Publication
Research Article
International Journal of MS Care
Effects of External Perturbations on Anticipatory and Compensatory Postural Adjustments in Patients with Multiple Sclerosis and a Fall History
Author(s):
Abstract
Background:
Although previous studies have investigated postural adjustment mechanisms in patients with multiple sclerosis (MS), it seems that no study has yet investigated the relationship between anticipatory and compensatory postural adjustments (APAs and CPAs, respectively) and falls.
Methods:
Seventeen MS fallers, 17 MS nonfallers, and 15 controls were exposed to a series of expected and unexpected backward pull perturbations applied at the trunk level. The electrical activity of 12 leg and trunk muscles as well as center of pressure displacement were recorded.
Results:
The MS fallers had delayed muscle activity onsets compared with MS nonfallers and controls. In addition, a significantly lower level of muscle activity during APAs was detected in MS fallers compared with controls. Moreover, in the unexpected condition of perturbation, significantly smaller CPA was observed in MS fallers compared with controls. Both groups of patients with MS required more time to stabilize their center of pressure after both types of perturbations compared with controls.
Conclusions:
The inability to produce efficient APAs and CPAs during perturbations may explain the high rates of postural instability and falls in patients with MS. Findings from this study provide a background for the development of perturbation-based training programs aimed at balance improvement and fall prevention by restoring mechanisms underlying balance impairments.
Impaired balance is a major feature of multiple sclerosis (MS) disease.1 Almost three-quarters of patients with MS experience some degree of postural instability even at the initial stage of the disease.2 Moreover, poor balance control has been identified as a significant risk factor for falls in patients with MS, leading to lower engagement in physical activity.3 4 Hence, comprehensive evaluation of the mechanisms underlying balance control in these patients is of great value because it can lead to improving balance control strategies and preventing falls.
Balance in patients with MS has been commonly assessed through performance-based clinical tests such as the Berg Balance Scale (BBS) and the Timed Up and Go (TUG) test and through patient-reported questionnaires such as the Activities-specific Balance Confidence (ABC) scale.5 6 These tools are generally used to monitor disease progression and to assess the efficacy of a proposed treatment plan.6 However, they do not always characterize the underlying balance impairments.6 Instead, laboratory measures of balance impairments can provide deeper insight into the underlying mechanisms of impairments, hence targeting fall interventions.6 Recently, it has been found that investigation of postural adjustment mechanisms can provide important information regarding postural control and falls.7 Anticipatory and compensatory postural adjustments (APAs and CPAs, respectively) are two main postural mechanisms used by the central nervous system (CNS) to maintain and restore balance during perturbations.8 9 The APAs are associated with activation of the postural muscles in a feed-forward manner before the body perturbations.8 9 The CPAs are associated with postural muscle activation in a feedback manner after a perturbation has happened.8 9 Small and/or predicted perturbations can only be counteracted with APAs.10 However, when perturbations are large and/or unexpected, CPAs are the main mechanism of balance restoration.10 11 These two types of postural adjustments interact to maintain equilibrium, and the availability of APAs determines the amount of compensatory muscle activity.10
Recent studies have shown that inefficient postural adjustment mechanisms in older adults can lead to accidental falls.12–14 Elderly individuals with a fall history demonstrated slow and inefficient APAs during the precrossing phase of obstacle walking.14 Similarly, the results of a recent randomized controlled trial suggest that a 4-week APA-based balance training program involving ball catching activities resulted in improved postural control, functional balance, mobility, and quality of life in older adults.13 In contrast, studies of APAs and CPAs during external lateral perturbations in patients with Parkinson disease revealed that the magnitude of CPAs, but not APAs, was reduced in these patients.15 Hence, it is of great value to determine the relationship between underlying balance impairments and falls in patients prone to fall injury.
Studies of postural adjustments in patients with MS have shown decreased magnitude and increased latency of muscle activations in APAs during voluntary load release tasks10 11 and also in response to external perturbations (ie, pendulum impact).16 Although these studies provide important information on the strategies of postural adjustments in patients with MS, it is still unknown whether there are differences in the impairments of underlying mechanisms of postural control between patients with a history of falls and those without falls. A better understanding of the APAs and CPAs between MS fallers, MS nonfallers, and controls may help improve fall prevention and management strategies, because postural adjustments represent the ability of the CNS to respond to postural disturbances and prevent loss of equilibrium and falls.17 Therefore, the present study aimed to investigate the main strategies of postural adjustments (APAs and CPAs) in patients with MS with and without a fall history and controls during a series of external expected and unexpected postural perturbations. Based on the previous literature, we hypothesized that muscle activations will be delayed in both groups of patients with MS compared with controls and that the delays in activations will be greater in patients with a fall history. Moreover, we predicted that the magnitude of compensatory muscle activity will be less in patients with MS, especially in those with a fall history. Information obtained from this study may assist clinicians in developing rehabilitation protocols aimed at improving balance control and reducing the risk of falls in patients with MS.
Methods
Participants
A total of 49 participants were divided into three groups: 17 MS fallers, 17 MS nonfallers, and 15 age- and sex-matched controls. Patients were recruited from the MS Society of Khuzestan (Iran). The inclusion criteria were 1) a confirmed diagnosis of relapsing-remitting MS as determined by a neurologist, 2) the ability to stand independently without any aids for at least 3 minutes, and 3) normal or corrected-to-normal vision. Patients were divided into two groups of fallers and nonfallers based on fall history. Patients with a history of at least one fall during the previous 6 months were known as fallers.18 A fall was characterized as an event in which the participant unintentionally came to rest on the ground or a lower level.4 19 Participants were excluded if they had any impairments in lower limb extremities that compromise their balance; also, those who were unable to perform the experimental procedure were excluded. All the participants signed an informed consent form approved by the internal review board of Ahvaz Jundishapur University of Medical Sciences.
For a more comprehensive assessment of the patients' clinical status, we examined both MS groups using the following clinical balance scales: BBS, TUG test, and ABC scale. The BBS and the TUG test have been reported to be valid and reliable tools for assessing balance in patients with MS.5 20 The ABC scale is a 16-item self-reported questionnaire that rates balance confidence in the performance of various ambulatory activities, with scores ranging from 0% (no confidence) to 100% (completely confident).21 Moreover, patients' demographic data, including MS duration, age, sex, and disability status, were collected. The Self-administered Expanded Disability Status Scale was used in this study to assess the disability status of the patients.22 The descriptive statistics, including means (SDs) of demographic and clinical data of the MS groups, are reported in Table 1.
Demographic and clinical data of MS and control groups
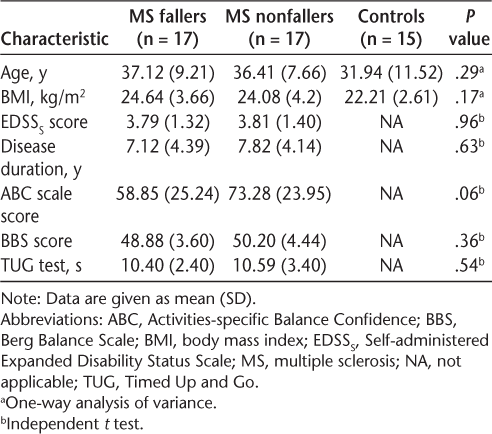
Experimental Set-up and Procedure
The type of perturbation used in this study was backward pull perturbation at the upper trunk level. Participants were instructed to stand barefoot on the force platform with their feet shoulder-width apart. All the participants wore an axillary belt connected to a system producing backward pull perturbation via a piece of cable and pulleys. The cable was terminated to a mobile plate, which received a ball impact and consequently produced a force to pull the participant backward. The mobile plate had a downward inclination, which caused the ball to slip immediately after impact. The weight of the ball was approximately 4% of the participant's body weight,23 and it was released by the same experimenter in each trial from the predefined distance. To determine the onset of perturbation (T0), a contact switch (foot switch) was situated into a compressive area between the axillary belt and the cable. Releasing the load resulted in pulling the cable, compressing the switch, and producing signal. For safety reasons, all the participants wore a harness with two straps attached to the ceiling to keep them safe from accidental falls. Figure 1 shows the schematic representation of the experimental set-up.
Schematic representation of experimental set-up
In this study, two types of external perturbations were delivered8: 1) expected perturbations were those applied immediately after verbal warning of an upcoming perturbation and 2) unexpected perturbations were those applied without verbal warning of an impending perturbation. In the unexpected condition, participants were listening to music via earphones to prevent them from obtaining auditory information about the moment of the perturbations. According to the previous literature, patients with MS are dependent on their visual information to maintain their balance, and eliminating visual information results in higher postural instability.24–26 Thus, for controlling this confounding effect, visual information was not manipulated in this study. Instead, all the participants performed both experimental conditions with open eyes, and perturbations were delivered from the back. Five trials were performed in each condition, and the order of experimental conditions was randomized. Moreover, before data collection, two practice trials were given to all the participants to familiarize them with the experimental protocol. Participants rested for at least 5 minutes between conditions, and they rested between trials as needed. The magnitude of the perturbations was adequate to evoke feet-in-place reactions.8
Instrumentation
Disposable self-adhesive electrodes (Skintact F-55; Leonhard Lang Plant, Austria) were placed bilaterally (right [r] and left [l]) on the following muscles: tibialis anterior (TAr and TAl), medial gastrocnemius (MGr and MGl), rectus femoris (RFr and RFl), biceps femoris (BFr and BFl), rectus abdominis (RAr and RAl), and erector spinae (ESr and ESl). Based on recommendations in the literature, the electrodes were attached to the bellies of the previously mentioned muscles, with an interelectrode distance of 20 mm.27 The electromyographic (EMG) signals were collected, filtered, and amplified (10–500 Hz, gain 2000) via an EMG system (ME6000; Mega Electronics Ltd, Kuopio, Finland) with a sampling frequency of 1000 Hz.8 28 A force platform (Bertec MIE, Leeds, United Kingdom) was used to record ground reaction forces and moments of forces. The signal of a foot switch (ME6000; Mega Electronics Ltd) was used to register the moment of perturbation.
Data Processing
All the signals were processed using MATLAB software (version R2012a; The MathWorks Inc, Natick, MA). The EMG signals were rectified and filtered using a 50-Hz low-pass and zero-lag Butterworth filter, whereas the force platform signals were filtered using a 20-Hz low-pass and zero-lag Butterworth filter.28
The onset latency of muscle activation/inhibition was detected in a time window from −250 to +250 milliseconds in relation to T0 (the moment of perturbation) by a combination of computer algorithm and visual inspection of the trial.11 It was defined as the instant when the amplitude of EMG was larger (activation) or smaller (inhibition) than the mean ±2 SD of its baseline (measured from −1000 to −850 milliseconds) and lasted for at least 25 milliseconds. Muscle onsets were averaged across trials in each experimental condition.
The integrated EMG (IEMG) activity signals were measured within the following time windows: APA phase, −100 to +50 milliseconds; and CPA phase, +50 to +350 milliseconds.8 29 They were corrected by the IEMG of baseline within a −1000 to −850-millisecond interval. Then the average of across-trial IEMG values was calculated for each muscle in each condition. At the end, IEMG values were normalized by the peak activity of muscle.
The displacement of center of pressure (COP) in the anterior-posterior direction was calculated as:
where My is the moment in sagittal plane, Fz and Fx are the vertical and anterior-posterior components of the ground reaction force, and d is the distance from the origin of the force platform to the surface.12 Anticipatory displacement of COP was defined as the mean displacement of COP 150 milliseconds before T0, and compensatory displacement of COP was the peak displacement of COP.11 Moreover, time to stabilization (TTS) in the COP signal was defined as the instant lasting for at least 500 milliseconds when the COP signal reached to the stable boundary after perturbation (mean COP position across trials 7–10 seconds after T0).
Statistical Analysis
The IEMG values and the latency of bilateral leg, thigh, and trunk muscles as well as COP displacements (mean anticipatory COP displacement, peak compensatory COP displacement, and TTS) were analyzed using the two-way mixed-design analysis of variance (ANOVA) with one between-subject factor of group (three levels: MS fallers, MS nonfallers, and controls) and one within-subject factor of condition (two levels: expected perturbation and unexpected perturbation). Tukey post hoc analysis was used to determine the differences between the pairs of groups with a level of significance of P < .05. Statistical significance was set at P < .05 in all the tests.
Results
Latency of EMG Activity
The results of the two-way mixed-design ANOVA revealed a significant interaction effect of group by condition for RAr, ESr, and ESl muscles. Further analysis with one-way ANOVA revealed that in the expected condition of perturbation, both ES muscles were significantly delayed in both MS groups compared with controls (P < .05). With respect to RAr latency, a significant difference was obtained between MS fallers and nonfallers in the unexpected condition. Moreover, there was a significant main effect of group for all the studied muscles except ESl, which was near significant (P = .06). The results of post hoc analysis with the Tukey test revealed that most studied muscles (RAr, RAl, ESr, RFl, BFl, MGr, and MGl) initiated significantly later in MS fallers compared with MS nonfallers and controls. Table 2 shows the ANOVA results with P values and F ratios. In addition, Table S1, which is published in the online version of this article at ijmsc.org, shows onset of muscle activity in all the studied groups and across the two experimental conditions.
Results of two-way mixed-design analyses of variances for muscle onsets, differences between groups, conditions, and their interaction effects
IEMG in APA Phase
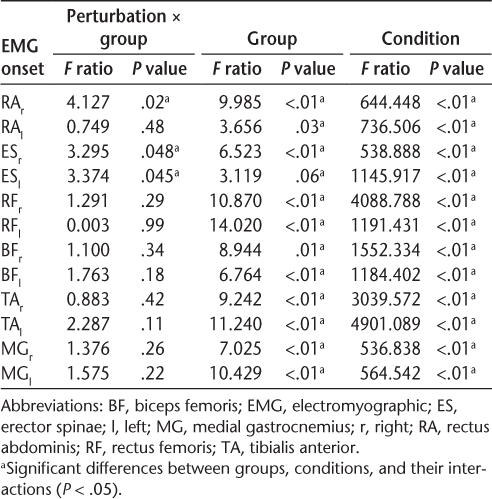
Figure 2 demonstrates IEMG values of the right muscles in the APA phase for all the studied groups and across the two experimental conditions. The results of the two-way mixed-design ANOVA revealed a significant interaction effect of group × condition for anticipatory IEMG of RFl, BFr, BFl, and MGr. The interaction revealed that the magnitude of APAs was small and could be ignored in all three groups in the unexpected condition of perturbation. However, further analysis with one-way ANOVA revealed that in the expected condition of perturbation, the anticipatory activity of RFl was significantly smaller in MS fallers compared with controls (P < .01). The main effect of group was also significant for RFl, BFl, and BFr and was close to the level of significance for ESr and MGr (P = .06). The MS nonfallers had significantly smaller APA activity for RFl, BFr, and BFl compared with controls. Table 3 shows the ANOVA results with P values and F ratios.
Anticipatory and compensatory postural adjustments (APAs and CPAs, respectively) represented by integrated electromyography (IEMG) values of the muscles on right side compared between conditions (expected × unexpected) for the three groups
Results of two-way mixed-design analyses of variances for anticipatory and compensatory integrated electromyography, differences between groups, conditions, and their interaction effects for each muscle

IEMG in CPA Phase
Figure 2 demonstrates IEMG values of the right muscles in the CPA phase for all the studied groups and across the two experimental conditions. There was a significant interaction effect of group × condition for compensatory IEMG. Further analysis with one-way ANOVA revealed that in the expected condition of perturbation, both groups of patients with MS had greater compensatory activity for the RFl and TAl muscles compared with controls (P < .05). Moreover, in the unexpected condition of perturbation, significantly smaller compensatory BFr activity was seen in MS fallers compared with controls (P < .05). A significant main effect of group was also found for the RAr, RAl, TAr, TAl, and MGr muscles. The results of post hoc analysis with the Tukey test revealed that irrespective of the perturbation condition, the compensatory activity of the RAr, RAl, TAr, and TAl muscles was greater in both MS groups compared with controls. Table 3 shows the ANOVA results with P values and F ratios.
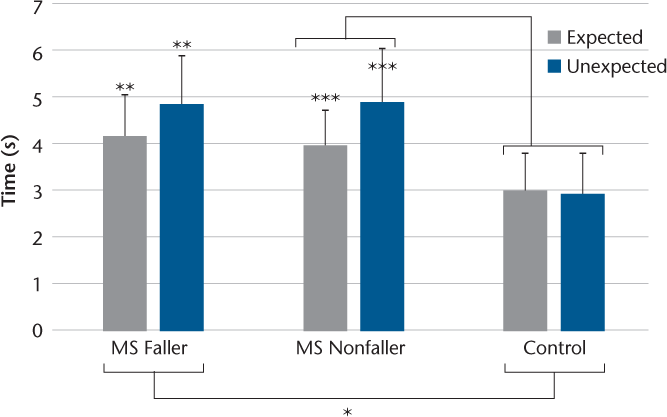
COP Displacement
With respect to COP variables, TTS was the only significant variable in the two-way mixed-design ANOVA. Hence, a variance analysis result is described for this variable. Figure 3 displays TTS across experimental conditions in all the studied groups. In addition, Figure S1 displays mean anticipatory COP displacement and peak compensatory displacement across both experimental conditions. The variance analysis results revealed a significant interaction effect of group × condition for TTS. Further analysis using one-way ANOVA revealed that in the experimental conditions, both groups of patients with MS required significantly more time to stabilize their COP after perturbations compared with controls (P < .01). Furthermore, the results of a series of paired t tests revealed that in both MS groups the required TTS was greater in the unexpected condition than in the expected condition (P < .05).
Differences in time to stabilization across all groups and conditions
Discussion
The present study investigated the APAs and CPAs during external predictable and unpredictable perturbations in patients with MS with and without a history of falls and in controls. We hypothesized that muscle activations would be delayed in patients with MS compared with controls in response to both types of perturbations. We also predicted that the possible delays in muscle activations would be greater in patients with a fall history than in those without a fall history. These hypotheses were supported. Most studied muscles (RAr, RAl, ESr, RFl, BFl, MGr, and MGl) initiated significantly later in MS fallers compared with MS nonfallers and controls. Moreover, lower levels of muscle activity (RFl, BFr, BFl, and MGr) during the APA interval were seen in MS fallers compared with controls. In addition, patients with MS, particularly those with a fall history, were not able to scale up their compensatory muscle activity in the unexpected condition compared with the expected condition. In contrast, controls increased their compensatory activity during the unexpected condition. The inability to produce efficient APAs during expected perturbations and CPAs during unexpected perturbations as the first line of defense against perturbations may explain the high rates of postural instability and falls in these patients.10 29
By inducing both predictable and unpredictable external perturbations in the present experiment, we were able to observe a sequence of events in the muscular activity, from APAs to CPAs. It has been shown that when the perturbation is predictable, APAs act as the first line of defense, preparing the body for the upcoming disturbance, followed by CPAs if the perturbation is large enough or poorly predicted.8 As expected, the use of robust APAs in controls in the present study considerably reduced the need for large CPAs. In contrast, patients with MS, particularly those with a fall history, were not able to produce APAs appropriately and tried to increase the compensatory muscle activity in the expected condition to confront with perturbation. Previous studies have shown that APAs are altered in patients with MS.16 Aruin et al.16 investigated APA and CPA onset timings during a pendulum impact task in a group of patients with MS and controls. They observed differences in APA latency between patients with MS and controls16; their findings were in agreement with those of the present study. However, in their study they investigated the onset of postural muscles unilaterally only.16 The present study aimed at investigating postural adjustment mechanisms and their relations to falls in a sample of patients with MS. Hence, patients in this study were divided into two groups based on their fall history. Interestingly, more deficits in the postural adjustment mechanisms were observed in MS fallers; ie, MS fallers exhibited significantly more delays and smaller integrals of muscle activities compared with nonfallers. However, note that both groups of patients with MS had similar clinical outcomes as determined by Self-administered Expanded Disability Status Scale, BBS, and TUG test scores. A possible explanation for this finding is that investigation of laboratory-based measures of balance (APAs and CPAs) may reveal subtle deficits in the underlying control mechanisms that may not be apparent in the clinical observation.30 This result parallels the findings obtained by Findling et al,31 who reported deficits in the laboratory-based measures of postural control (posturography) in patients with MS, even in those with normal clinical balance test findings. Therefore, the present results suggest that inability to respond efficiently to postural perturbations may be one of the underlying balance impairments associated with falling. Similar results have been obtained in recent studies of postural adjustment deficits in older adults, suggesting that an inability to produce APAs is related to an increased likelihood of falls in this population, whereas older adults using APAs showed no difference in stability compared with young adults.14
The type of postural perturbations used in this study was different than that in previous studies.16 In previous studies, predictable and unpredictable perturbations were delivered through pendulum impact with open and closed eyes.16 The pendulum impact perturbation paradigm has the advantage of mimicking perturbations commonly occurring in daily life, for example, colliding with someone while walking. However, to apply unpredictable types of perturbations, patients were instructed to close their eyes during those experiments.16 Considering that closing the eyes limits the available sensory information of posture and may alter postural control of patients differently in the unexpected conditions, visual information was not manipulated in this study.25 26 Instead, postural perturbations of this study were delivered backwardly via a system of pulleys and loads that was independent of visual manipulation. A similar backward pulling system was used to apply unexpected external perturbation in children with hemophilia.23 The present results may help develop therapeutic interventions aimed to reduce falls and improve balance through perturbation training. The need to develop evidence-based interventions to effectively manage falls has been highlighted by the fact that most treatment protocols to date seem not to be effective in reducing future falls in patients with MS.32
Finally, some limitations of the present study need to be discussed. Although this study was the first to investigate the relationship between postural adjustment mechanisms and falls in patients with MS, fall status was determined based on patients' fall history. Ideally, the best design for investigating fall risk factors is a prospective model with a future fall assessment. Future studies shall investigate patients' postural and muscle responses to perturbations in a prospective design to better illustrate further association between the underlying mechanisms of balance impairments and future falls.
PRACTICE POINTS
Inability to produce efficient mechanisms of postural adjustments during external perturbations may explain the high rates of postural instability and falls in patients with MS.
Despite their clinical similarities, there were major differences in anticipatory and compensatory postural adjustments between MS fallers and nonfallers.
Findings from this study provide a background for the development of perturbation-based rehabilitation programs to improve balance and prevent falling in patients with MS.
Financial Disclosures
The authors declare no conflicts of interest.
References
Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010;10:407–412.
Sosnoff JJ, Socie MJ, Boes MK, et al. Mobility, balance and falls in persons with multiple sclerosis. PLoS One. 2011;6:e28021.
Gunn H, Creanor S, Haas B, et al. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler. 2013;19:1913–1922.
Nilsagård Y, Denison E, Gunnarsson L-G, et al. Factors perceived as being related to accidental falls by persons with multiple sclerosis. Disabil Rehabil. 2009;31:1301–1310.
Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006;28:789–795.
Kanekar N, Aruin AS. The role of clinical and instrumented outcome measures in balance control of individuals with multiple sclerosis. Mult Scler Int. 2013;2013:190162.
Kanekar N, Aruin AS. Improvement of anticipatory postural adjustments for balance control: effect of a single training session. J Electromyogr Kinesiol. 2015;25:400–405.
Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture, 1: electromyographic analysis. J Electromyogr Kinesiol. 2010;20:388–397.
Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture, 2: biomechanical analysis. J Electromyogr Kinesiol. 2010;20:398–405.
Mehravar M, Yadollah-Pour N, Tajali S, et al. The role of anticipatory postural adjustments and compensatory control of posture in balance control of patients with multiple sclerosis. J Mech Med Biol. 2015;15:1550087.
Krishnan V, Kanekar N, Aruin AS. Feedforward postural control in individuals with multiple sclerosis during load release. Gait Posture. 2012;36:225–230.
Claudino R, dos Santos EC, Santos MJ. Compensatory but not anticipatory adjustments are altered in older adults during lateral postural perturbations. Clin Neurophysiol. 2013;124:1628–1637.
Jagdhane S, Kanekar N, Aruin A. The effect of a four-week balance training program on anticipatory postural adjustments in older adults: a pilot feasibility study. Curr Aging Sci. 2016;9:295–300.
Uemura K, Yamada M, Nagai K, et al. Older adults at high risk of falling need more time for anticipatory postural adjustment in the precrossing phase of obstacle negotiation. J Gerontol A Biol Sci Med Sci. 2011;66:904–909.
Claudino R, Conceição JS, Swarowsky A, et al. Anticipatory and compensatory postural adjustments in response to external lateral shoulder perturbations in subjects with Parkinson's disease. PLoS One. 2016;11:e0155012.
Aruin AS, Kanekar N, Lee Y-J. Anticipatory and compensatory postural adjustments in individuals with multiple sclerosis in response to external perturbations. Neurosci Lett. 2015;591:182–186.
Aruin AS, Kanekar N, Lee Y-J, et al. Enhancement of anticipatory postural adjustments in older adults as a result of a single session of ball throwing exercise. Exp Brain Res. 2015;233:649–655.
Cameron MH, Thielman E, Mazumder R, Bourdette D. Predicting falls in people with multiple sclerosis: fall history is as accurate as more complex measures. Mult Scler Int. 2013;2013:496325.
Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. PMR. 2011;3:624–632.
Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil Rehabil. 2007;29:1920–1925.
Nilsagård Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int. 2012;2012:613925.
Bowen J, Gibbons L, Gianas A, et al. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler. 2001;7:201–206.
De Souza F, Pereira R, Minuque N, et al. Postural adjustment after an unexpected perturbation in children with haemophilia. Haemophilia. 2012;18:311–315.
Huisinga JM, Yentes JM, Filipi ML, et al. Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett. 2012;524:124–128.
Kanekar N, Lee Y-J, Aruin AS. Frequency analysis approach to study balance control in individuals with multiple sclerosis. J Neurosci Methods. 2014;222:91–96.
Van Emmerik R, Remelius J, Johnson M, et al. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture. 2010;32:608–614.
Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374.
Mohapatra S, Krishnan V, Aruin AS. Postural control in response to an external perturbation: effect of altered proprioceptive information. Exp Brain Res. 2012;217:197–208.
Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett. 2012;506:256–260.
Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239–248.
Findling O, Sellner J, Meier N, et al. Trunk sway in mildly disabled multiple sclerosis patients with and without balance impairment. Exp Brain Res. 2011;213:363–370.
Gunn H, Markevics S, Haas B, et al. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil. 2015;96:1898–1912.







