Publication
Research Article
International Journal of MS Care
Perspectives About Time Frames in Stem Cell Research for Multiple Sclerosis
Author(s):
CME/CNE Information
Activity Available Online:
To access the article, post-test, and evaluation online, go to http://www.cmscscholar.org.
Target Audience:
The target audience for this activity is physicians, physician assistants, nursing professionals, and other health care providers involved in the management of patients with multiple sclerosis (MS).
1) Demonstrate knowledge of MS patient and clinician perspectives about the time frames associated with stem cell research and development.
2) Integrate evidence-informed clinical communication strategies to clarify translational time frames and promote informed hope about stem cell research into his/her own clinical practice.
Learning Objectives:
Accreditation Statement:

In support of improving patient care, this activity has been planned and implemented by the Consortium of Multiple Sclerosis Centers (CMSC) and Delaware Media Group. The CMSC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Credit
The CMSC designates this journal-based activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurse Credit
The CMSC designates this enduring material for 1.0 contact hour (none in the area of pharmacology).
Disclosures:
Editor in Chief of the International Journal of MS Care (IJMSC), has served as physician planner for this activity. He has disclosed relationships with Springer Publishing (royalty); Qr8 (receipt of intellectual property rights/patent holder); Abide Therapeutics, GW Pharma (consulting fee); Biogen (speakers' bureau); and Adamas Pharmaceuticals (contracted research).Francois Bethoux, MD,
has served as reviewer for this activity. She has disclosed no relevant financial relationships.Laurie Scudder, DNP, NP,
has disclosed no relevant financial relationships.Shelly Benjaminy, PhD,
has disclosed no relevant financial relationships.Cody Lo, BSc,
has disclosed no relevant financial relationships.Andrew Schepmyer, MD,
has disclosed relationships with Biogen, Teva, Roche, Merck/EMD Serono, Sanofi Genzyme, Chugai (consulting fee); Sanofi Genzyme (speakers' bureau); and Chugai, Roche, Sanofi Genzyme (contracted research [principal investigator]).Anthony Traboulsee, MD,
has disclosed no relevant financial relationships.Judy Illes, CM, PhD,
The peer reviewer for IJMSC has disclosed no relevant financial relationships.
The staff at IJMSC, CMSC, and Delaware Media Group who are in a position to influence content have disclosed no relevant financial relationships.
Note: Financial relationships for some authors may have changed in the interval between listing these disclosures and publication of the article.
Method of Participation:
Release Date: August 1, 2019
Valid for Credit Through: August 1, 2020
In order to receive CME/CNE credit, participants must:
1) Review the continuing education information, including learning objectives and author disclosures.
2) Study the educational content.
3) Complete the post-test and evaluation, which are available at http://www.cmscscholar.org.
Statements of Credit are awarded upon successful completion of the post-test with a passing score of >70% and the evaluation.
There is no fee to participate in this activity.
Disclosure of Unlabeled Use:
This educational activity may contain discussion of published and/or investigational uses of agents that are not approved by the FDA. CMSC and Delaware Media Group do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of CMSC or Delaware Media Group.
Disclaimer:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any medications, diagnostic procedures, or treatments discussed in this publication should not be used by clinicians or other health-care professionals without first evaluating their patients' conditions, considering possible contraindications or risks, reviewing any applicable manufacturer's product information, and comparing any therapeutic approach with the recommendations of other authorities.
Abstract
Background:
Stem cell research has been a focus of inquiry in the field of neurology for nearly 3 decades and has led to much hope for people with multiple sclerosis (MS). Previous studies, however, demonstrate that information about the pace of developments in the stem cell arena is less accessible than are representations of potential benefits.
Methods:
To explore the understanding and perspectives of adult patients with MS and MS clinicians about the time frames associated with stem cell research, we conducted semistructured interviews with 20 patients with MS across Canada and 15 clinicians who specialize in MS. Patients who participated did not have any previous stem cell interventions. Interviews were analyzed for recurring themes and individual variations using the constant comparative approach.
Results:
We found that patients with MS have a limited understanding about the time that it takes for stem cell research to reach the clinic. In parallel, they express a desire to know more than they do about the translational process. Clinicians offer strategies to address patients' questions about the pace of stem cell research and to promote informed hope about experimental interventions.
Conclusions:
These results underscore opportunities to promote transparency in clinical discourse about the pace of stem cell research for MS and other progressive neurologic diseases.
Multiple sclerosis (MS) has been the subject of clinical inquiry in regenerative medicine and in the neurologic sciences since the late 1990s.1 Although stem cell applications for MS have been the focus of hope for nearly 3 decades, and advances have resulted in human trials using both hematopoietic and mesenchymal approaches,2,3 efforts have not yielded a widely available treatment to date. While patients await stem cell therapies, studies suggest that information about the pace of research and development may be less accessible than representations of the potential benefits of stem cell interventions, which have been widely disseminated through print and online media.4–6 In addition, substantial marketing forces have been associated with the premature application of unproven and unregulated stem cell interventions, a phenomenon colloquially termed stem cell tourism. Such interventions are often marketed directly to consumers and presented as bona fide therapies despite limited or no preclinical or clinical evidence to that effect. Dubious interventions exploit the urgency of affected individuals to access meaningful therapeutics and may contribute to confusion about the readiness of stem cell interventions for clinical uptake.6,7 Clear messaging about the time frames associated with stem cell research may be particularly important given the complexity of navigating both scientifically rigorous clinical trials and unregulated interventions.7,8 Context around translation may be particularly helpful for patients with MS, not only given the progressive nature of their illness but also because purveyors of unproven interventions predominantly target patients with MS and other diseases of the brain.9
Herein we explore the perspectives of stakeholders about the pace of this translational area guided by the following research question: What are the perspectives of patients with MS and MS clinicians about the time frames associated with the research and development of stem cell interventions?
Methods
Study Participation
We recruited affected individuals (patients) from across Canada through online advertisements on patient advocacy group websites and through a local MS clinic. We recruited MS clinicians through advertisements on a professional Canadian listserv and through e-mail invitations. The inclusion criteria for patients with MS were a diagnosis of MS, age 19 years or older, ability to provide informed consent, and ability to speak English. The inclusion criteria for MS clinicians were specialization in the care of patients with MS, ability to provide informed consent, and ability to speak English.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was reviewed and approved by the University of British Columbia Behavioral Research Ethics Board. Written informed consent was obtained from all the research participants.
One of us (S.B.) conducted a series of in-depth semistructured interviews. The patient and clinician interview guides (Appendix S1, which is published in the online version of this article at ijmsc.org) were developed using previous studies of patient perspectives about novel biotechnologies.10,11 Interviews began with conversations about patient familiarity with stem cell research and clinical applications, and then probed for receptivity, estimated time frames for the clinical implementation, and factors affecting the pace of translation of stem cell interventions.
Interviews were conducted by telephone or in person between May 23, 2014, and February 20, 2017. Interviews were ongoing until no new major themes emerged, operationally marking theoretical saturation.12 Verbatim interview transcripts were verified for accuracy and then managed using NVivo 11 software (QSR International, Melbourne, Australia). Data analysis was concurrent with data collection in an effort to accommodate emergent themes.13
Using standard qualitative content analysis methods,14 three of us (S.B., C.L., and A.S.) developed a codebook that reflected the emerging phenomena and the hierarchy of codes in the patient and clinician data sets. Deliberations about the organization of the codebook were iterative until consensus was reached. Data were analyzed line by line initially, noting remarkable phenomena and primary codes. Similarities and differences were probed within and between transcripts for the patient and clinician data sets, and primary codes were then organized into major themes and subthemes through a constant comparative approach.12 The final codebook reflects the narrative across themes for the patient and clinician cohorts. Two of us (S.B. and C.L.) independently coded 10% of the sample. A Cohen's kappa test performed on this sample yielded coefficients of 0.93 and 0.83 for the patient and clinician samples, respectively, indicating substantial intercoder agreement.15 After coding was finalized, data were analyzed to identify patterns corresponding with demographic information (e.g., age, sex, MS subtype) using the NVivo query function. Responses that segregated by demographic patterns are noted in the Results section.
We provided synthesized results to all participants who agreed to be recontacted and invited them to provide feedback on data interpretation. Twelve participants responded to this call (seven patients and five clinicians), representing an acceptable sample for this member checking data verification process.16 Participants indicated that the data represent their views authentically, and no major revisions were suggested.
Results
Study Participants
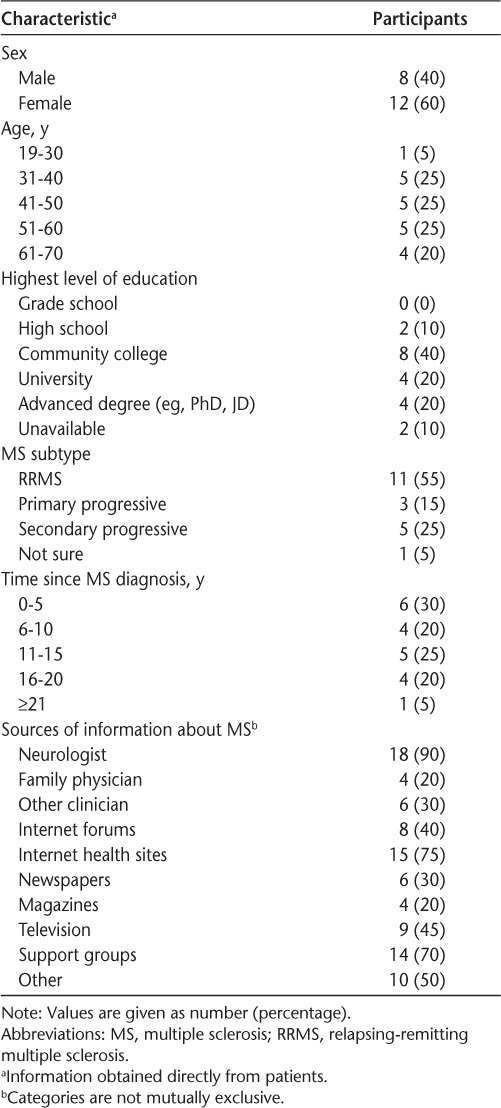
Twenty patients (Table 1) and 15 clinicians (Table 2) were interviewed. None of the patients had received a stem cell intervention. Sample sizes are consistent with standard qualitative methods.16 Interviews ranged from 12 to 80 (mean, 31) minutes, for a total of 18 hours of audio-recorded data.
Patient characteristics (n = 20)
Clinician characteristics (n = 15)

Themes
The final codebook consisted of four major themes: receptivity, translational pace, accelerators and barriers to translation, and clinical communication strategies (Table 3). Major themes were defined by their prominence and relevance to the objectives of the study.
Emergent themes

Receptivity
Patients expressed support for stem cell research. Some individuals with progressive or poorly controlled disease indicated a desire to undergo stem cell interventions. Patient 1 (male, relapsing-remitting MS [RRMS]): “I know about stem cell research … I absolutely believe in it and I absolutely support it … if they [researchers] did a clinical trial I would absolutely participate.”
Patients expressed optimism that stem cell research would someday produce a treatment for MS. Many cited other recent advancements to support these views, including examples from medical interventions that had successfully made the transition to the clinic after years of research. Patient 13 (female, RRMS): “I have great hopes that it [stem cell interventions] will be a very viable treatment for MS … oncology took a long time before they got to the transplant stage.” Likewise, many clinicians noted that patients are receptive to learning about stem cell research and often ask about advancements in the field.
Some patients explained that they would not consider undergoing stem cell interventions at the time of their interview because their disease was stable. They further explained that they would consider having a stem cell intervention if they were to experience progressive disease. Patient 2 (female, RRMS): “When I hear you talking about stem cell clinical trials and their relation to MS, the first thing that comes to my mind is patients who are in wheelchairs, or … have permanent disability due to their MS … I imagine that [stem cell interventions] would be most relevant and most effective and most needed … by them.” In contrast, clinicians explained that stem cell interventions, particularly mesenchymal and hematopoietic, would likely offer the most therapeutic benefit to young patients who are in earlier stages of the disease. Clinician 3 (male, neurologist): “I think it [stem cell interventions] certainly shouldn't be given to patients who are too advanced in their disease, because there's fibrosis, astrocytosis that will block the multiplication of those cells.”
Translational Pace
Clinicians explained that urgency often motivates patients to inquire about the pace of stem cell research, particularly with respect to the therapeutic window they face. Clinician 5 (male, neurologist): “I think many of them [patients] feel a sense of urgency to do something about their disease … [patients] know that time is brain and they want to get on board [with stem cell interventions] as fast as they can.”
Clinicians explained that the stem cell tourism phenomenon often underlies patients' inquiries about the time frames for clinical application. Clinician 2 (male, neurologist): “… they're [patients] fully aware that there are people around the world that are willing to inject … stem cells into them and they're … wanting an honest answer as to is this technology at the point where they can trust it.” On the other hand, a few patients commented on stem cell tourism and expressed skepticism about stem cell interventions that are offered on the global front but remain unavailable through the Canadian health system, and they questioned their ripeness for clinical uptake. Patient 5 (female, RRMS): “There's people out there sneaking off to these, you know, far off places and spending a ton of money to have it [stem cell intervention] done … I'm kind of skeptical because I don't really know if stem cell treatment procedures or, you know, whatever you want to label them, are at the point where we should all be running off to have them done.”
Clinicians noted the challenges of responding to questions about time frames given the unpredictable nature of the research and development process. Clinician 1 (male, physiatrist): “… they ask me … ‘when is it going to be kind of standard treatment?’ But I myself don't know the answer.” While acknowledging the uncertain time frames associated with research, some clinicians provided estimates for when stem cell interventions will be available as standard-of-care therapies. Estimates ranged from 5 to 25 years. Clinicians were most optimistic about the translational pace of autologous hematopoietic stem cell approaches. Some had referred their patients to centers that provide the intervention through an expanded access program after phase 1/2 clinical trials. They provided more uncertain and distant time frames for the clinical application of mesenchymal and neural approaches than for the application of hematopoietic approaches. Clinician 5 (male, neurologist): “With the mesenchymal [approach] …we have to follow up with the study and see if there's … early data that suggests it's helpful. And then the restorative stuff [neural stem cell approach] … I think that we're 20 years out.” Likewise, patients provided heterogeneous estimates for stem cell therapies to become widely available that ranged from 2 to 30 years. Some expressed hopes that stem cell therapies will be available to them personally. Others explained that by the time stem cell therapies are developed, their disease would likely have progressed too far. Patient 5 (female, RRMS): “I would like to think that there will be stem cell therapies available for me in my lifetime … you can never really predict what course your disease is going to take. So, I like to remain hopeful that my disease will stay sort of at the course it's at and stem cell therapies will help me at some point.” Patient 12 (male, secondary progressive MS): “I'm 60 and a cure might not be found in my lifetime, but hopefully it's going to be found for a kid.”
Many patients explained that they know little about the translational process, and that this makes predicting time frames for clinical application challenging. Patient 2 (female, RRMS): “I don't know how far along they [researchers] are with the research. I don't know what they've done or what the next steps are.” Indeed, although many patients indicated a limited understanding of different stem cell sources and the stages of clinical research and their goals, they also expressed a desire to learn more about the research process. Patient 15 (male, primary progressive MS): “How long does the process take to get stem cells done [approved for clinical use]?”
Accelerators and Barriers to Translation
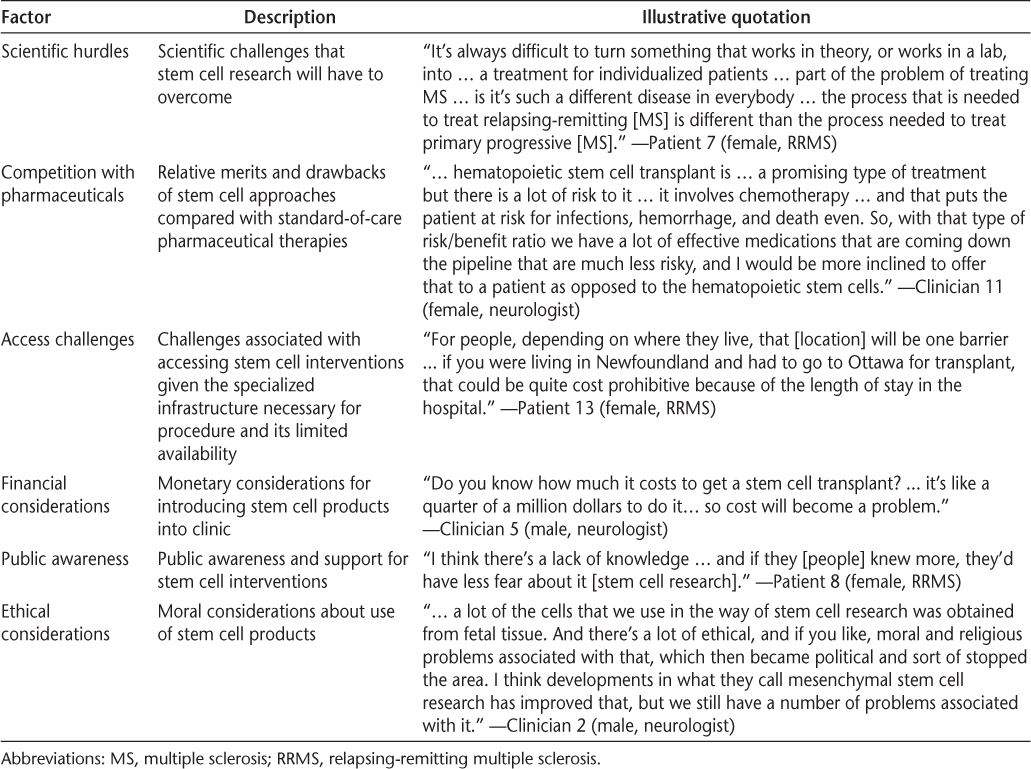
Patients and clinicians described similar factors that may speed up or slow down the translation of stem cell research. The major factors were scientific hurdles, competition with pharmaceuticals, financial considerations, access challenges, ethical considerations, and public awareness (Table 4).
Accelerators and barriers to translation of stem cell interventions for MS
Clinical Communication Strategies
Clinicians suggested communication approaches to address patient inquiries about stem cell research and elucidate the pace of research and development. Strategies focus on two main goals: promoting informed hope and clarifying time frames for the clinical implementation of stem cell research. Clinical communication strategies were not explored in patient interviews but emerged as a major theme in clinician narratives.
Promoting Informed Hope. Clinicians expressed that it is important to help patients establish a sense of informed hope that is grounded in scientific promise and acknowledges the caveats of research. At the same time, clinicians explained that it is also essential to honor patient hope despite scientific uncertainty. Clinician 2 (male, neurologist): “I try not to … create false hopes … but on the other hand, you don't want to take all hope away from individuals. Patients … cling to hope and you want to … [promote] reasonable, realistic hopes.”
Clinicians explained that it is important to help patients anchor expectations in current clinical realities. They described strategies for informing patients about current therapies (eg, pharmaceuticals, physical or occupational therapy) to help manage MS while research is underway. Clinician 4 (female, physiatrist): “… [It's] about not taking away their hope … but to explain to them that right now the focus is more on the things that we can do something about, like managing their spasticity, managing their pain, optimizing their functioning. And maintaining their body ready for any potential cure that may come out of stem cells.”
Clarifying Time Frames. Consistent with a stance of epistemic humility,17 clinicians explained that they should be upfront with their patients about the scientific uncertainty associated with stem cell research, particularly about whether stem cell interventions will be available within their therapeutic windows. Clinicians also reinforced the importance of engaging with patients about the process of research and associated time frames. Clinicians indicated that patients are often unfamiliar with the stages of clinical trials. To this end, they suggested that dialogue could explore clinical trial phases, their goals, and the time frames historically associated with each phase of clinical research. Clinician 5 (male, neurologist): “I just explain ... you do the sort of open-label early stuff to find out if the intervention's safe at all in the human body. And then you do the phase 2 study, which generally is shorter and has less patients and is generally supposed to show that there might be some effect…. And then they do a larger … phase 3 studies, where we get a sense of if the intervention works.”
Clinicians indicated that explaining the heterogeneity of stem cell sources (eg, hematopoietic, mesenchymal, neural) would clarify why different stem cell types are at different stages of development. Clinicians also suggested that it is important to highlight both the progress achieved in the stem cell arena and the challenges that remain. Clinician 14 (female, neurologist): “We talk about that study [a phase 1/2 autologous hematopoietic clinical trial] and how it was published, how it was indicated for a very sort of small percentage of people and why and how it was helpful for that group. And then we talk about other stem cell work going on, and we usually talk about the study in Ottawa with the mesenchymal stem cells.”
Discussion
The data suggest that patients with MS are receptive to stem cell interventions. At the same time, patients and clinicians agreed that stem cell interventions would not be appropriate first-line approaches given their risk profile. Nevertheless, clinical equipoise remains as research comparing hematopoietic stem cell transplant and pharmaceutical approaches is currently underway.18
A divergence between the receptivity of patients and clinicians exists with respect to the timing of stem cell transplantation. Although many patients indicated that they would be receptive to stem cell approaches if they were to become disabled, clinicians indicated that hematopoietic stem cell interventions would be more optimally suited for patients who are younger and who have less severe disability. Indeed, clinical trials of autologous hematopoietic stem cell transplantation have found that patients who have milder disease (Multiple Sclerosis Severity Scale score ≤8.3) experience more stabilization or improvement than individuals with more severe MS (Multiple Sclerosis Severity Scale score >8.3) after transplantation2 and that patients who are younger and undergo transplantation closer to the time of diagnosis have better outcomes than older patients.19
These findings align with those of a study that explored the perspectives of patients with spinal cord injury. Patients with subacute spinal cord injury (injured 1–7 months before the study) expressed hesitation to participate in stem cell clinical trials, whereas those with chronic injury (injured >18 months before the study) expressed greater readiness to enroll in stem cell research.11 In the same study, patients with more severe disease (cervical spinal cord injury) were less receptive to stem cell interventions than were patients with less severe disease (thoracic spinal cord injury).11 In the present study, patient receptivity to stem cell interventions did not segregate by MS subtype. This finding is consistent with a recent autologous hematopoietic stem cell clinical trial that showed no significant difference on Expanded Disability Status Scale scores between patients with RRMS and those with secondary progressive MS after transplantation.2 Differences in patient perspectives in the context of MS and spinal cord injury may be due to the progressive nature of MS compared with the more static nature of impairment in spinal cord injury.
The present results reveal patients' desire to learn more about the translational process and the pace of research and development. The complex translational landscape in the stem cell arena is a salient challenge in clarifying such time frames. Models of translation have been historically described as a linear, single-track path from preclinical studies to clinical trials and finally to an approved therapy.20 In contrast, the contemporary landscape of translation in the stem cell arena is a far more complex, multitrack pipeline. This pipeline is characterized by several points of access to stem cell interventions from both within and outside of clinical trials.21 For example, stem cell interventions may be offered outside of clinical trials through regulated routes such as the expanded access program of the US Food and Drug Administration and through unregulated avenues, such as stem cell tourism. Indeed, clinicians noted that patient inquiries about the local availability of stem cell treatments for MS are often fueled by marketing efforts for stem cell tourism. Findings about the heterogeneity of patient estimates for the clinical availability of stem cell applications are, therefore, not surprising given the diversity of routes to access stem cell interventions.
The challenge of clarifying research time frames may necessitate additional dialogue about the richness of translation in the stem cell arena. Findings about heterogeneous patient estimations of time frames and their appetite to learn more about the research process correspond well with clinician recommendations for further dialogue about clinical translation. These conversations may include clarification about diverse stem cell sources and clinical trial phases and their goals. Clinician recommendations to approach conversations through a posture of epistemic humility may serve to nurture the physician-patient relationship17,22 and to promote trust in local health care providers and resources.23–25 In addition to these recommendations, clinical communication may address the various routes to access stem cell interventions outside of clinical trials (eg, stem cell tourism). Conversations ought to include clarification about the level of evidence and regulation associated with routes of administration outside of clinical trials. This will serve to contextualize premature and illegitimate forms of stem cell application that detract from the translational process of stem cell biotechnologies, prey on patient urgency to access a therapy,6 and reframe the rigorous process of scientific evaluation as an access hurdle.
This study has some limitations. As is standard in qualitative inquiry, this study has a limited sample size and represents the views of participants at a snapshot in time. It does not aim to be generalizable. Because this study explores the perspectives of patients with MS only, study findings may not be transferable to neurologic diseases characterized by a stationary prognosis, such as those demonstrated by patients with spinal cord injury.11 Recruitment strategies generated a study sample that may be affected by self-selection bias. Finally, 50% of the patients in this study were diagnosed within 10 years of study participation, and 55% had the relapsing-remitting form of the disease. This sample may be indicative of a demographic with a lower level of disability. In the absence of self-reported MS-related disability severity data, such as Expanded Disability Status Scale scores, the findings cannot be reliably interpreted in reference to disease severity.
In conclusion, regenerative medicine has been a focus of hope in the MS community for nearly 3 decades. Although patients still anticipate the therapeutic applications of stem cell research, information about the time frames associated with the translation of research into clinically applicable treatments is less accessible. This study characterizes patients' receptivity to stem cell interventions and their desire to learn more about the process and pace of research and development. These results highlight opportunities to promote transparency in clinical discourse that aims to clarify translational time frames and promote informed hope.
PRACTICE POINTS
Clinicians have an opportunity to promote informed hope about experimental stem cell interventions by discussing scientific promise, acknowledging the caveats of research, and anchoring the conversation in currently available clinical resources.
To promote informed hope, clinicians can describe the process of translation from bench to bedside by explaining clinical trial phases and their goals, the heterogeneity of stem cell sources, the progress achieved in the stem cell arena, and the challenges that remain.
Clinical conversations about standards of evidence and regulatory protections may provide clarity about premature and illegitimate forms of stem cell application.
Acknowledgments
The authors thank Michelle Eisner for assisting with participant recruitment.
References
Burt R, Burns W, Hess A. Bone marrow transplantation for multiple sclerosis. Bone Marrow Transplant. 1995;16:1–6.
Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388:576–585.
Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156.
Benjaminy S, Lo C, Illes J. Social responsibility in stem cell research: is the news all bad? Stem Cell Rev Rep. 2016;12:269–275.
Kamenova K, Caulfield T. Stem cell hype: media portrayal of therapy translation. Sci Transl Med. 2015;7:278ps4.
Petersen A, MacGregor C, Munsie M. Stem cell miracles or Russian roulette? patients' use of digital media to campaign for access to clinically unproven treatments. Health Risk Soc. 2016;17:592–604.
Caulfield T, Sipp D, Murry CE, et al. Confronting stem cell hype. Science. 2016;352:776–777.
Petersen A, Munsie M, Tanner C, et al. Stem Cell Tourism and the Political Economy of Hope. London, England; Palgrave Macmillan; 2017.
Berger I, Ahmed A, Bansal A, et al. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell. 2016;19:158–162.
Benjaminy S, MacDonald I, Bubela T. “Is a cure in my sight?” multi-stakeholder perspectives on phase I choroideremia gene transfer clinical trials. Genet Med. 2014;16:379–385.
Illes J, Reimer JC, Kwon BK. Stem cell clinical trials for spinal cord injury: readiness, reluctance, redefinition. Stem Cell Rev. 2011;7:997–1005.
Charmaz K. Constructing Grounded Theory. Thousand Oaks, CA: Sage Publications; 2014.
Mayan MJ. Essentials of Qualitative Inquiry. 2nd ed. New York, NY: Routledge; 2016.
Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
Neuendorf KA. The Content Analysis Guidebook. Thousand Oaks, CA: Sage Publications; 2016.
Guest G, Bunce A, Johnson L. How many interviews are enough? an experiment with data saturation and variability. Field Methods. 2006;18:59–82.
Schwab A. Epistemic humility and medical practice: translating epistemic categories into ethical obligations. J Med Philos. 2012;37:28–48.
Stem cell therapy for patients with multiple sclerosis failing alternate approved therapy: a randomized study. ClinicalTrials.org website. https://clinicaltrials.gov/ct2/show/NCT00273364?term=BURT&cond=Multiple+Sclerosis&rank=1. Accessed July 23, 2017.
Muraro P, Pasquini M, Atkins H, et al. Long-term outcomes after autologous haematopoietic cell transplantation for multiple sclerosis: a joint study from the Center for International Blood and Marrow Research (CIBMTR) and the European Group for Blood and Marrow Transplant. Bone Marrow Transplant. 2013;48:S1.
Kimmelman J, London AJ. The structure of clinical translation: efficiency, information, and ethics. Hastings Cent Rep. 2015;45:27–39.
Hyun I. Allowing innovative stem cell-based therapies outside of clinical trials: ethical and policy challenges. J Law Med Ethics. 2010;38:277–285.
Benjaminy S, Traboulsee A. At the crossroads of civic engagement and evidence-based medicine: lessons learned from the chronic cerebrospinal venous insufficiency experience. In: Illes J, ed. Neuroethics: Anticipating the Future. Oxford, UK: Oxford University Press; 2017:264–273.
Crooks VA, Li N, Snyder J, et al. “You don't want to lose that trust that you've built with this patient…”: (dis)trust, medical tourism, and the Canadian family physician-patient relationship. BMC Fam Pract. 2015;16:25.
Petersen A, Tanner C, Munsie M, et al. Between hope and evidence: how community advisors demarcate the boundary between legitimate and illegitimate stem cell treatments. Health. 2015;19:188–206.
Snyder J, Adams K, Crooks VA, et al. “I knew what was going to happen if I did nothing and so I was going to do something”: faith, hope, and trust in the decisions of Canadians with multiple sclerosis to seek unproven interventions abroad. BMC Health Serv Res. 2014;14:445.







