Publication
Research Article
International Journal of MS Care
Depression and Age at First Neurology Appointment Associated with Receipt of Behavioral Medicine Services Within 1 Year in a Multiple Sclerosis Population
Background:
Patients with multiple sclerosis (MS) newly seen by a neurologist may benefit from early psychological intervention owing to the reciprocal relationship between stress and disease progression. However, it is uncertain what factors contribute to patients' receiving these services.
Methods:
Logistic regression analysis of prospectively gathered data evaluated how demographic and disease characteristics and emotional/physical health factors contributed to referral to receive behavioral medicine (BM) services within 1 year of their first neurology appointment at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. Survival analyses then evaluated whether this resulted in earlier receipt of services.
Results:
Although many factors were associated with receiving BM services during univariate analyses (age, race, marital status, years since MS onset, depression, stress, and quality of life), when considering multivariable interactions, only two variables remained significant: age (odds ratio [OR] = 0.86, 95% confidence interval [CI], 0.80–0.92) and depression (OR = 1.56, 95% CI, 1.39–1.75). Survival analyses did not show differences in time to BM services for stratifications of age or depression scores.
Conclusions:
Younger patients and patients with more severe depression were more likely to receive BM services within 1 year of their first neurology appointment. Future research will focus on evaluating whether these are also the patients in greatest need of services or whether they are simply more open to receiving them.
Considerable evidence supports a reciprocal relationship between stressful life events and multiple sclerosis (MS) symptoms and exacerbations, possibly mediated by immune function and autonomic imbalance.1–6 There is also a reciprocal relationship between disease progression and emotional distress such that disease progression can result in increased emotional distress, and greater emotional distress can increase the risk of further disease progression.7
Increased severity of depression during acute relapses of MS symptoms correlates with overactivation of the inflammatory response as measured by increased production of cytokines such as tumor necrosis factor alpha and interleukin-10.8 Because this increased depression results in increased immune dysfunction and risk of relapse or disease progression, interventions that improve a patient's depression or emotional distress could also help improve symptoms such as cognitive deficits, fatigue, and other disease-related outcomes.9 Nonpharmacologic interventions such as cognitive-behavioral therapy10 11 and stress reduction12 have been shown to be beneficial in the treatment of MS. These psychological interventions have resulted in improvements in emotional distress,13 quality of life,14 and fatigue11 and in the reduced occurrence of new brain lesions.12
Further evidence shows that disease severity and time since diagnosis are strongly correlated with emotional distress in patients with MS.15 Patients' cognitive, emotional, and behavioral reactions to diagnosis and disease progression (eg, preoccupation with disability status, reduction in social interactions) can also predict illness-related functional impairment.10 Therefore, patients newly diagnosed as having MS or who experience worsening disability may be particularly at risk for emotional distress and impaired physical functioning, which may worsen their health status as well.16
The increased emotional distress that patients may experience throughout the course of their MS, combined with the reciprocal relationships among stress, symptom burden, and immune function, suggests that early interventions with cognitive-behavioral therapy and stress management techniques may substantially benefit patients by reducing depression and other emotional distress. Despite an understanding of the benefit of psychological intervention, it remains unclear what factors prompt a referral and acceptance of a referral for psychological services with behavioral medicine (BM) in patients newly seen by a neurologist specializing in the treatment of MS. This study sought to identify patient characteristics measured at the time of their first neurology appointment at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic (Cleveland, OH) that increase the likelihood of their being seen by BM within 1 year of this first neurology appointment.
Methods
The data were collected as part of the Knowledge Program Data Registry (KP)17 at the Cleveland Clinic. The KP is a systematic administration of questionnaires to patients at each appointment that helps track patients' physical and emotional functioning over time.17 Questionnaires from patients' initial neurology appointment at the Mellen Center were the primary data source for this study. The KP data accuracy was corroborated by a review of patients' electronic medical records. This study was approved by the institutional review board of the Cleveland Clinic Foundation.
Data Reduction of Patients
The initial KP data extraction included questionnaires from all in-person office visits at the Mellen Center between January 1, 2010, and June 30, 2015. This extraction yielded 14,656 unique adult patients who were diagnosed as having MS and 111,768 office visits within the specified dates. Of this initial data pull of all patients with MS seen at the Mellen Center, 1001 (6.8%) met with BM. After this initial extraction, a series of data reduction steps were conducted to refine the sample. First, patients who had their first neurology appointment at the Mellen Center before 2010 were excluded from data analysis because they would not have had the opportunity to receive BM services after their first appointment. Second, patients who had their first neurology appointment after July 2014 were excluded from the analysis because 1 year had not passed between the date of their first neurology appointment and the time of this analysis. Third, only patients who resided in Ohio and had more than one office visit at the Mellen Center were included. These exclusion criteria were included to capture only patients who were more likely to return for regular follow-up. Fourth, patients who were missing demographic data (sex, race, or marital status) were excluded from data analysis. Fifth, only patients who had a stable and clearly diagnosed course of MS were included for data analysis to allow for the most accurate comparison between courses of MS. Sixth, participants who met with BM but had a BM appointment more than 1 year after their first neurology appointment were excluded from data analysis. The final data sample included 1740 unique patients (11.9% of the original extraction), who had 19,848 office visits (17.8% of the original extraction). Within this reduced sample, 176 patients received BM services (17.6% of the original sample who received BM services vs. 1.2% of the total sample of the original extraction).
Measures
Multiple Sclerosis Performance Scales
The Multiple Sclerosis Performance Scales (MSPS) originally included eight items of domains that were affected by MS (mobility, hand function, vision, fatigue, cognition, bladder/bowel, sensory, and spasticity).18 Over time, three additional domains were included (pain, depression, and tremor/coordination).19–21 Each domain is scored on a 6-point ordinal scale (ranging from 0 = normal to 5 = total disability) except for the mobility domain, which is measured on an ordinal scale from 0 to 6. Test-retest reliability for the MSPS is relatively high across each measured domain (range, 0.65–0.91).18 The various domains measured by the MSPS can also be added into a single scale score, the MSPS-Performance Scale Sum (MSPS-PSS),18 which has shown good test-retest reliability (0.89), internal consistency (Cronbach α = 0.78), and criterion validity compared with the Expanded Disability Status Scale (0.62–0.64) and the Multiple Sclerosis Functional Composite (MSFC) (0.58).18 22
Timed 25-Foot Walk Test
The Timed 25-Foot Walk test is used to measure walking speed in MS and is part of the MSFC.23 During this task, the patient is instructed to walk 25 feet as quickly as possible but safely. Two trials are conducted, and the average time is calculated.
Nine-Hole Peg Test
The Nine-Hole Peg Test is used to measure upper-extremity function and is also part of the MSFC.23 During this task, the patient is instructed to transfer nine pegs one at a time into a block with nine holes. Patients complete this task twice with both the dominant and nondominant hands. For the purposes of this study, only the results from the dominant hand are included in the analysis.
EuroQol–5 Dimension
The EuroQol–5 Dimension (EQ-5D)24 25 consists of five questions on different domains of functioning (mobility, self-care, ability to engage in usual activities, pain, and anxiety/depression) that are rated on ordinal scales unique to each item (eg, 0 = no problems in walking about; 1 = unable to walk without a stick, crutch, or walking frame; and 2 = confined to bed). Test-retest reliability of the EQ-5D is high (0.73).24
Health Index
The Health Index is a component of the EQ-5D.24 25 Patients are instructed to rate their overall perceived health status on a visual analogue scale in the appearance of a thermometer that ranges from 0 (worst imaginable health state) to 100 (best imaginable health state). Test-retest reliability for the Health Index was high (0.78).24
Patient Health Questionnaire-9
Patients' depression was measured by the Patient Health Questionnaire-9 (PHQ-9),26 27 which consists of nine items that ask patients how often they have experienced a variety of symptoms of depression rated on 4-point ordinal scales (0 = not at all to 3 = nearly every day). Internal reliability of the PHQ-9 was high (Cronbach α = 0.86–0.89), as was test-retest reliability (0.84).26 A total score on the PHQ-9 ranges from 0 to 27. Cutoff scores of 5, 10, 15, and 20 represent mild, moderate, moderate-severe, and severe depressive symptoms, respectively. Scores of 10 or higher are indicative of a depressive disorder, with good sensitivity and specificity.26 The PHQ-9 has also been evaluated specifically in the MS population, and it was shown that no modified approaches to scoring are necessary.28
Generalized Anxiety Disorder Scale-7
Patients' anxiety was measured by the Generalized Anxiety Disorder Scale-7 (GAD-7),29 30 a seven-item scale that asks patients how often they have experienced a variety of symptoms of anxiety in the past 2 weeks rated on 3-point ordinal scales (0 = not at all, 1 = several days, and 2 = more than half the days). A total scale score ranges from 0 to 14. The GAD-7 showed good internal consistency (Cronbach α = 0.75) and convergent validity with the anxiety scale of the Hospital Anxiety and Depression Scale.30 The GAD-7 has been evaluated specifically in an MS population, and the authors did not recommend the use of MS-specific norms.30
Pain Disability Index
The Pain Disability Index (PDI)31–33 is a seven-item scale that asks patients to rate the level of functional impairment caused by their pain in different domains of their life (family/home responsibilities, recreation, social activities, occupation, sexual behavior, self-care, and life-support activities) on an 11-point scale ranging from 0 to 10. The PDI total score ranges from 0 to 70. Internal consistency of the PDI is high (Cronbach α = 0.86), but test-retest reliability may be more moderate (0.44).33
Results
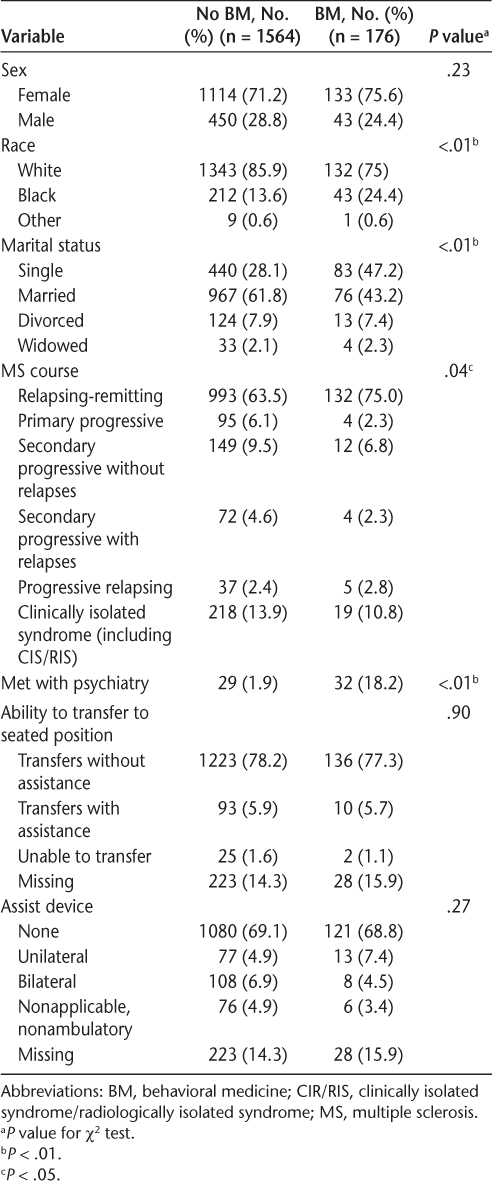
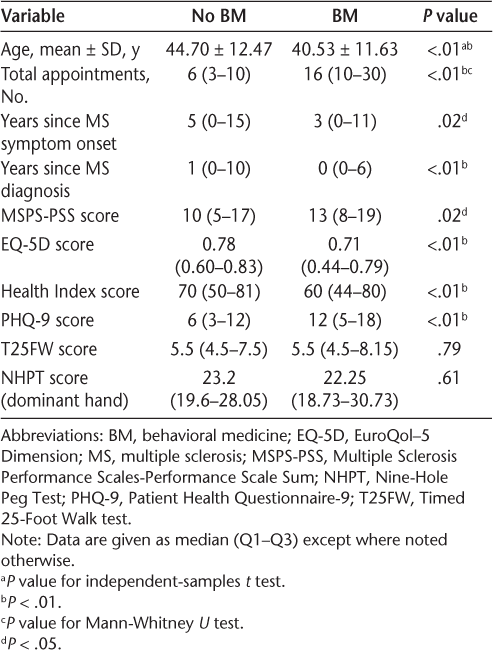
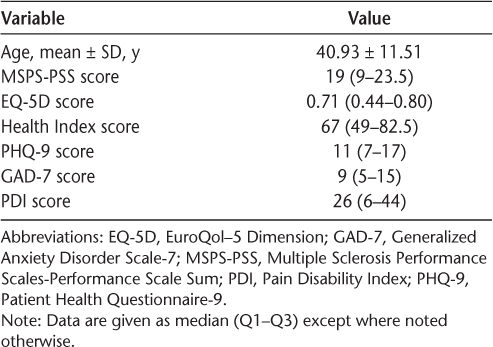
Data were analyzed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY). Chi-square tests for categorical variables (Table 1) and independent-samples t tests and Mann-Whitney U tests for continuous variables (Table 2) were conducted to compare differences in demographic information between patients who received BM services and those who did not at their first neurology appointment. The results of these analyses showed that at the time of their first neurology appointment, patients who would later receive or not receive BM services differed from one another in race, marital status, course of MS, having an appointment with psychiatry at any point, age, total number of appointments during the 5-year period, years since the onset of MS symptoms or formal diagnosis of MS, MSPS-PSS score, EQ-5D score, Health Index score, and PHQ-9 score. For the 176 patients who received BM services, descriptive statistics for questionnaires at the time of their first BM appointment are included in Table 3.
Categorical demographic data for 1740 participants at their first neurology appointment
Numeric demographic data for participants at their first neurology appointment
Data for 176 participants at first behavioral medicine appointment
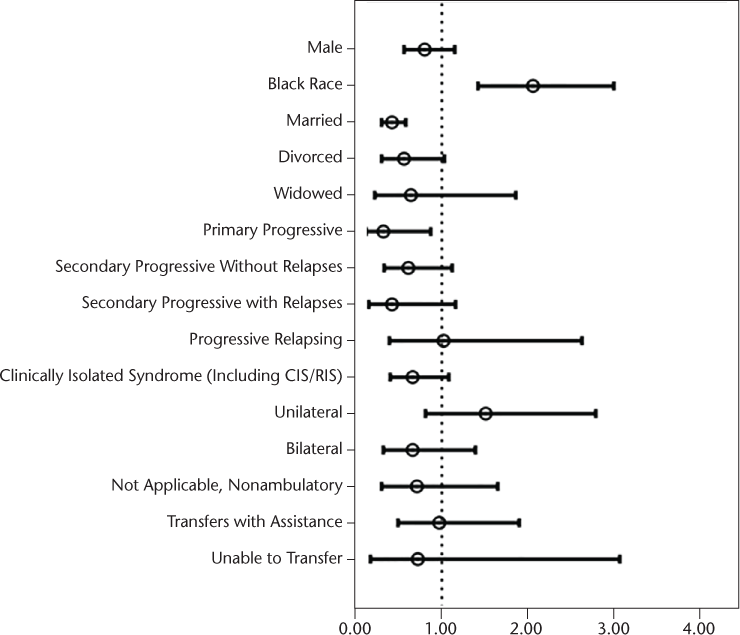
Next, a series of univariate binary logistic regressions were conducted, with receipt of BM services within 1 year of patients' first neurology appointment as the outcome variable and each of the demographic variables, disease characteristics, functional status, and KP questionnaires as independent predictors. For some of the continuous predictors, a transformation was used. Point estimates and confidence intervals (CIs) for patients' age, Health Index score, PHQ-9 score, and GAD-7 score reflect a 5-point increase in each of these measures. Point estimates and CIs for the EQ-5D reflect a 0.1-point increase. Patients' race, marital status, course of MS, age, years since symptom onset or diagnosis, MSPS-PSS score, EQ-5D total score, Health Index score, PHQ-9 score, and GAD-7 score were all independently significantly associated with receipt of BM services in univariate analyses. See Figure 1 for odds ratios (ORs) and 95% CIs for all categorical predictors and Figure 2 for ORs and 95% CIs for all continuous predictors. The vertical dotted line on each graph indicates an OR of 1, or no differences. Point estimate ORs and CIs to the right of the line (>1) indicate an increased likelihood of receiving BM services, and those to the left of the line indicate that patients with those characteristics are less likely to receive services.
Odds ratios and 95% confidence intervals for categorical predictors in univariate analyses on receipt of behavioral medicine within 1 year of the first neurology appointment
Odds ratios and 95% confidence intervals for numeric predictors in univariate analyses
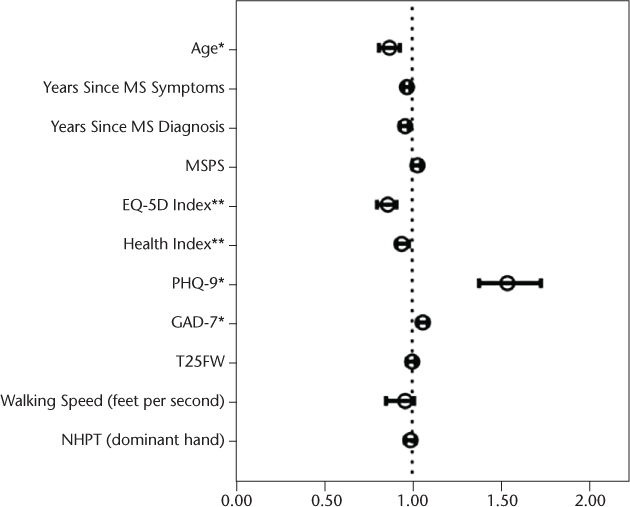
After the univariate analyses, variables that were significant were entered into a forward stepwise multivariable logistic regression. This analysis revealed that only two variables at the time of the first neurology appointment remained significantly associated with whether a patient received BM services within 1 year: age (OR = 0.86, 95% CI, 0.80–0.92, P < .01) and PHQ-9 score (OR = 1.56, 95% CI, 1.39–1.75, P < .01). For these two variables, effect measure modification was assessed for each of the other variables using the categorical groupings or stratification split on the mean value for continuous variables. However, the estimates and confidence intervals from the stratified groupings were not significantly different from the unadjusted estimates. Because of this, independent estimates were not generated for separate groupings. Confounding effects were also assessed. Because the two identified predictors were both continuous, the Cochran-Mantel-Haenszel estimate for confounding could not be used with these data. A multivariable logistic regression assessing for confounding effects was computed, but the point estimates and CIs for the adjusted and unadjusted models were again very similar, with overlapping CIs for each point estimate. Therefore, the unadjusted estimates are reserved for the final model, without effect modification or confounding effects from other variables.
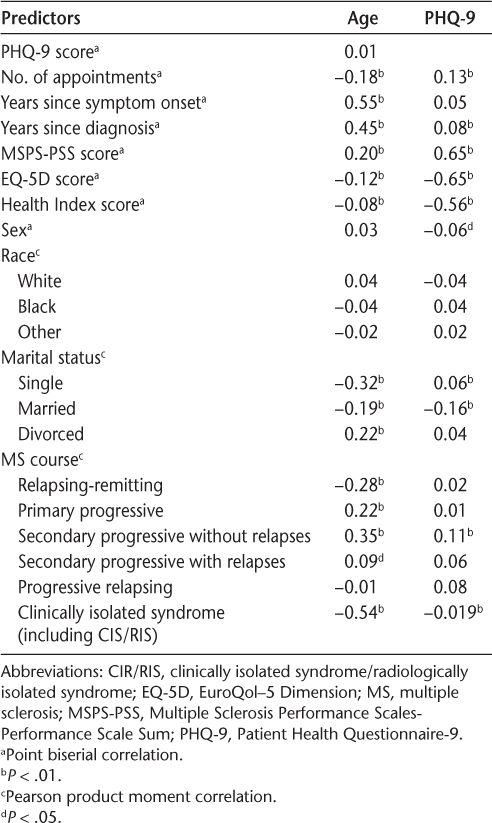
Point biserial and Pearson product moment correlations were computed comparing age, PHQ-9 score, and other statistically significant measures from the univariate analyses (Table 4). The results of these correlations showed that age and PHQ-9 score were not significantly associated with one another. However, increased age was correlated with fewer appointments, greater number of years since symptom onset and diagnosis, higher MSPS scores, lower EQ-5D and Health Index scores, increased likelihood of being divorced instead of single or married, and increased likelihood of having a primary progressive or secondary progressive course of MS instead of a relapsing-remitting course or clinically isolated syndrome. Higher PHQ-9 scores were associated with more appointments, more years since diagnosis, higher MSPS scores, lower EQ-5D and Health Index scores, being female, being single rather than being married, and having a secondary progressive course of MS.
Correlations among age, PHQ-9, and other predictors
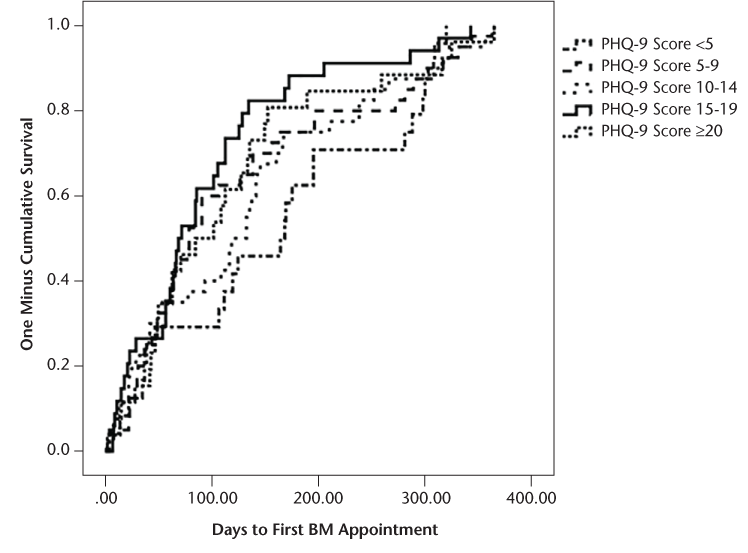
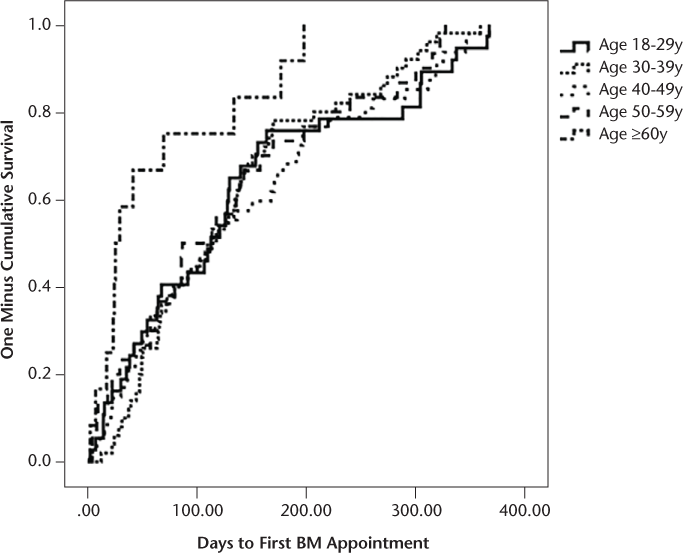
Finally, survival analyses were conducted based on stratified age groups and PHQ-9 scores to evaluate whether specific groups received BM services earlier or later than other groups. The PHQ-9 score was stratified based on established criteria for severity level of depression. Age was stratified by decade of life, with the exception that the youngest group of patients included adults aged 18 to 29 years, and given a more limited sample size of older adults, all patients 60 years and older were included in the eldest stratified group. The results of the survival analyses comparing the stratified groups were nonsignificant for PHQ-9 score (F 4,159 = 1.48, P = .21) and age (F 4,171 = 1.45, P = .22). Kaplan-Meier reversed survival curves were also generated showing the time between the first neurology appointments until patients were seen at the first appointment with BM based on stratification of patients' PHQ-9 scores and age (Figures 3 and 4).
One minus cumulative survival curve for Patient Health Questionnaire-9 (PHQ-9) scores
One minus cumulative survival curve for age group
Discussion
This study was a focused evaluation of factors that contribute to a patient with MS receiving BM services within 1 year of their first neurology appointment in a clinical setting rather than a research setting. The results of the study demonstrated that younger patients and those with higher levels of depression were more likely to receive services during this period. Although several other variables were considered for their contribution, ultimately, it was found that age and PHQ-9 score better accounted for patients receiving services. Age and PHQ-9 score were not correlated with one another (Table 4). However, there were a variety of connections between age and PHQ-9 score with other demographic and clinical data.
Consistent with our expectations, patients' depression was significantly correlated with other measures of emotional distress and quality of life. Higher PHQ-9 scores were associated with higher MSPS-PSS scores and lower EQ-5D and Health Index scores. In addition, higher PHQ-9 scores were associated with patients having more health-care utilization. The fact that PHQ-9 score rather than these other measures was associated with a patient receiving BM services suggests that the other measures capture physical functioning rather than purely emotional distress and depression. This result may indicate that patients are correctly identified to receive BM services for emotional distress rather than solely physical limitations.
The finding that younger patients were more likely to receive BM services warrants future study. There are multiple plausible explanations for this finding. First, younger patients may experience greater emotional distress with a diagnosis of MS earlier in their life. Second, they may have fewer supports (social, financial, etc.) or coping skills to help manage the stress of living with MS. Third, younger patients may have equal referral rates for BM services but are more open to receiving services. Clarification of this issue would help to best identify specific causes that led younger patients to be more likely to accept BM services. Although survival analyses investigating the effect of age on the number of days between the first neurology appointment and the first BM appointment were non-significant, it seemed that there was some association between age and time to receive services. Patients 60 years and older waited 61 days on average to meet with BM after their first neurology appointment, whereas other age groups waited for considerably longer (123–139 days on average) (Figure 4). An interesting finding not captured in the original goal of the study was the correlations between diagnosed course of MS and age. Younger patients were more likely to be diagnosed as having either a clinically isolated syndrome or a relapsing-remitting course, whereas older patients were more likely to be diagnosed as having a primary or secondary progressive course of MS. This is consistent with the literature and MS-specific disease characteristics.34
There were several limitations to this study. One of the most noteworthy is that the database used for analysis did not distinguish between patients who were referred for BM services and elected to receive them versus those referred who elected not to receive them. Future research evaluating this distinction would help identify patients who may be lost to treatment by not following through on their referral for BM services. A second limitation is that patients seen by the neurology department at the Mellen Center varied in whether they were newly receiving a diagnosis and treatment for MS or whether they had been previously diagnosed or treated and were transferring services to a specialist. This limitation confuses the concept of early psychological interventions but may still be appropriate for patients new to the practice. Another limitation is that this study focused on identifying which patients received BM services within 1 year of their first neurology appointment but did not assess what factors contributed to how many sessions with BM they had or whether participation in treatment improved their depression or other emotional distress. Although this study had planned not to address these questions to remain tightly focused, these areas remain interesting subjects for future exploration. Despite these limitations, this study offers a first step at better understanding how patient characteristics contribute to receiving BM services as an early intervention when newly receiving neurologic treatment for MS and that providers could take into consideration referrals to BM services that do not fall into the statistically significant groups.
PracticePoints
Patients who are younger and have more severe depression were more likely to receive psychological services within 1 year of their first neurology appointment. However, disease characteristics and other demographic characteristics and other measures of emotional/physical functioning are not significantly associated with receiving psychological services.
Although age and depression were associated with receiving psychological services within 1 year, stratification of age/depression groups revealed that there were no disparities in the length of time within that year between the first neurology appointment and the psychology appointment
Financial Disclosures
Dr. Sullivan has received funding from Novartis, the MS Cure Fund, and the National Multiple Sclerosis Society. The other authors have no conflicts of interest to disclose.
References
Reynard AK, Sullivan AB, Rae-Grant A. A systematic review of stress-management interventions for multiple sclerosis patients. Int J MS Care. 2014; 16:140–144.
Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011; 36:109–120.
Bennett JL, Stüve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: therapeutic implications. Clin Neuropharmacol. 2009; 32:121–132.
Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler. 2001; 7:327–334.
Flachenecker P, Rufer A, Bihler I, et al. Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology. 2003; 61:851–853.
Gunal DI, Afsar N, Tanridag T, Aktan S. Autonomic dysfunction in multiple sclerosis: correlation with disease-related parameters. Eur Neurol. 2002; 48:1–5.
Pittion-Vouyovitch S, Debouverie M, Guillemin F, Vandenberghe N, Anxionnat R, Vespignani H. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci. 2006:243:39–45.
Kahl KG, Kruse N, Faller H, Weib H, Rieckmann P. Expression of tumor necrosis factor-α and interferon-γ mRNA in blood cells correlates with depression scores during an acute attack in patients with multiple sclerosis. 2002;27:671–681.
Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008; 23:189–199.
Dennison L, Moss-Morris R. Cognitive-behavioral therapy: what benefits can it offer people with multiple sclerosis? Expert Rev Neurother. 2010; 10:1383–1390.
van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008; 70:205–213.
Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012; 79:412–419.
Artemiadis AK, Vervainioti AA, Alexopoulos EC, Rombos A, Anagnostouli MC, Darviri C. Stress management and multiple sclerosis: a randomized controlled trial. Arch Clin Neuropsychol. 2012; 27:406–416.
Sung C, Chiu C, Lee E, Bezyak J, Chan F, Muller V. Exercise, diet, and stress management as mediators between functional disability and health-related quality of life in multiple sclerosis. Rehabil Couns Bull. 2013; 56:85–95.
Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002; 159:1862–1868.
Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009; 29:141–153.
Katzan I, Speck M, Dopler C, et al. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011; 2011:683–692.
Schwartz CE, Vollmer T, Lee H; North American Research Consortium on Multiple Sclerosis Outcomes Study Group. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999; 52:63–70.
Chamot E, Kister I, Cutter GR. Item response theory-based measure of global disability in multiple sclerosis derived from the Performance Scales and related items. BMC Neurol. 2014;14:192.
Marrie RA, Cutter G, Tyry T, Hadjimichael O, Vollmer T. Validation of the NARCOMS Registry: pain assessment. Mult Scler. 2005; 11:338–342.
Marrie RA, Goldman M. Validation of the NARCOMS Registry: Tremor and Coordination Scale. Int J MS Care. 2011; 13:114–120.
Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007; 13:1176–1182.
Fischer JS, Rudick RA, Cutter GR, Reingold SC; National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler. 1999; 5:244–250.
Brooks R. EuroQol: the current state of play. Health Policy. 1996; 37:53–72.
EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. 1990;16:199–208.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606–613.
Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006; 28:71–77.
Sjonnesen K, Berzins S, Fiest KM, et al. Evaluation of the 9-item Patient Health Questionnaire (PHQ-9) as an assessment instrument for symptoms of depression in patients with multiple sclerosis. Postgrad Med. 2012; 124:69–77.
Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006; 166:1092–1097.
Terrill AL, Hartoonian N, Beier M, Salem R, Alschuler K. The 7-item Generalized Anxiety Disorder Scale as a tool for measuring generalized anxiety in multiple sclerosis. Int J MS Care. 2015; 17:49–56.
Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59:974.
Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987; 68:438–441.
Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. 1990; 40:171–182.
Rae-Grant A, Fox R, Bethoux F. Multiple Sclerosis and Related Disorders: Clinical Guide to Diagnosis, Medical Management, and Rehabilitation. New York, NY: Demos; 2013.







