients with multiple sclerosis. J Clin Exp Neuropsychol. 1998;20:98–106.
Duque B, Sepulcre J, Bejarano B, Samaranch L, Pastor P, Villoslada P. Memory decline evolves independently of disease activity in MS. Mult Scler. 2008;14:947–953.
Jønsson A, Andresen J, Storr L, Tscherning T, Sørensen PS, Ravnborg M. Cognitive impairment in newly diagnosed multiple sclerosis patients: a 4-year follow-up study. J Neurol Sci. 2006;245:77–85.
Deloire MS, Bonnet MC, Salort E, et al. How to detect cognitive dysfunction at early stages of multiple sclerosis? Mult Scler. 2006;12:445–452.
McIntosh-Michaelis SA, Roberts MH, Wilkinson SM, et al. The prevalence of cognitive impairment in a community survey of multiple sclerosis. Br J Clin Psychol. 1991;30:333–348.
Anhoque CF, Biccas Neto L, Domingues SCA, Teixeira AL, Domingues RB. Cognitive impairment in patients with clinically isolated syndrome. Dement Neuropsychol. 2012;6:266–269.
Morrow SA, Weinstock-Guttman B, Munschauer FE, Hojnacki D, Benedict RH. Subjective fatigue is not associated with cognitive impairment in multiple sclerosis: cross-sectional and longitudinal analysis. Mult Scler. 2009;15:998–1005.
Morrow SA, Rosehart H, Pantazopoulos K. Anxiety and depressive symptoms are associated with worse performance on objective cognitive tests in MS. J Neuropsychiatry Clin Neurosci. 2015;28:118–123.
Blair M, Gill S, Gutmanis I, Smolewska K, Warriner E, Morrow SA. The mediating role of processing speed in the relationship between depressive symptoms and cognitive function in multiple sclerosis. J Clin Exp Neuropsychol. 2016;38:782–794.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis. Ann Neurol. 2011;69:292–302.







