Publication
Research Article
International Journal of MS Care
Predictors of Multiple Sclerosis Severity Score in Patients with Multiple Sclerosis
Author(s):
Abstract
Background:
Multiple sclerosis (MS) is a demyelinating autoimmune disorder. Several factors have been shown to associate with MS clinical severity. The influence of different lifestyle factors on MS clinical severity as assessed by the Multiple Sclerosis Severity Score (MSSS) was investigated.
Methods:
A questionnaire was administered to 128 Kuwaiti MS patients to assess the association of smoking, nutritional supplement use, food allergy, physical activity (PA), and educational level with MSSS. A multiple linear regression model was used to test for associations. Regression model results were adjusted for sex, history of blood transfusion, age at MS onset, and marital status.
Results:
Smoking status, passive smoking, and food allergy are not associated with MSSS. Patients with MS with a college education and graduate/professional degrees score, on average, 2.56 lower on the MSSS compared with those with less than a high school education (β= −2.22, P = .045; and β = −2.90, P = .048, respectively). Patients who perform PA score, on average, 2.32 lower on the MSSS compared with those with no PA (moderate exercise, P = .003; rigorous exercise, P = .001), and PA correlated significantly with MSSS outcomes (r2 = 0.452, P < .001).
Conclusions:
Educational level and PA are significantly associated with reduced MSSS, and both contribute to a less severe MS clinical course. Current MS management protocols should consider lifestyle changes to improve the quality of life of patients with MS.
Multiple sclerosis (MS) is a complex chronic demyelinating autoimmune disorder of the central nervous system. Onset of MS usually occurs at 18 to 40 years of age, but pediatric and late-onset forms of MS also exist.1,2 Multiple sclerosis exhibits many aspects of a hereditary disease, such as familial transmittance and anticipation3,4; however, sporadic forms of MS are creating an exponential burden, suggesting environmentally triggered forms of MS in genetically susceptible individuals.5 Although genetic factors underlie the cause and possibly the clinical course of MS, they do not do so alone. Environmental factors have been shown to be equally important in controlling MS prevalence and distribution, as well as some aspects of its clinical course and disability progression.6 Particulate matter pollution is one of many environmental factors associated with MS risk and relapse.7 Other MS environmental-associated factors include smoking, passive or prenatal smoking, deficient sun exposure or vitamin D deficiency, viral infections, obesity, poor lifestyle and dietary habits, and gut microbiota.8,9 Disability in MS is generally assessed using the Expanded Disability Status Scale (EDSS), which measures physiologic system dysfunction over time from the initial MS diagnosis. Another scale system was devised using real-world data of patients with MS to synthesize a Multiple Sclerosis Severity Score (MSSS) that quantifies MS disability (EDSS) in relation to disease duration.10 Assessing MS severity in patients is important to make decisions regarding clinical interventions and disease-modifying drug regimens. Several lifestyle factors have been implicated in influencing MS clinical severity. Smoking, alcohol consumption, and obesity, among others, have been shown to associate with fast disease progression in different MS populations.11–14
Kuwait was considered a low-risk area for MS; however, within a decade MS prevalence increased to 85 cases per 100,000 Kuwaiti individuals in 2014.15 In the previous 2 decades several poor environmental and lifestyle habits have been introduced in the country that have resulted in an associated increase in the incidence of chronic disorders, including MS, and suboptimal health indices in Kuwait after 1991.16,17 Herein we assessed several environmental and lifestyle factors that might contribute to MS severity in a Kuwaiti MS cohort. We focused on several possibly contributing factors to MS severity, including current lifestyle habits, such as physical activity (PA), smoking, food allergy, and nutritional supplement use; history of post-MS blood transfusion that may impart alloimmunity; presence during the Iraqi invasion, where extreme stress/anxiety and air pollution occurred that might have a progressive effect18; and educational level, which might influence patients’ outlook and management of their disease.
Methods
Study Design and Participants
This was a cross-sectional study conducted during 2013–2015 in which all patients with MS gave their written consent before participating in any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by Kuwait University Health Sciences Center’s Joint Committee for the Protection of Human Subjects and by the ethical review committee of Dasman Diabetes Institute (Dasman, Kuwait), where patients were recruited. Patients with MS were selected based on the following criteria: 1) availability of a detailed clinical history, 2) being a Kuwaiti citizen and residing in Kuwait from birth to at least early adult life, 3) a clear MS clinical course defined as relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS) or primary progressive MS (PPMS) in accordance with the modified McDonald criteria,19 4) at least 1 year since initial diagnosis, and 5) willingness to complete the study questionnaire. In total, 200 patients with MS were approached for participation.
Data Collection
The study questionnaire was designed to analyze modifiable lifestyle factors that might contribute to MS severity and quality of life. The questionnaire was a modified version of the International Agency for Research on Cancer’s epidemiologic questionnaire, inclusive of demographics, MS-specific environmental factors, and related quality-of-life factors. The questionnaire was tested for clarity and duration on a sample of ten individuals, with an average response time of 12 minutes. Demographic factors assessed included age, sex, educational level, marital status, presence during the Iraqi invasion, and employment status. Lifestyle factors assayed included current exercise type and duration (hours per week), smoking habits, history of passive smoking in immediate family, history of a post–MS diagnosis blood transfusion, and food and other allergies.
Data Analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). Descriptive statistics, including frequencies, percentages, means, medians, SDs, and interquartile ranges, were calculated. To test the relationship between MSSS as an outcome and an array of covariates, a multiple linear regression model was used. To estimate and test the effects of these covariates on MSSS, a multivariable linear regression model was implemented using the “enter” method to account for confounders’ effects. Because the goal is not to build a regression model for the purpose of prediction but to gauge the contribution of the covariates on the outcome, a hierarchical linear regression modeling was implemented. To trust the estimated parameters of the multivariable linear regression model, the following assumptions were carefully assessed. The continuous covariates were linearly related to the outcome, and the residuals were independent according to the Durbin-Watson test. Also, the residuals were normally distributed as indicated by the Kolmogorov-Smirnov test of normality with Lilliefors correction. Finally, according to the Breusch-Pagan and Koenker tests, the assumption of homoscedasticity (the error variances are homogeneous) is satisfied. The multivariable linear regression model was further assessed for multicollinearity using the variance inflation factor (VIF). All the covariates had a VIF less than 10; in fact, the maximum VIF is 4.53. Therefore, we have full faith in the regression model estimates. For all the analyses, exercise levels were classified into no PA, mild/moderate (walking, 1–3 hours per week), and vigorous PA (outdoor sports and bodybuilding, >3 hours per week) categories based on the physical intensity of the activity and its duration. All tests are two tailed, with a P value less than .05 considered statistically significant.
Results
Of 200 patients approached for participation, 135 provided written consent to participate, and 128 of these returned a completed study questionnaire. Table 1 presents the main characteristics of the study participants. Of the 128 patients with MS included in the study, 83 (64.8%) were females and 124 (96.9%) had RRMS. The mean ± SD for age at MS onset was 26.07 ± 6.70 years for males and 25.93 ± 7.97 years for females. Both males and females had a median MS disease duration of 3 years. An MSSS greater than 4 (5th–10th decile of the MSSS) was found in 47.6% of the MS cohort. The mean ± SD MSSS for males was 4.49 ± 2.37 and for females was 3.84 ± 2.23. Of the study sample, a mean ± SD of 42% ± 38.5% had relatives with a neurologic disorder, whereas 29% ± 67.4% had MS as the neurologic disorder in their family.
Demographic and clinical characteristics of the MS study cohort (N = 128)
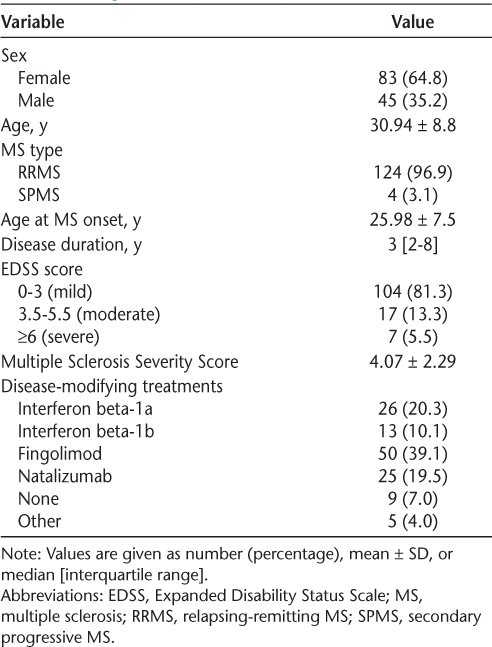
We used a multivariable linear regression model to assess the influence of the following factors on MS severity: age at onset of MS, sex, passive smoking, presence during the Iraqi invasion, history of blood transfusion after MS diagnosis, nutritional supplement use, marital status, food allergy, smoking status, and PA. There were 35.9% of patients with MS reporting exposure to passive smoking, and 28.9% were current smokers. Sixty-one percent of patients were in Kuwait during the Iraqi invasion, and 82.8% were in Kuwait during the 3 years after the Iraqi invasion (1991–1994). Only five patients reported a post-MS history of blood transfusion, and 33.6% had allergies, of which 35% were food allergies. Nutritional supplement use was common in the present cohort (49.2%), with vitamins, minerals, and omega fatty acids as the supplements used most. No current regular PA was reported in 48.4% of the MS cohort, whereas 38.2% were regular exercisers, of which 45% were performing rigorous physical exercise according to the study criteria (>3 hours per week). The results of the multivariable linear regression model are presented in Table 2. The covariates of age at onset, sex, passive smoking, presence during the Iraqi invasion, history of blood transfusion after an MS diagnosis, marital status, food allergy, and smoking status were not significantly associated with MSSS. Patients with MS who currently use supplements have, on average, 1.23 higher MSSS compared with those not using supplements (β = 1.232, P = .026). Patients with MS with graduate or undergraduate degrees have, on average, 2.90 and 2.22 lower MSSS compared with those with less than a high school education (β = −2.90, P = .048; β = −2.22, P = .045, respectively). Finally, patients who perform rigorous PA score, on average, 2.56 lower on the MSSS compared with those with no PA (β = −2.56, P = .001).
Associations between Multiple Sclerosis Severity Score and study variables using multiple linear regression model (N = 128)
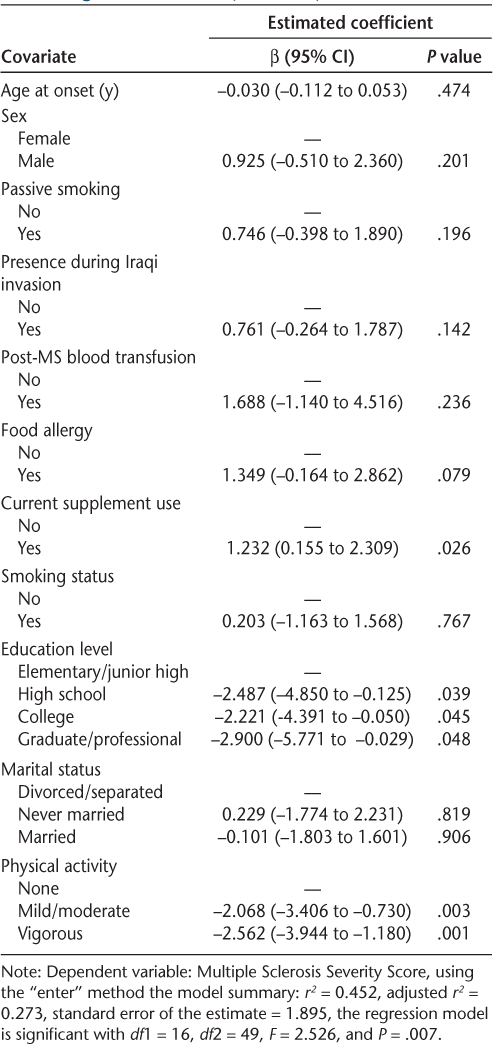
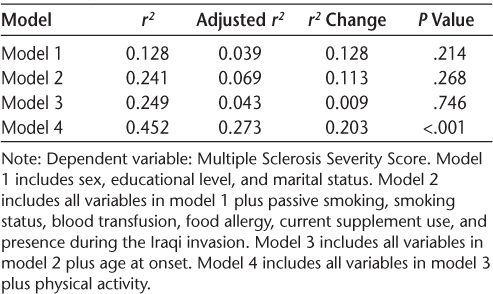
Table 3 presents the coefficient of determination, its adjusted value, the change in its value, and a test for the significance of such a change. The hierarchical linear regression model revealed that age at onset made the least change in the amount of variation explained in MSSS values, which is supported by their insignificance as indicated by their large P value in the linear regression model. On the other hand, PA made the largest change in the amount of variation explained in the outcome, which is supported by the high significance of the parameters of PA as indicated by their very small P values.
Results of hierarchical multiple regression analysis of Multiple Sclerosis Severity Scores (N = 128)
Discussion
Progression of MS disability is inevitable despite development of a variety of disease-modifying drugs and management regimens. The average life expectancy of a patient with MS has been shown to be 7 to 14 years less than that of the average person; however, this reduction in life expectancy differs across MS populations.20 Several factors contribute to MS life expectancy, including early age at MS onset, sex, MS comorbidities, lifestyle, accidents, and suicide.20,21 Because MS is a complex disorder, management regimens should implement proactive lifestyle modifications as a part of treatment. In recent years, patient awareness of lifestyle and dietary factors affecting MS severity has increased as reflected by better MS survival rates; however, this observation remains limited to developed countries.22,23 In Kuwait, obesity and a sedentary lifestyle have become commonplace, and in 2016 the prevalence of obesity in Kuwait was estimated to be 42.4%.24 In an observational study of 212 Kuwaiti patients with MS, we noted that 60.8% of patients with MS were overweight/obese (R.A.-T., unpublished data, 2019).
Herein we investigated whether other factors influence MS severity. The present findings suggest that although educational level and supplement use may contribute to MS severity, a sedentary lifestyle contributed the most. We found no effects of allergy, smoking, presence during the Iraqi invasion, or history of post-MS blood transfusion on MS severity. The lack of MSSS association with allergy might be explained by evidence that T helper 2 allergic disorders are thought to impart a protective effect against MS, which is a T helper 1 disorder.25 Smoking status did not influence MS severity despite its established role as an MS risk factor.26 Cigarette smoking has been reported recently to be associated with MS severity.27 However, previous reports have repeatedly shown that smoking affects MS disability rather than severity.11,28 Note that in Kuwait, female smoking imposes a social and cultural stigma that prevents females from taking up smoking. In addition, the present Kuwaiti MS cohort had more females than males, as expected for the MS incidence ratio, and the influence of smoking was probably masked by sex.13,29 Presence during or after the Iraqi invasion of Kuwait did not influence MSSS, and neither did history of blood transfusion. The effect of nutritional supplement use on MS severity was significant. Supplement use was high in the present MS cohort, with most patients taking vitamins, minerals, and fatty acid supplements along with a few patients taking herbal extracts and amino acids. Supplement preparations vary, and some may contain trace amounts of compounds that interact with MS treatments, which may potentially explain its association with higher MSSS values. On the other hand, it is probable that supplement association with MS severity is a byproduct of patient response to increases in MS severity. Higher educational degrees were associated with lower MS severity. It is possible that these patients have maintained their cognitive and functional reserve by the learning activity fostered by educational systems. In addition, these patients may have a clearer understanding of and an enhanced conscientiousness toward their disease and seek information through educational resources, thus affecting their lifestyle and dietary choices and improving their MS outcomes.30 Exercise levels had the most profound effects on MS severity and disability. Patients with MS who reported no weekly exercise had the worst MSSS, whereas patients who performed rigorous exercise had the lowest MS severity; those performing mild/moderate exercise also had low MS severity, albeit not as profound as rigorous exercisers. Whether this finding is a result of disability interference or is sex specific is unclear because 80.6% of patients reporting no exercise were females. Moreover, 40.8% of patients with MSSS greater than 4 (n = 61) were regular exercisers, half of which were performing rigorous exercise, and were females. The nonexercisers in the moderate/high MSSS category (MSSS >4) were 76% female; therefore, sex is the stronger discriminatory factor for exercise potential in this MS cohort, not MSSS. The benefit of exercise for patients with MS has been reported repeatedly to be associated with better disease outcomes.31,32 Exercise effect investigations have provided supporting evidence of the need for exercise regimens to be part of the clinical management of MS.33,34 Most significantly, strength training and routine exercise have proved beneficial for improving quality of life in patients with MS, but more applied research is needed to establish clear interventional guidelines.35,36
Although the present study findings support many reports from other MS populations, this study is not without limitations. This study was limited by sample size; despite 200 patients with MS having been approached, only 135 patients (67.5%) consented to participate, and incomplete questionnaires (5.2%) were also a limiting factor. In addition, as in every qualitative study there is a chance of human bias in describing personal information. That the cohort mostly had RRMS of low/moderate EDSS category and were mostly females is another limitation. However, we would argue that RRMS is the most common MS type and that SPMS and PPMS already exhibit high MSSS and might bias the analysis, and also that low/moderate EDSS score would be the best disability range to find predictors for MS severity. Moreover, females are affected twice as often as males, and due to the random sampling study design, the cohort reflected this phenomenon. On the positive side, this study is novel because it investigated the association of modifiable lifestyle factors with MS severity in a culturally distinct MS population and should add supporting evidence to the value of educational level and physical exercise in MS management.
In conclusion, PA and educational level are significantly associated with reduced MSSS. Physical activity and educational level contribute to a less severe MS clinical course in patients with RRMS. Current MS clinical management protocols should consider lifestyle changes to improve the quality of life of patients with MS and their physical rehabilitation.
PRACTICE POINTS
Modifiable lifestyle factors that predict MS severity were investigated to discern disease interventional management plans that reduce disease severity and maximize quality of life for patients with MS.
These findings suggest that physical exercise should be integrated into the management of MS and that information regarding the use of nutritional supplements should be systematically collected.
Patients with MS of lower educational levels and their families should be referred to counseling services to educate them about their disease and guide them to information resources that aid in better management of their disease.
Financial Disclosures
The authors declare no conflicts of interest.
References
Reinhardt K, Weiss S, Rosenbauer J, Gartner J, von Kries R. Multiple sclerosis in children and adolescents: incidence and clinical picture: new insights from the nationwide German surveillance (2009–2011). Eur J Neurol. 2014; 21: 654– 659.
Vaughn CB, Jakimovski D, Kavak KS, . Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019; 15: 329– 342.
Romero-Pinel L, Martinez-Yelamos S, Gubieras L, . Anticipation of age at onset in familial multiple sclerosis. Eur J Neurol. 2010; 17: 572– 575.
Katsavos S, Artemiadis A, Davaki P, Stamboulis E, Kilindireas K, Anagnostouli M. Familial multiple sclerosis in Greece: distinct clinical and imaging characteristics in comparison with the sporadic disease. Clin Neurol Neurosurg. 2018; 173: 144– 149.
Haines JL, Terwedow HA, Burgess K, ; The Multiple Sclerosis Genetics Group. Linkage of the MHC to familial multiple sclerosis suggests genetic heterogeneity. Hum Mol Genet. 1998; 7: 1229– 1234.
Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008; 7: 268– 277.
Calderon-Garciduenas L, Leray E, Heydarpour P, Torres-Jardon R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: the clinical impact on children and beyond. Rev Neurol. 2016; 172: 69– 80.
Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015; 14: 263– 273.
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018; 391: 1622– 1636.
Roxburgh RH, Seaman SR, Masterman T, . Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005; 64: 1144– 1151.
D’Hooghe MB, Haentjens P, Nagels G, De Keyser J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur J Neurol. 2012; 19: 616– 624.
Weiland TJ, Hadgkiss EJ, Jelinek GA, Pereira NG, Marck CH, van der Meer DM. The association of alcohol consumption and smoking with quality of life, disability and disease activity in an international sample of people with multiple sclerosis. J Neurol Sci. 2014; 336: 211– 219.
Paz-Ballesteros WC, Monterrubio-Flores EA, de Jesus Flores-Rivera J, Corona-Vazquez T, Hernandez-Giron C. Cigarette smoking, alcohol consumption and overweight in multiple sclerosis: disability progression. Arch Med Res. 2017; 48: 113– 120.
Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015; 18: 125– 136.
Alroughani R, Ahmed SF, Behbahani R, . Increasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult Scler. 2014; 20: 543– 547.
Al-Afasy HH, Al-Obaidan MA, Al-Ansari YA, . Risk factors for multiple sclerosis in Kuwait: a population-based case-control study. Neuroepidemiology. 2013; 40: 30– 35.
Cange CW. The life course model as a framework for post-conflict health analysis: reflections on the Gulf War critical period. Med Confl Surviv. 2016; 32: 282– 294.
O’Donovan A, Cohen BE, Seal KH, . Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015; 77: 365– 374.
Thompson AJ, Banwell BL, Barkhof F, . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17: 162– 173.
Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Mortality in patients with multiple sclerosis. Neurology. 2013; 81: 184– 192.
Marrie RA, Patten SB, Greenfield J, . Physical comorbidities increase the risk of psychiatric comorbidity in multiple sclerosis. Brain Behav. 2016; 6: e00493.
Gitto L. Living with multiple sclerosis in Europe: pharmacological treatments, cost of illness, and health-related quality of life across countries. In: Zagon IS, McLaughlin PJ, eds. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis . Codon Publications; 2017: 17– 37.
Warren SA, Janzen W, Warren KG, Svenson LW, Schopflocher DP. Multiple sclerosis mortality rates in Canada, 1975–2009. Can J Neurol Sci. 2016; 43: 134– 141.
Nutrition landscape information system: Kuwait. Global Health Observatory data repository. World Health Organization website. http://apps.who.int/nutrition/landscape/mddetails.aspx?sourcecode=300303. Published 2016.
Monteiro L, Souza-Machado A, Menezes C, Melo A. Association between allergies and multiple sclerosis: a systematic review and meta-analysis. Acta Neurol Scand. 2011; 123: 1– 7.
Degelman ML, Herman KM. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord. 2017; 17: 207– 216.
Ivashynka A, Copetti M, Naldi P, D’Alfonso S, Leone MA. The impact of lifetime alcohol and cigarette smoking loads on multiple sclerosis severity. Front Neurol. 2019; 10: 866.
Heydarpour P, Manouchehrinia A, Beiki O, . Smoking and worsening disability in multiple sclerosis: a meta-analysis. Acta Neurol Scand. 2018; 138: 62– 69.
Mandia D, Ferraro OE, Nosari G, Montomoli C, Zardini E, Bergamaschi R. Environmental factors and multiple sclerosis severity: a descriptive study. Int J Environ Res Public Health. 2014; 11: 6417– 6432.
Fuchs TA, Benedict RH, Wilding G, . Trait Conscientiousness predicts rate of brain atrophy in multiple sclerosis [published online ahead of print June 20, 2019]. Mult Scler. doi: 10.1177.1352458519858605.
Motl RW, Pilutti LA. Is physical exercise a multiple sclerosis disease modifying treatment? Exp Rev Neurother. 2016; 16: 951– 960.
Doring A, Pfueller CF, Paul F, Dorr J. Exercise in multiple sclerosis: an integral component of disease management. EPMA J. 2011; 3: 2.
Veldhuijzen van Zanten JJ, Pilutti LA, Duda JL, Motl RW. Sedentary behaviour in people with multiple sclerosis: is it time to stand up against MS? Mult Scler. 2016; 22: 1250– 1256.
Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017; 17: 185.
Mañago MM, Glick S, Hebert JR, Coote S, Schenkman M. Strength training to improve gait in people with multiple sclerosis: a critical review of exercise parameters and intervention approaches. Int J MS Care. 2019; 21: 47– 56.
Kim Y, Lai B, Mehta T, . Exercise training guidelines for multiple sclerosis, stroke, and Parkinson disease: rapid review and synthesis. Am J Phys Med Rehabil. 2019; 98: 613– 621.







