Publication
Research Article
International Journal of MS Care
Preferences for Multiple Sclerosis Treatments
Background:
The growing number of treatments for relapsing multiple sclerosis (MS) provides opportunities to consider patient preferences in treatment decisions.
Methods:
We designed a Web-based, discrete-choice experiment survey to analyze treatment preferences in patients with relapsing-remitting MS (RRMS). The survey presented hypothetical MS treatments defined by six attributes: risk of MS progression, time between relapses, risk of serious infection, treatment-related flu-like symptoms and gastrointestinal symptoms, and route and frequency of administration. Preference weights estimated with random-parameters logit were used to calculate importance scores and preference shares among three pairs of subsamples.
Results:
Patients with a self-reported physician diagnosis of RRMS (N = 301) completed the survey: 56% rated their disability level as normal or mild; 43% currently used a self-injectable treatment. Respondents with normal or mild disability levels placed greater weight on avoiding injections with flu-like symptoms and risk of progression, whereas patients with worse disability placed greater weight on reducing risk of progression and risk of serious infection. Patients taking injectables placed the most weight on risk of progression and risk of serious infection, whereas respondents not taking injectables placed the most weight on route and frequency of administration. Differences in preferences between subgroups were significant (P < .05). The presence of common adverse events associated with daily pills and injectables altered predicted preferences for route of administration.
Conclusions:
Preferences of patients with RRMS varied depending on current treatment and disability level, especially regarding mode of administration. Considering patient preferences for treatment features may lead to higher treatment satisfaction and adherence.
Multiple sclerosis (MS) is a progressive disease of the central nervous system, and most patients initially receive a diagnosis of relapsing MS (RMS). The number of treatments for RMS has increased substantially in the past 4 years, and 13 disease-modifying therapies (DMTs) are now approved by the US Food and Drug Administration.
Several newer DMTs have similar efficacy profiles but vary in adverse events (AEs) and dosing. In a recent review, the MS Coalition highlighted the variability of preferences in the MS population—regarding response to treatment, risk tolerance, and willingness to tolerate AEs—as a reason to maintain access to a full range of DMTs.1 The report listed inadequate adherence as a reason to consider alternative DMTs, and research suggests that AEs and route of delivery can affect adherence.1 2
The growing choice of DMTs suggests an important role for patient preferences regarding dosing and associated AEs, especially given similar efficacy profiles across several DMTs. Discrete-choice experiments (DCEs) provide a method of quantifying patient preferences and the trade-offs that patients are willing to make among treatment features. A variety of DCE surveys have investigated various features of DMTs for MS, including risk tolerance for severe AEs.3–10 These studies have found that patients are willing to trade efficacy, AE risks, and features of DMTs such as the route of treatment administration. Only two previous studies compared oral, injection, and intravenous (IV) therapies, and in these studies, IV therapy was described as being administered every month.9 10 The existing studies do not include DMT options under development that offer different efficacy/safety profiles and administration and dosing options.
In this study, we explored treatment preferences in patients with RMS using a DCE with attributes related to efficacy, serious infection risk, common AEs, and four routes of administration and dosing schedules. The attributes included an additional dosing schedule that has not been evaluated before. The DCE allowed us to look at preferences for a range of DMTs, including newly approved treatments and treatments under development. We examined the effect of current disability level and current treatment on relative preferences through subgroup analyses and examined how the presence of AEs can change patient preferences for route of treatment administration.
Methods
Study Design
The online survey was built around a DCE, which is based on the hedonic principle that products are composed of a set of attributes and that people value these attributes individually. The DCE contained a series of treatment-choice questions between pairs of hypothetical RMS treatments presented as combinations of attributes with varying levels, allowing us to quantify the relative value that patients place on individual MS treatment features.
The survey collected disease- and treatment-related information from respondents, presented the treatment attributes and the DCE treatment-choice questions, asked questions about likely adherence to medications with different features, and concluded with socio-demographic questions. Respondents indicated their current level of disability using an eight-level scale based on the Kurtzke Expanded Disability Status Scale.11 12 Levels defined in the scale were combined to create eight levels of disability, each presented with a picture and a short description in Supplementary Table 1, which is published in the online version of this article at ijmsc.org.
The DCE treatment attributes and levels within attributes used to build the hypothetical MS treatments in this study were determined by considering existing MS treatments and clinical input from experts in MS treatment. We selected six treatment attributes: risk that MS symptoms worsen in the next year, average time between relapses, risk of serious infection, flu-like symptoms after injection or IV infusion, gastrointestinal (GI) symptoms, and mode and frequency of administration. Table 1 presents the attributes and levels included in the survey, along with the descriptions presented to the respondents.
Attributes and levels for the treatment-choice questions
The attribute “risk that MS symptoms worsen in the next year” described the probability that a respondent's level of disability would worsen by one level from their current level of disability on the eight-level scale presented in the survey. The levels for risk of progression were derived from data on disability progression for a range of DMTs. The attribute “average time between relapses” was extrapolated from data on the average annual relapse rate, which is typically less than 1 and would be difficult for patients to understand. Respondents were asked to assume that, after a relapse, the severity of their MS symptoms would return to the same level as before the relapse.
For the two risk-based attributes, “risk of progression in the next year” and “risk of serious infection,” the risk was presented both numerically and graphically. Risk levels were presented graphically using risk grids, each including 1000 dots, in which each dot represented one person who takes the medication. Dots presented in color represented patients for whom the event occurred.13 Respondents were asked a comprehension question about the risk grid.
In the final online survey, each of the six attributes was described and defined individually (see Table 1 for the attribute descriptions used in the survey). The descriptions were followed by questions about the attribute and the respondent's experience with the attribute to encourage the respondent to think about each attribute. To gain experience with the DCE format, respondents answered two practice DCE questions and two comprehension questions. The survey text was pretested in semistructured, face-to-face interviews before survey administration.
Each respondent answered ten DCE treatment-choice questions. They were asked to choose which treatment they would prefer between two specific hypothetical treatments. A screenshot of a treatment-choice question from the online survey is included in Supplementary Figure 1. The experimental design used to create the hypothetical treatments followed good practice guidelines14 and was generated using SAS version 9.3 (SAS Institute Inc, Cary, NC). The design contained the restriction that a daily pill was always presented with no flu-like symptoms. The experimental design comprised 48 different treatment pairs. To maximize the quality of response and minimize fatigue or cognitive burden,15 the 48 paired comparisons were divided into six survey versions, each version containing eight treatment-choice questions. After the blocks were tested for balance in the levels of the attributes, two additional treatment-choice questions taken from other blocks were added to each block for a total of ten treatment-choice questions. The new design and each block were evaluated for correlations and level balance. Each patient was randomly assigned to one of the six versions, and the order of the ten treatment-choice questions was randomized for each patient.
The survey included questions related to medication adherence. After introducing flu-like symptoms and GI symptoms, respondents were asked whether they had experienced these symptoms when receiving an MS treatment, and if they had, whether they had stopped taking an MS medicine, skipped a dose, or reduced their dose because of these symptoms. After responding to the DCE questions, respondents were asked to look at a list of different modes of administration and dosing frequencies and select the one that would make them most and least likely to miss or skip treatment doses.
Study Sample
The target sample size for this study was 300 respondents.14 The sample size in this study was within the range of sample sizes observed in most published studies.16 Patients were recruited by All Global (New York, NY), a health-care research firm, from an online panel of US consumers engaged for the purpose of survey research who had identified themselves as having MS. All respondents were 18 years or older, had a self-reported physician diagnosis of RRMS, and could read and understand English to provide informed consent and complete the 30-minute online survey instrument. The study was approved by the institutional review board of RTI International (Research Triangle Park, NC). All the patients provided informed consent before their inclusion in the study.
Analysis
Treatment-choice data were analyzed following good research practices.17 To estimate preferences for the attribute levels, a random-parameters logit (RPL) model was estimated using NLOGIT version 5.0 (Econometric Software Inc, Plainview, NY). The RPL model accounts for respondent heterogeneity by estimating a distribution of preferences for each parameter as well as a mean preference parameter.18 19 The treatment choice, medicine A or medicine B, was regressed against the independent variables, which were the levels for each of the attributes included in the study. Results from the RPL model produce mean preference-weight estimates interpreted to indicate the relative strength of preference for each attribute level compared with the other attributes and levels. All the parameters were specified to be normally distributed random parameters.
All the attributes listed in Table 1 were included in the model as effects-coded categorical variables except the attribute “risk that MS symptoms worsen in the next year.” With effects coding, zero indicates the mean effect across all levels of an attribute rather than the omitted level, as in dummy coding.20 This procedure produces a parameter estimate for all level categories whereby the omitted-category parameter is the negative sum of the included-category parameters. The resulting log-odds parameter estimates can be interpreted as relative preference weights, where t statistic values indicate whether attribute-level estimates differ significantly from the attribute mean effect (zero) rather than from other parameter estimates or an omitted category. The attribute “risk that MS symptoms worsen in the next year” was modeled as a linear continuous variable, and the resulting estimates for the attribute levels were centered at zero. The 95% confidence interval was calculated for each mean preference weight using the standard error of the mean attribute-level estimates.
On the basis of the pretests, preferences for avoiding flu-like symptoms were expected to depend on how frequently respondents were told the flu-like symptoms were going to be experienced. The model included an interaction term between “flu-like symptoms after each injection or infusion” and the frequency of flu-like symptoms, which depends on the level of the attribute “how you take the medicine.” The variable in the RPL model for frequency of flu-like symptoms is a continuous variable for the number of times per year a patient would experience flu-like symptoms defined as follows: 0 when “how you take the medicine” is “pill every day,” 2 when “how you take the medicine” is “IV infusion every 6 months,” 12 when “how you take the medicine” is “IV infusion every month,” and 156 when “how you take the medicine” is “injection 3 times per week.” A Wald test was used to test for differences between adjacent attribute levels.
Three different subgroup pairs were considered in subgroup analyses. The subgroup analyses examined whether answers to choice questions provided evidence of systematic variations in preferences across all attributes for respondents in each subgroup pair. To test for differences in preferences, we created a dummy-coded variable for each subgroup category that was equal to 1 if the respondent belonged to one of the two subgroups and generated a set of interaction variables. Each interaction variable was the product of the dummy-coded variable and one of the treatment attribute-level variables. Each of these interaction terms can be interpreted as the difference between the preference weights for the two subgroups considered. A joint test of significance of all interaction terms was used to determine whether preferences across all the attributes were different between the subgroups.
The first subgroup analysis looked at differences in preferences between respondents who self-reported normal or mild disability and those who reported moderate or worse disability based on the scale included in the survey (Supplementary Table 1). The second subgroup analysis looked at differences in preferences in respondents who are currently taking interferon beta-1a (Avonex; Biogen Idec, Cambridge, MA; or Rebif; EMD Serono, Inc, Rockland, MA), interferon beta-1b (Betaseron; Bayer HealthCare Pharmaceuticals, Montville, NJ), glatiramer acetate (Copaxone; Teva Pharmaceutical Industries Ltd, North Wales, PA), or peginterferon beta-1a (Plegridy; Biogen Idec) (known collectively as self-injectables) and those who were not taking self-injectables. The third subgroup analysis looked at respondents who had ever asked their doctors about switching medicines to assess whether these patients might be looking for different attributes in their next treatment compared with patients who had never asked to switch.
The preference weights for each subgroup were used to calculate conditional relative importance weights, which indicate the relative importance of each attribute included in the survey over the full range of levels of each attribute. Conditional relative importance was scaled so that the difference between the best and worst levels of “risk that your MS symptoms worsen in the next year” was equal to 10, with the differences in the levels of all the remaining attributes scaled relative to this attribute.
Finally, the RPL model for the full sample was used to calculate relative preference shares to evaluate preferences for combinations of attribute levels in a medicine profile. Using the attributes and levels in Table 1, two sets of medicine profiles were created for the four possible modes of administration in the survey and the most common AEs associated with the daily pill and injection modes. In the first set, each mode of administration (daily pill, IV infusion every 6 months, IV infusion every month, and injection 3 times per week) was presented with no AEs. In the second set, the daily pill was associated with moderate GI AEs, and the injection was associated with flu-like symptoms.
To calculate the preference shares across the different modes for the two sets of assumptions, we calculated a clinical benefit score for each profile that weighted each profile feature by the RPL preference estimate for that feature. With the clinical benefit scores, we calculated the predicted preference share for the two sets of profiles assuming that respondents would select one profile from among the four choices under each of the two sets of assumptions.
Results
E-mail invitations were sent to 2345 panel members identified by All Global as having MS. Of those invited, 475 individuals responded to the invitation by accessing the survey link, and 317 of those who responded (67%) were eligible to participate and gave their informed consent. In all, 301 individuals (95%) completed the survey in May 2015.
Table 2 displays the demographic characteristics and disease experiences of the sample. The respondents were mostly women, had a mean age of approximately 54 years, and rated their disability level as normal or mild (56%). Most respondents had had their disease diagnosed at least 10 years earlier and were not experiencing a relapse at the time of the survey. In addition, 62% of respondents reported ever asking their doctor about switching medicines, and 43% were currently taking a self-injectable for their treatment. The risk grid comprehension question was answered correctly by 91% of the respondents; the other two comprehension questions were answered correctly by 82% of the respondents.
Respondent characteristics

Respondent characteristics (Contd.)
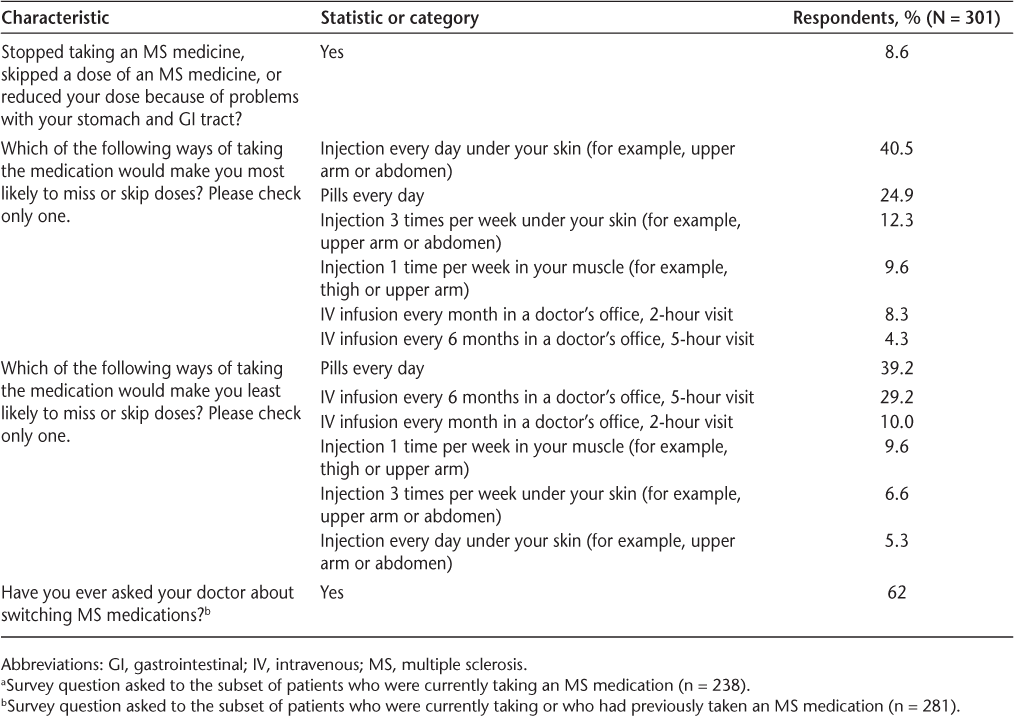
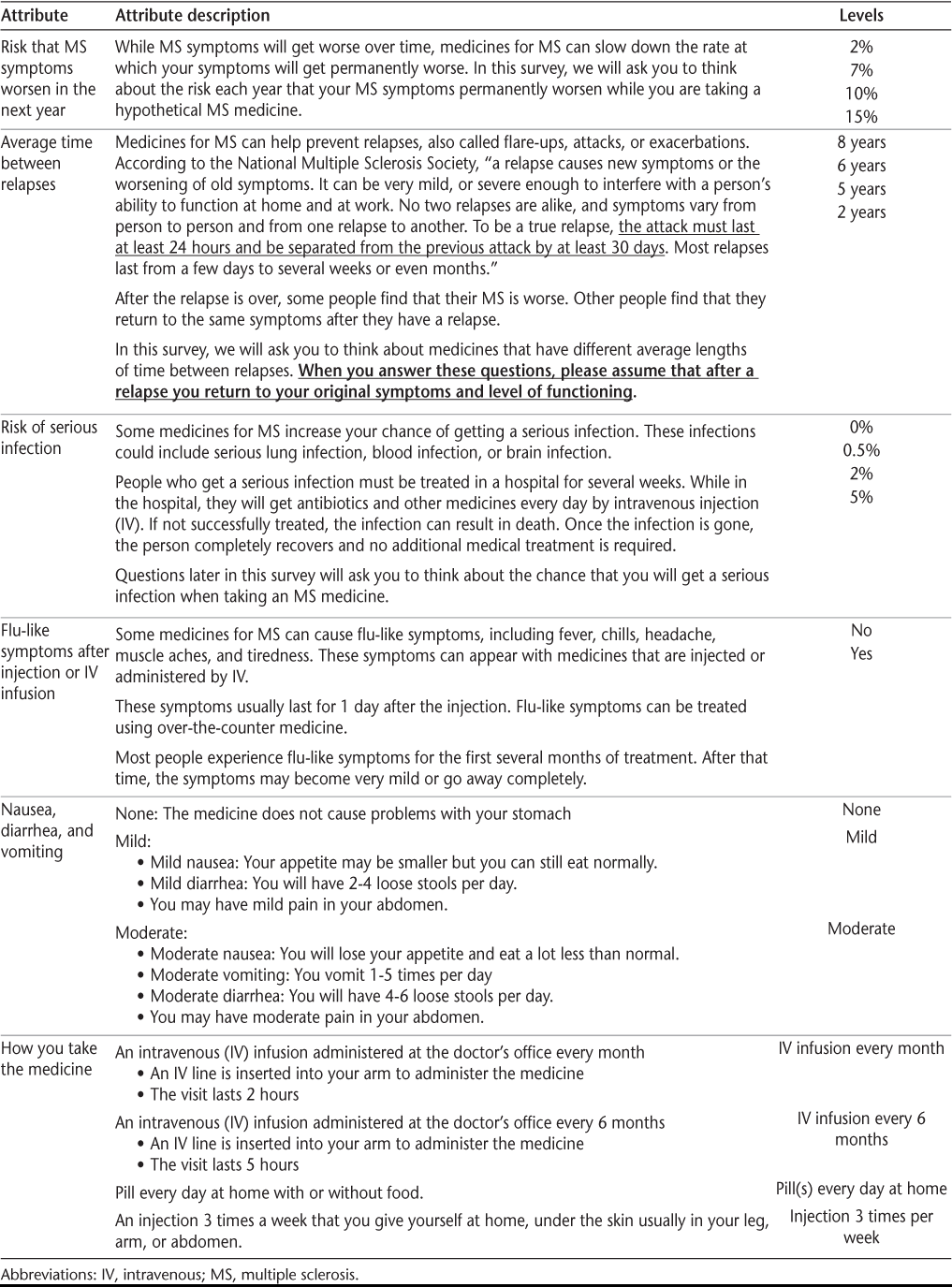
Figure 1 displays mean preference-weight estimates relative to the mean attribute effect, which is normalized at zero, and 95% confidence intervals. The preference weights indicate the relative strength of preference for each attribute, where larger, positive numbers indicate greater preference and smaller, negative numbers indicate less preference. The preference weights for avoiding flu-like symptoms combine the results for the attributes “how you take the medicine” and whether you have “flu-like symptoms” with the interaction between flu-like symptoms and frequency of flu-like symptoms into two attributes: “how you take the medicine; no flu-like symptoms” and “how you take the medicine; flu-like symptoms.”
Preference weights
Where there was a natural ordering, the estimated preference weights for all attributes were consistent with the natural ordering of the levels—that is, better outcomes were preferred to worse outcomes. All adjacent levels within attributes were significantly different from one another (P < .05), except for three levels of the attribute “average time between relapses” (“8 years,” “6 years,” and “5 years”; P = .24 to .56) and two levels of the attribute “how you take the medicine; no flu-like symptoms” (“IV infusion every month; no flu-like symptoms” and “injection 3 times per week; no flu-like symptoms”; P = .12).
Within each attribute, the vertical distance between preference weights in Figure 1 indicates the relative importance of moving from one level of the attribute to another. When the change from the least-preferred to the most-preferred level of each attribute was examined, the biggest difference, on average, was associated with a change in the risk that MS symptoms would worsen in the next year from 15% to 2% and a change in the mode of administration from an injection 3 times per week with flu-like symptoms to a daily pill with no flu-like symptoms. Respondents placed the least weight on a change in the average time between relapses from 2 to 8 years.
Figure 2 presents preference shares for combinations of mode of administration and two common AEs associated with specific modes: GI symptoms and flu-like symptoms. When no AEs were associated with the mode of administration, daily pill received the highest preference share (56%), followed by an IV infusion every 6 months (21%). However, when the injection was associated with flu-like symptoms and the daily pill was associated with moderate GI AEs, the preference share for IV infusion every 6 months (38%) was higher than that for the daily pill (32%), although the difference was not significant (P > .05).
Preference shares for the full sample
When asked which mode and frequency of administration they would be most likely to adhere to of a list of six options (Table 2), 39% of respondents reported being most likely to adhere to daily pills and 29% to IV infusion every 6 months. Nearly 41% reported that they would be least likely to adhere to a daily injection under the skin, and 25% reported being least likely to adhere to a daily pill. In addition, when asked about stopping or skipping treatment doses, 19% of respondents reported stopping or skipping treatment doses due to flu-like symptoms, and 9% reported stopping or skipping doses due to GI symptoms.
In the subgroup analyses, we found significant differences (P < .05) in overall preferences between the two disability subgroups (normal or mild vs. moderate or worse) and between the self-injectable and non–self-injectable subgroups. The third subgroup analysis, between respondents who had asked their doctors about switching medicines and those who had not, did not find statistically significant differences in overall preferences between the subgroups.
Figure 3 displays the conditional relative importance weights for the disability split-sample subgroups. The conditional relative importance of an attribute depends on the range of levels for each attribute and the set of attributes included. Over the range of levels included in this survey, respondents who rated their disability level as normal or mild placed greater weight on the mode and frequency of administration and the risk that their MS progressed in the next year than on the risk of serious infection and nausea attributes. Patients with moderate or worse disability placed the most weight on risk of progression and risk of a serious infection.
Disability subgroups: relative importance
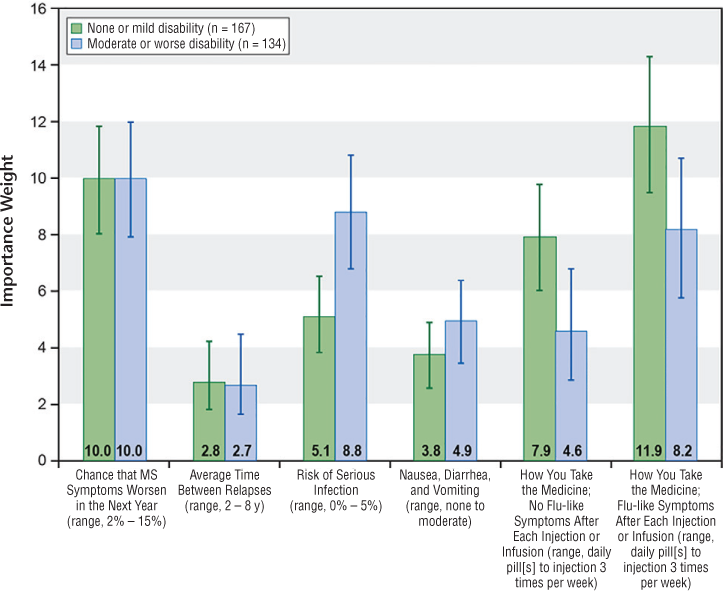
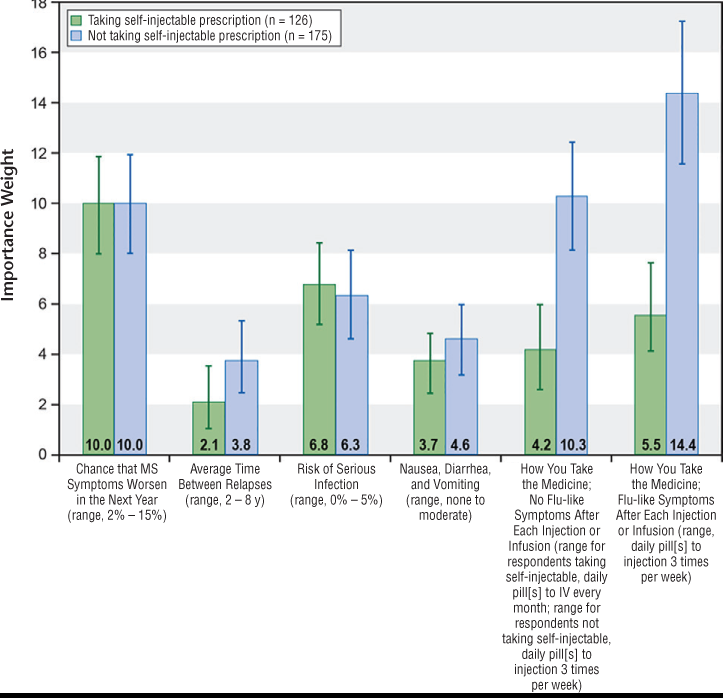
Figure 4 presents the conditional relative importance of the attributes for respondents currently taking a self-injectable and respondents not currently taking a self-injectable. Over the range of levels included in the survey, respondents not currently taking a self-injectable placed more weight on the mode and frequency of administration and the risk that their MS would progress in the next year than on the risk of serious infection and nausea attributes. Respondents currently taking a self-injectable placed more weight on risk of progression and risk of a serious infection than on mode of administration. Respondents currently taking a self-injectable preferred an injection three times per week or an IV infusion every 6 months compared with an IV infusion every month (P < .05) when there were no flu-like symptoms associated with the injection or the IV infusion. Respondents who were not taking a self-injectable rated injection three times per week as the least-preferred option.
Self-injectable treatment subgroups: relative importance
Discussion
Based on the DCE results, on average, respondents placed relatively more weight on improving the dosing method and frequency than on almost any other attribute given the set of attributes included and the ranges of levels of the attributes. However, the subgroup analyses suggested that relative preferences for route and frequency of administration depended on a respondent's disability level and current treatment. Respondents who rated their disability level as normal or mild or who were not currently receiving a self-injectable treatment had much stronger relative preferences for avoiding injectables than did respondents with moderate or severe disability or respondents currently taking a self-injectable. Respondents were indifferent between changes in the average time between relapses beyond 5 years, which is consistent with the finding of Wilson et al.10 The survey text specified that relapses can sometimes lead to a permanent increase in disability (Table 1) and were instructed to assume that they would return to their baseline disability level after the relapse ended. Given the importance of reducing the risk of permanent worsening of the disease, preferences for avoiding relapses might have been stronger if the respondents had been instructed that relapses would result in a permanent increase in disability.
Although there are other DCE studies of DMTs for MS, not all included multiple routes of administration. Utz et al.5 found that while orals were preferred to injectables when other attributes were held constant, preferences for oral versus injectable treatments changed when the level of AEs and the dosing frequency were varied. Wilson et al.9 10 found that oral was the most preferred mode, and monthly IV was preferred to either injection.
The survey also found evidence suggesting that route and frequency of administration play a role in a patient's perceptions of likely adherence. Although self-reported adherence can be a poor predictor of actual adherence, the present study looked at how respondents think mode of administration might influence likelihood of adherence.21 Laba et al.22 reviewed stated-preference surveys on adherence and found that mode and cost may become more important than efficacy or safety of a drug. In the present sample, respondents reported missing or skipping doses due to AEs such as GI and flu-like symptoms, and most had, at some point, asked their doctor about switching therapies. The factors that influence a patient's stated preferences for medication features may be different from those that influence likely adherence, and the adherence questions in this study focused on a single attribute (mode). Based on the DCE question responses, daily pills had the highest preference weight. However, in questions about adherence and mode of administration, a daily pill was both the mode that the highest percentage reported being most likely to adhere to and the mode that the second highest percentage reported being least likely to adhere to. An IV infusion every 6 months had the second highest percentage of respondents selecting it as the most likely to be adhered to and the lowest percentage selecting it as the least likely to be adhered to. Similarly, a recent study found that injectable and IV DMTs had better reported adherence rates than oral drugs.23
The results of the present study should be interpreted in light of several limitations. The sample was recruited from an online panel and may not be representative of the US population of patients with RRMS. Female respondents are overrepresented relative to the general MS population.24 Studies suggest that disease prevalence in MS peaks between 50 and 60 years of age,24 25 and, therefore, the average age of the sample (54 years) might be representative of the MS population. Most respondents in the sample rated their MS symptoms as normal, mild, or moderate, so the results may not be generalizable to patients who need to rely on a cane or a wheelchair or who are bedridden. We analyzed the data using RPL models, which control for preference heterogeneity even if we cannot identify the source. To the extent that an underrepresented group has systematically different preferences, the average preference weights may not be representative of the expected preferences from the population.
The scenarios presented to the respondents were hypothetical, which allowed for the evaluation of preferences for attribute levels of treatments that are not currently available but that may not carry the weight of actual decisions. In addition, the number of attributes in a DCE must be limited or the trade-off questions become too difficult, so the attributes do not include the full set of possible benefits and risks. Last, the estimated preference shares should not be interpreted as market share predictions. The study was not designed to estimate market shares, and other considerations beyond those presented in the survey may influence DMT prescription practices.
As more DMTs for RMS become available, patient preferences can play a larger role in treatment decisions; consistent with the findings of previous studies, features such as AEs, risk of severe AEs, and mode and frequency of administration may be important differentiators to patients.3–5 9 10 The results of this study suggest that patients with different treatment histories and different levels of disability may place more emphasis on different features of medicines. In addition, given the problems with adherence, new DMTs with alternative routes of administration and dosing frequencies—such as less frequent IV infusions—may provide an alternative that will improve adherence. Based on these findings, physicians should consider previous treatment experiences and disability levels, among other patient features, in the context of the unique attributes of DMTs, such as route of administration and dosing frequency, when prescribing DMTs to patients with RRMS.
PracticePoints
Overall, patients preferred medicines with better efficacy and daily pills or intravenous infusions every 6 months over self-injectables.
Patients with more severe disability or those who were currently taking self-injectables placed more weight on avoiding the risk of serious infection, whereas patients who did not have these characteristics placed more weight on avoiding injections.
Among a set of modes and schedule of administration, daily pills were selected by the most respondents to be the most likely mode to result in greater adherence; however, they were also selected by the second highest number of respondents as likely to result in lower adherence. Intravenous infusion every 6 months was the second most likely mode to result in greater adherence.
Acknowledgments
Third-party writing support for this manuscript was provided by Health Interactions and Articulate Science and was funded by Genentech Inc.
References
MS Coalition. The use of disease-modifying therapies in multiple sclerosis: principles and current evidence. http://www.nationalmssociety.org/getmedia/5ca284d3-fc7c-4ba5-b005-ab537d495c3c/DMT_Consensus_MS_Coalition_color. Updated March 2015. Accessed November 3, 2015.
Webb UH. Early interferon beta treatment in multiple sclerosis: nursing care implications of the BENEFIT study. J Neurosci Nurs. 2008; 40:356–361.
Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients' benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009; 256:554–562.
Poulos C, Kinter E, Yang JC, Bridges JF, Posner J, Reder AT. Patient preferences for injectable treatments for multiple sclerosis in the United States: a discrete-choice experiment. Patient. 2016; 9:171–180.
Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014; 7:263–275.
Rosato R, Testa S, Oggero A, Molinengo G, Bertolotto A. Quality of life and patient preferences: identification of subgroups of multiple sclerosis patients. Qual Life Res. 2015; 24:2173–2182.
Shingler SL, Swinburn P, Ali S, Perard R, Lloyd AJ. A discrete choice experiment to determine patient preferences for injection devices in multiple sclerosis. J Med Econ. 2013; 16:1036–1042.
Wicks P, Brandes D, Park J, et al. Preferred features of oral treatments and predictors of non-adherence: two web-based choice experiments in multiple sclerosis patients. Interact J Med Res. 2015;4:e6.
Wilson L, Loucks A, Bui C, et al. Patient centered decision making: use of conjoint analysis to determine risk-benefit trade-offs for preference sensitive treatment choices. J Neurol Sci. 2014; 344:80–87.
Wilson LS, Loucks A, Gipson G, et al. Patient preferences for attributes of multiple sclerosis disease-modifying therapies: development and results of a ratings-based conjoint analysis. Int J MS Care. 2015; 17:74–82.
Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33:1444–1452.
Haber A, LaRocca NG, eds. Minimal Record of Disability for Multiple Sclerosis. New York, NY: National Multiple Sclerosis Society; 1985.
Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011; 103:1436–1443.
Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013; 16:3–13.
Bech M, Kjaer T, Lauridsen J. Does the number of choice sets matter? results from a web survey applying a discrete choice experiment. Health Econ. 2011; 20:273–286.
Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health: how are studies being designed and reported? an update on current practice in the published literature between 2005 and 2008. Patient. 2010; 3:249–256.
Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016; 19:300–315.
Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, eds. Applications of Simulation Methods in Environmental and Resource Economics. Dordrecht, the Netherlands: Springer; 2005:117–134.
Train K. Discrete Choice Methods with Simulation. Cambridge, United Kingdom: Cambridge University Press; 2003.
Hensher DA, Rose JM, Greene WH. Applied Choice Analysis: A Primer. Cambridge, United Kingdom: Cambridge University Press; 2005.
Bruce JM, Hancock LM, Lynch SG. Objective adherence monitoring in multiple sclerosis: initial validation and association with self-report. Mult Scler. 2010; 16:112–120.
Laba T, Essue B, Kimman M, Jan S. Understanding patient preferences in medication nonadherence: a review of stated preference data. Patient. 2015; 8:385–395.
Dionne CA, Ganguly R, Camac A, Chaves C. Do oral disease-modifying agents (DMTs) improve adherence to MS treatment? a comparison of oral and injectable drugs [CMSC abstract DX19]. Int J MS Care. 2015;17(suppl 1):38.
Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010; 9:520–532.
Mackenzie IS, Morant SV, Bloomfield GA, et al. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry. 2014; 85:76–84.







