Publication
Research Article
International Journal of MS Care
Evaluating the Effect of Functional Electrical Stimulation Used for Foot Drop on Aspects of Health-Related Quality of Life in People with Multiple Sclerosis
Author(s):
Abstract
Background:
Multiple sclerosis (MS) is a common degenerative neurologic condition resulting in walking difficulties. Foot drop is a common walking impairment in MS that can affect health-related quality of life (HRQOL). Functional electrical stimulation (FES) can improve walking in people with MS, but its effect on HRQOL is not well established. This review investigated the effect of FES used for foot drop on HRQOL in adults with MS.
Methods:
A systematic search was performed using CINAHL, MEDLINE, Cochrane Library, PubMed, and PEDro online databases. Inclusion and exclusion criteria were applied to select eligible studies. Data were extracted, and two reviewers independently rated the quality of the studies using the Effective Public Health Practice Project assessment tool.
Results:
Eight studies were eligible for review; seven were of moderate-to-strong methodological quality and one was weak. Seven studies demonstrated significant positive effects of FES on different aspects of HRQOL as measured by the 29-item Multiple Sclerosis Impact Scale, 36-item Short Form Health Status Survey, Canadian Occupational Performance Measure, and Psychosocial Impact of Assistive Devices Scale.
Conclusions:
This review provides preliminary evidence that FES has a positive effect on aspects of HRQOL in people with MS; however, the variety of HRQOL outcomes used makes it difficult to determine definitive conclusions. Future larger-scale randomized studies with long-term follow-up are recommended to better understand the effect of FES on HRQOL. This will inform prescribing decisions and support compliance with FES over the longer-term.
Multiple sclerosis (MS), a chronic, progressive, autoimmune disease of the central nervous system, results in demyelination and axonal loss in the brain, spinal cord, and optic nerves.1,2 The disease has an unpredictable course and is usually progressive in nature. Gait disturbances are reported as one of the most disabling activity limitations and highest priorities for people with MS due to its profound effect on daily activities.3
Foot drop is a common gait abnormality experienced by people with MS and presents as a loss of or inefficient dorsiflexion in the swing phase of gait. This loss of foot clearance results in an increased effort of walking,4 risk of trips and falls,5 and subsequent reduction in activity restriction and independence,6 affecting health-related quality of life (HRQOL), which is a potent measure of the performance of health care interventions. Health-related quality of life incorporates multiple domains (eg, physical, psychological, and social functioning) and involves the patient's own perspective.7 Health-related quality of life goes beyond direct measures of health and life expectancy and focuses on the effect that health status has on perceived quality of life (QOL), incorporating perceptions of role functions, social health, general well-being, and life satisfaction.7–9 In terms of measurement, generic or specific instruments can be used. Generic instruments, such as the 36-item Short Form Health Survey (SF-36), provide a summary of HRQOL, whereas measures such as the 29-item Multiple Sclerosis Impact Scale (MSIS-29) focus on problems associated with single disease states, patient groups, or areas of function.7,10,11 Using a combination of generic and specific HRQOL measures is considered more appropriate for monitoring change in health status after intervention.11
Functional electrical stimulation (FES), an assistive device used in the treatment of foot drop, produces a muscle contraction that lifts the foot during the swing phase of gait by electrically stimulating the common peroneal nerve via either surface or implanted electrodes.12 Functional electrical stimulation has been found to be an effective treatment to correct foot drop and improve walking in people with MS.4,13,14 People with MS have reported positive benefits with FES, such as improvements in fatigue and confidence in walking and increased participation in physical activity.15 Most studies investigating FES in people with MS have evaluated the effect on gait parameters such as speed, energy expenditure, walking distance, and gait kinematics. A recent systematic review and meta-analysis found clinically meaningful orthotic effects of FES on walking speed in people with MS.16 Although it is important to evaluate the effect of FES on these aspects of walking, understanding the effect of FES on HRQOL will provide clinicians with greater insight into the potential effect on an individual's daily life. This review aimed to systematically review evidence of the effect of FES on aspects of HRQOL in people with MS who present with foot drop.
Methods
Search Strategy
A systematic search of electronic databases was conducted in March 2017 by accessing a variety of online databases (CINAHL, Cochrane Library, MEDLINE, PubMed, and PEDro). The databases were searched using a PICO (population, intervention, comparison, and outcome) approach.17 After an initial search, no articles published before 2010 using HRQOL measures to evaluate the effect of FES were identified. Thus, for the purpose of this review, articles published in English from 2010 onward were considered. The keywords used for population were multiple sclerosis OR MS AND foot drop (unilateral/bilateral) OR dropped foot. The keywords for intervention were functional electrical stimulation OR FES OR neuromuscular stimulation OR electrical stimulation OR e-stim OR drop foot stimulator OR foot drop stimulator OR peripheral nerve stimulation OR peroneal nerve stimulation. Studies that included usual care as a control or alternative treatments for foot drop, such as ankle-foot orthoses or exercise interventions, as a comparator were included. The keywords for the outcome were health-related quality of life OR HRQOL OR quality of life OR QOL. A hand search of the reference lists of relevant articles was also undertaken (A.C.L. and J.W.).
Eligibility Criteria
After the systematic search of databases, duplicates were removed. Studies of empirical or observational design were included. Participants were older than 18 years, had a diagnosis of MS (or were part of a neurologic sample where MS data could be extracted), presented with foot drop (unilateral or bilateral), and had used FES (surface or implanted electrodes). Selected studies required at least one validated HRQOL outcome measure that assessed the effect of FES to be reported. Articles that did not investigate FES as an intervention (eg, systematic reviews or opinion pieces) were excluded. Articles were initially screened by title and then by abstract by three of the authors (A.C.L., J.W., and R.H.). Where there was disagreement over inclusion, a fourth author (L.R.) was consulted. Articles that met the inclusion criteria were then read in full to confirm eligibility (Figure).
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Diagram
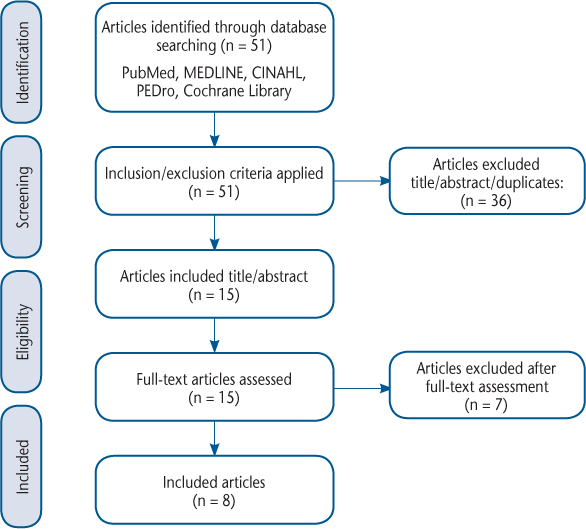
Data Extraction
One reviewer (J.W.) extracted data from the articles, including details on participant characteristics, methods and study design, interventions, outcomes, and results. A second reviewer (A.C.L.) checked the extracted data. Authors were contacted when clarification of study details was required.
Methodological Quality
Articles that met the predefined inclusion criteria were independently rated by two reviewers (A.C.L. and R.H.) using the Effective Public Health Practice Project assessment tool, which was developed to appraise studies of any public health topic area and provides a valid and reliable assessment of a range of study designs.18 Discrepancies between the reviewers were resolved by consulting a third independent assessor (L.R.) to reach consensus.
Results
Study Design
Eight studies were found to be eligible for this review (Figure). The articles for review included one randomized controlled trial,19 one randomized crossover trial,20 three experimental nonrandomized studies,13,14,21 and three observational studies22–24 (Table 1). The crossover study by Taylor et al20 involved two groups. For the purposes of this review we review the results from only the first 6 weeks of group 1, who received FES for 6 weeks followed by 6 weeks of physiotherapy, to allow for comparison with the other studies.
Features of the eight studies eligible for review
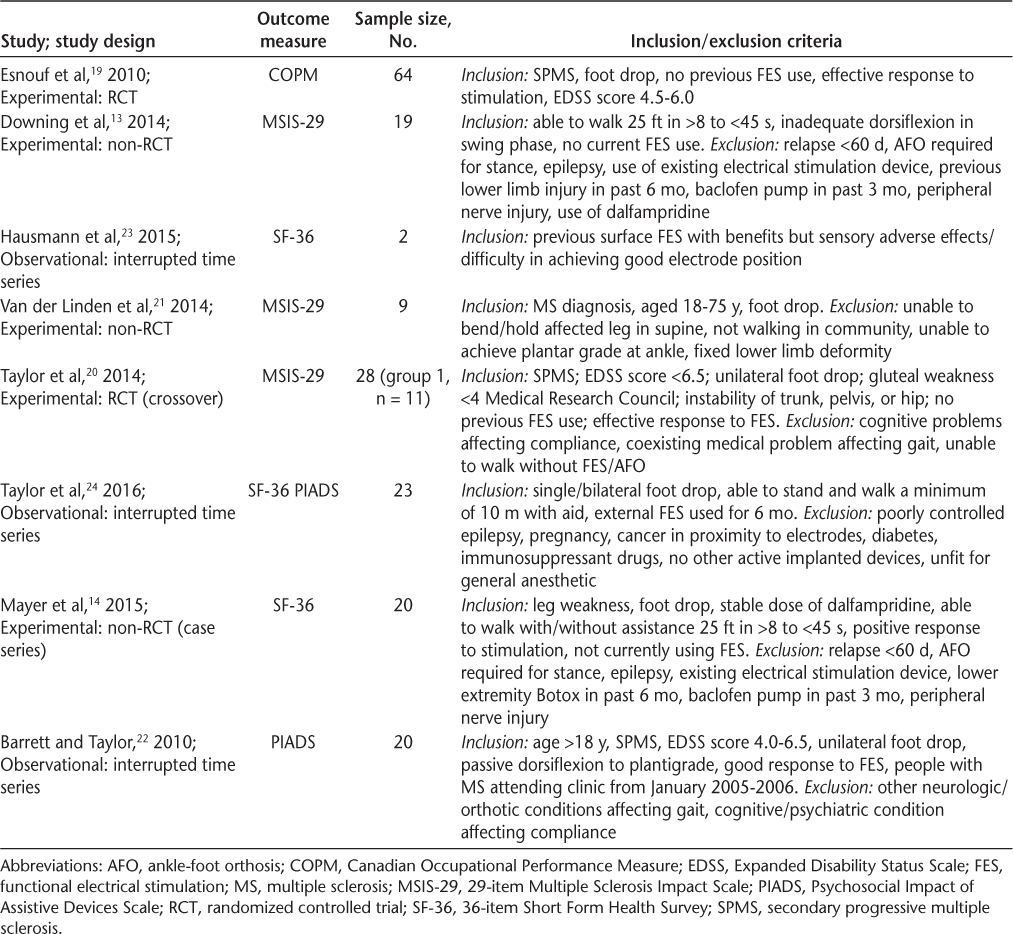
Recruitment
One article did not specify the method of recruitment.23 The seven remaining studies recruited people with MS via referrals,19,21 waiting lists,20 MS and FES clinics,14,22,24 or orthopedic and prosthetic clinics for the management of foot drop.13 The inclusion and exclusion criteria for participant eligibility are detailed in Table 1.
Participants
The total number of participants included in the eight studies in this review was 168, and the individual sample sizes varied from 223 to 6419 (Table 2). Seven studies recruited both male and female participants. One study did not specify the sex of participants.24 Of the studies reporting sex, all but one23 recruited more female than male participants. In total, 63% of participants (n = 94) were female and 37% were male (n = 55).
Participant demographic characteristics, interventions, and assessment frequency
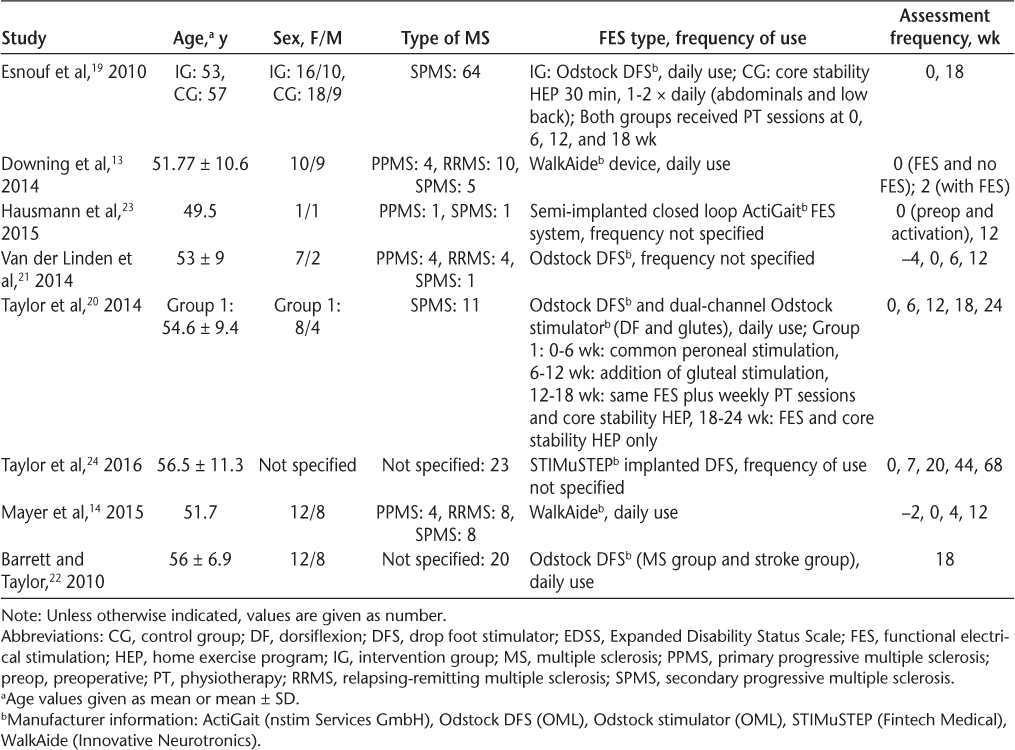
Two studies did not report MS subtype.22,24 Two studies exclusively recruited people diagnosed as having secondary progressive MS (SPMS),19,20 and the remaining studies recruited participants with various MS subtypes. In total, 104 participants presented with SPMS, 23 with relapsing-remitting MS (RRMS), and 13 with primary progressive MS (PPMS). The diagnosis was unknown for 43 participants.
Methodological Quality
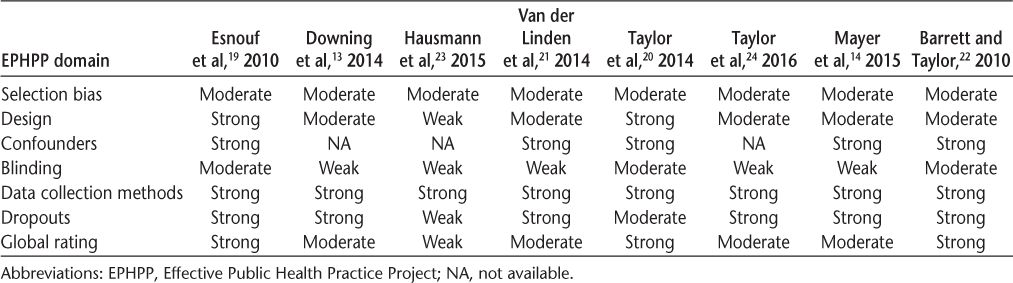
The methodological quality of the studies is summarized in Table 3. The global rating of quality was found to be strong in three articles,19,20,22 moderate in four articles,13,14,23,24 and weak in one article due to selection bias, confounding variables, and poor blinding.23 Double blinding in rehabilitation studies is difficult, and it is suggested that such bias is unavoidable in assistive technology studies.25 Nevertheless, researchers should consider novel ways of overcoming this issue to improve the quality of future research.
Methodological quality: EPHPP assessment
Outcome Measures and Effect of FES Intervention
Four measures were used to assess the effect of FES on aspects of HRQOL in people with MS: the MSIS-29, the SF-36, the Canadian Occupational Performance Measure (COPM), and the Psychosocial Impact of Assistive Devices Scale (PIADS). Of the eight studies, seven demonstrated significant positive effects of FES on aspects of HRQOL in people with MS.13,14,19,20,22–24 The remaining study found no significant effect on MSIS-29 scores after 12 weeks of FES,21 and Taylor et al24 reported no significant difference in SF-36 scores 128 days after implantable FES (Table 4). Most of the studies evaluated the ongoing orthotic/therapeutic effect of FES on HRQOL. Mayer et al14 also investigated the initial orthotic effect by applying the SF-36 before using FES.
Summary of effects of FES on HRQOL
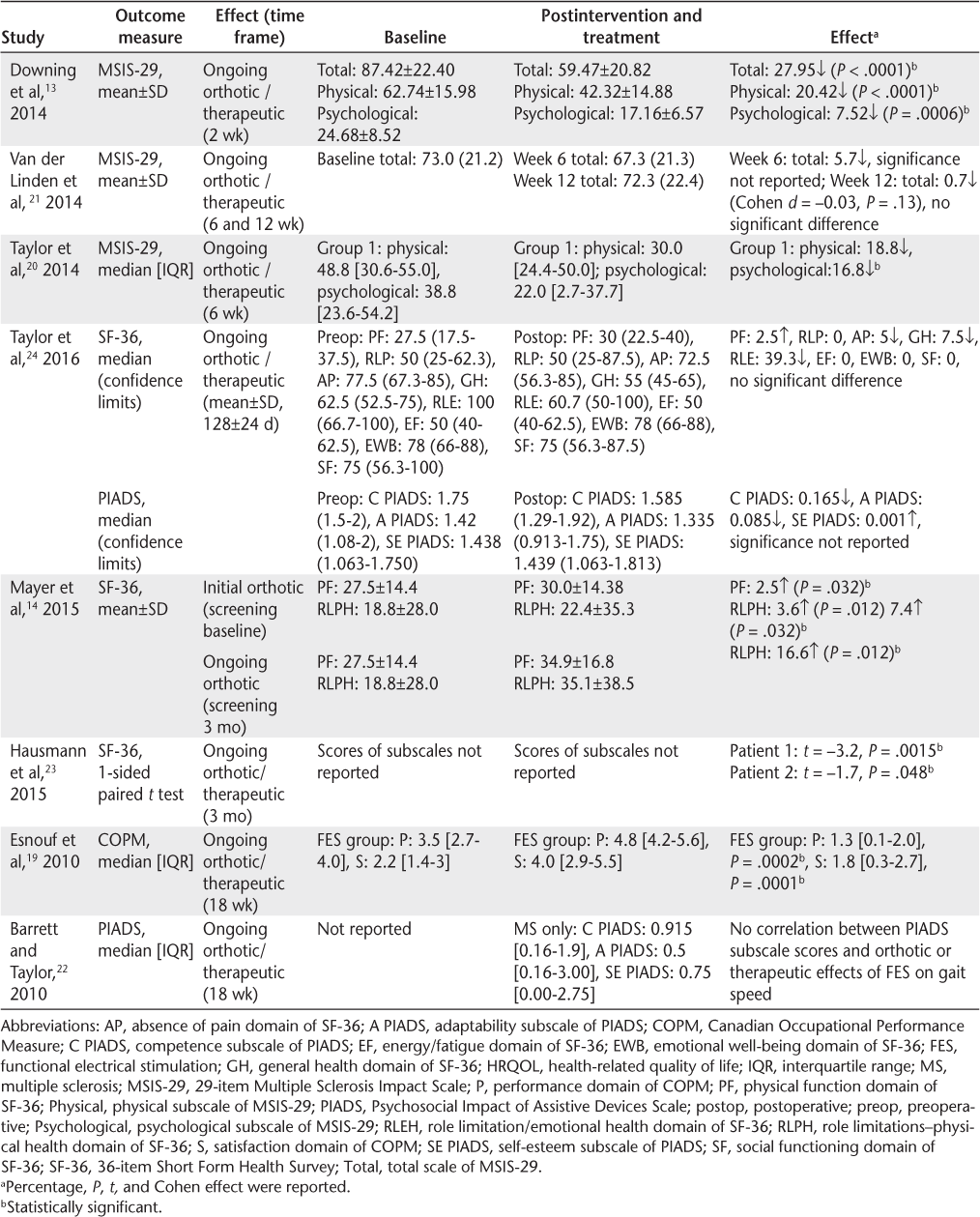
Three studies used the MSIS-29.13,20,21 Downing et al13 reported significant reductions in the total (27.95, P < .0001), physical subscale (20.42, P < .0001), and psychological subscale (7.52, P = .0006) scores of the MSIS-29 after 2 weeks of FES. Similar improvements were noted by Taylor et al,20 where a significant (P < .05) reduction in the psychological subscale score of the MSIS-29 was observed after 6 weeks of FES. Van der Linden et al21 found no significant reductions in either the physical or psychological subscale scores at 6 or 12 weeks.
The SF-36 was used in three studies.14,23,24 Mayer et al14 noted significant changes after 3 months of FES in both the physical function (P = .032) and role limitations–physical health (P = .012) domain scores. Hausmann et al23 reported significant improvements in both participants (participant 1, t = −3.2, P = .0015; participant 2, t = −1.7, P = .048) in SF-36 scores after 12 weeks of FES, particularly regarding pain, energy/fatigue, and the role limitations–physical health domains. Taylor et al24 found small, nonsignificant changes in the pain, general health, and energy/fatigue domains of the SF-36 and no changes in any of the other domains using the STIMuSTEP (Fintech Medical, UK) FES device.
Two studies used the PIADS to evaluate the psychosocial impact of FES after 18 weeks.22,24 Barrett and Taylor22 reported the scores after 18 weeks of FES. Medians and interquartile ranges for the domains were reported as follows: competence, 0.915 (0.16–1.9); adaptability, 0.5 (0.16–3); and self-esteem, 0.75 (0–2.75), which are indicative of a positive effect of FES on HRQOL. Taylor et al24 reported PIADS scores before and after STIMuSTEP, with some small improvements being observed in the self-esteem domain, but reductions in the adaptability and competence domains were observed. The median scores of the domains after STIMuSTEP were significantly higher in this study compared with those reported by Barrett and Taylor.22 These results are, nevertheless, misleading, as participants in the study by Taylor et al24 had previously used surface FES for a minimum of 6 months before receiving their implantable FES device.
The COPM measure was used in one study where significant improvements in the performance (P = .0002) and satisfaction (P = .0001) scores of the COPM after 3 months of FES were observed.19 The exercise control group in this study demonstrated improvements in the satisfaction scores only (P = .0437).
Discussion
This review of eight articles aimed to appraise the effect of FES for foot drop on aspects of HRQOL in adults with MS. Seven studies were found to be of either moderate or strong methodological quality, and one study was weak. This review found evidence of a positive effect in most of the studies included. Ongoing orthotic or therapeutic effects of FES were investigated, while one study evaluated the initial orthotic effect.14 The HRQOL measures are not able to differentiate between ongoing orthotic or therapeutic effects of FES; therefore, it is impossible to determine whether results can be specifically attributed to either effect.
To our knowledge, this is the first systematic review to specifically evaluate the effect of FES for foot drop on aspects of HRQOL in MS. None of the studies selected for review had a HRQOL measure as its primary outcome. Nevertheless, a better understanding of the impact of FES on HRQOL is critical in providing insight into how previously identified improvements in gait parameters16 might affect an individual's QOL, thus improving compliance with and acceptance of such an intervention over the long-term. Improvements in aspects of HRQOL with FES, such as fatigue, fitness, confidence in walking, and increased engagement in physical activity and participation in daily activities, have been identified in an interpretative phenomenological analysis focus group study15 and a clinical audit.26 Such changes give premise to how FES may affect the range of different HRQOL measures identified in this review. Future research should make the impact of FES on HRQOL its primary focus. There has been limited qualitative research in this topic to date, and further in-depth qualitative studies are required to fully understand the experiences and views of people with MS using FES. This will help determine the most appropriate HRQOL outcomes to be used, thus facilitating the future investigation of the effect of FES on HRQOL.
Three studies investigated the effect of FES on the MSIS-29 score, a measure of the effect of MS on physical and psychological well-being. Higher scores are indicative of a greater effect of the disease.27 The MSIS-29 has excellent correlation with fatigue in MS28 and disability as measured by the Expanded Disability Status Scale.29 Reductions in the energy cost of walking with FES have been previously identified4; therefore, changes in fatigue or the effort and distance walking may account for significant reductions in the physical subscale of the MSIS-29 observed.13,20 It is also important to consider the clinical significance of these changes, and two of the studies13,20 demonstrated changes that were greater than the minimal clinically important difference identified for the MSIS-29 physical subscale.29 Taylor et al,20 however, recruited only participants with SPMS; therefore, the results may not be applicable to other MS subtypes. Overall, there was a much greater number of people with SPMS than other MS subtypes in this review; therefore, further research is required to establish the effect on HRQOL across the full range of MS subtypes. In contrast to the other two studies, Van der Linden et al21 noted small, nonsignificant changes in the MSIS-29 scores after up to 12 weeks of FES. This study, however, recruited only nine participants and, thus, was underpowered to detect changes in HRQOL. Participants in the study by Van der Linden et al21 presented with lower levels of disability compared with those in the study by Taylor et al.20 Miller et al30 previously noted that people with MS walking at slower gait speeds, and thus presenting with greater disability, had most to gain from FES in terms of improvements in walking. This may also be the case for HRQOL; however, these effects are not fully understood and warrant further investigation.
The effect of FES on the SF-36, which has been found to be a valid and reliable QOL instrument in MS,31 was investigated in three studies.14,23,24 This measure, consisting of 36 items resulting in eight subscales and two composite QOL measures, is a generic, not a disease-specific, HRQOL measure. Generic measures may fail to address clinically important impact aspects of a specific disease. Nevertheless, the physical functioning domain score of the SF-36 has been found to be lower in people with MS compared with other chronic neurologic conditions. Those requiring support for walking were the most impaired in the role limitations–physical health dimension of the SF-36.32 Two of the studies reported significant improvements in aspects of the SF-36 after 3 months of FES.14,23 Mayer et al14 investigated the effect of FES on the physical function and role limitations–physical health domains only, which specifically assess the effect of an individual's health on work or activities of daily living such as dressing and bathing, walking, using the stairs, or undertaking leisure activities. Such aspects of HRQOL that incorporate tasks involving lower limb function and mobility are those that might be expected to improve with FES. The minimal clinically important difference for SF-36 has not been determined in MS; however, a change of more than 10 points is considered to be clinically significant in other health conditions.33 Most of the changes observed were small, but clinically significant changes were found in the role limitations-emotional24 and role limitations–physical health14 subscales. Participants in the study by Mayer et al14 were also prescribed dalfampridine in addition to FES. Dalfampridine is a drug that has been shown to increase gait performance by approximately 25% in people with MS34; thus, this may have influenced the results.
Two studies investigated the effect of two different implantable FES systems on SF-36 scores.23,24 Hausmann et al23 found significant effects, whereas Taylor et al24 did not. Both of these studies applied surface FES before implantable FES, which meant that baseline scores were not completely before FES intervention. This may have resulted in a smaller increase in domain scores, particularly in the study by Taylor et al,24 where participants had used surface FES for a considerable period (6 months) before receiving an implantable device. The study by Hausmann et al23 was a small feasibility study (n = 2); therefore, although they reported significant results for the total SF-36 score, it is difficult to draw any definitive conclusions.
Two studies investigating the effect of FES on PIADS scores reported positive results.22,24 The PIADS is designed to measures the effect of rehabilitative technologies and assistive devices on HRQOL and comprises three domains measuring competence, adaptability, and self-esteem.35 Each item is scored from −3 (maximum negative impact) to +3 (maximum positive impact), indicating the impact of using an assistive device.35 Taylor et al24 reported scores double those reported by Barrett et al.22 Differences between the device type (implantable: surface FES) and intervention time (128 days: 18 weeks) may have accounted for the enhanced results observed by Taylor et al.24 In a study investigating the effect of mobility assistive devices in older adults (>45 years),36 the PIADS domain scores were lower than those reported by Taylor et al,24 suggesting that FES may have equivocal effects compared to other assistive device interventions. The PIADS is responsive to variables such as device stigma and the functional features of the device.37 It reflects the perceived experiences of people using an assistive device and is, therefore, helpful in understanding an individual's ability to cope with and adapt to using FES. It may also aid in predicting device use and discontinuance, thus supporting long-term compliance with FES.
The COPM, which was used in one study included in this review,19 applies a semistructured interview where participants identify and then rate important activities within self-care, leisure, and productivity from 1 to 10 based on their perceptions of and satisfaction with these activities.38 Validity has been previously established in MS populations.39 Significant improvements in the perceived performance and satisfaction of activities such as walking, balance, climbing stairs, and managing steps and curbs were observed in people with SPMS after 18 weeks of FES.19 Such effects are similar to those identified in previous FES studies.15,16,29 Similar changes in the COPM scores were reported after a 4-week interdisciplinary inpatient rehabilitation program with people with MS,40 suggesting that an FES intervention may produce comparable effects.
This review has some limitations. Despite an extensive literature search, only eight studies were found that investigated the effect of FES on HRQOL. Articles reporting negative findings are less likely to be published and cited, which can result in publication bias.41 The authors did not approach FES researchers for unpublished data; thus, studies may have been missed, and this must be considered a limitation. Most studies in this review had small sample sizes, and, therefore, it is difficult to generalize findings to people with MS per se. Although most studies reported positive results, further studies fully powered for HRQOL outcomes are undoubtedly required before any conclusions or recommendations can be made. Despite the progressive nature of MS, none of these studies investigated FES use beyond 24 weeks. An observational study that investigated the effect of FES on a range of measures over 5 years in a large cohort of participants with MS (n = 145) reported a statistically significant positive orthotic effect of FES on walking speed and joint pain, but there was no effect on QOL as measured by a self-reported visual analogue scale.42 Future studies should consider investigating the effect of FES using a valid, clinically meaningful HRQOL over longer periods. Such studies, however, are challenging to undertake due to the potentially high dropout rate experienced in assistive device trials of longer duration. Street and Singleton42 observed 57% dropout of participants at 5 years.
In conclusion, to our knowledge this is the first review investigating the effect of FES for foot drop on HRQOL in people with MS. A small number of studies, recruiting small numbers of participants with no control comparators, used a range of HRQOL measures as secondary outcomes. There is preliminary evidence of a positive effect of FES on some aspects of HRQOL, such as the impact of MS, perceived activities of daily living performance, competence, self-esteem, and confidence. Interpreting changes in HRQOL, a complex, multidimensional construct, is challenging, particularly in a progressive neurologic condition such as MS. The variety of HRQOL outcomes used in the studies, each measuring different domains, makes it difficult to determine definitive conclusions from this review. Further qualitative investigation is required primarily to understand how FES affects HRQOL, before the most appropriate HRQOL measures can be identified to fully investigate the effectiveness of FES on HRQOL in people with MS. Further high-quality research should aim to capture the effect of FES on clinically meaningful aspects of HRQOL in longer-term studies. Such investigations will help inform future device development and prescribing decisions and more clearly define the support required for people with MS, thus optimizing compliance, satisfaction, and efficacy over the long-term.
PRACTICE POINTS
Functional electrical stimulation (FES) used for the treatment of foot drop has a positive effect on the speed and energy cost of walking in MS.
This review found that FES has a positive effect on aspects of health-related quality of life, such as the impact of MS, perceived activities of daily living performance, competence, self-esteem, and confidence in adults with MS.
The current evidence for the effect of FES on health-related quality of life is limited by studies recruiting small numbers of participants, investigating impact over the short-term, and lacking control comparators.
Financial Disclosures
The authors declare no conflicts of interest.
References
MacLean R. Multiple sclerosis: understanding a complex neurological condition. Nurs Stand. 2010;24:50–56.
Rejdak K, Jackson S, Giovannoni G. Multiple sclerosis: a practical overview for clinicians. Br Med Bull. 2010;95:79–104.
Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26:109–119.
Paul L, Rafferty D, Young S, et al. The effect of functional electrical stimulation on the physiological cost of gait in people with multiple sclerosis. Mult Scler. 2008;14:954–961.
Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. PM R. 2011;3:624–632.
Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler. 2007;13:1168–1175.
Health-related quality of life and well-being. HealthyPeople.gov website. https://www.healthypeople.gov/2020/about/foundation-health-measures/Health-Related-Quality-of-Life-and-Well-Being. Updated January 2018. Accessed January 31, 2018.
Health-related quality of life (HRQOL). Centers for Disease Control and Prevention website. https://www.cdc.gov/hrqol/index.htm. Updated May 27, 2016. Accessed January 31, 2018.
Kyrkou MR. Health-related quality of life. Int Public Health J. 2014;6:355.
Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622.
Chen T, Li L, Kochen MM. A systematic review: how to choose appropriate health-related quality of life (HRQOL) measures in routine general practice? J Zhejiang Univ Sci B. 2005;6:936–940.
Dapul GP, Bethoux F. Functional electrical stimulation for foot drop in multiple sclerosis. US Neurol. 2015;11:10–18.
Downing A, Van Ryn D, Fecko A, et al. Effect of a 2-week trial of functional electrical stimulation on gait function and quality of life in people with multiple sclerosis. Int J MS Care. 2014;16:146–152.
Mayer L, Warring T, Agrella S, et al. Effects of functional electrical stimulation on gait function and quality of life for people with multiple sclerosis taking dalfampridine. Int J MS Care. 2015;17:35–41.
Bulley C, Mercer TH, Hooper JE, et al. Experiences of functional electrical stimulation (FES) and ankle foot orthoses (AFOs) for foot-drop in people with multiple sclerosis. Disabil Rehabil Assist Technol. 2015;10:458–467.
Miller L, McFayden A, Lord A, et al. Functional electrical stimulation for foot drop in multiple sclerosis: a systematic review and meta-analysis of the effect on gait speed. Arch Phys Med Rehabil. 2017:98:1435–1452.
Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31:47–50.
EPHPP. Effective Public Health Practice Project. https://merst.ca/ephpp/ Published 2009. Accessed January 31, 2018.
Esnouf J, Taylor P, Mann G, Barrett C. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Mult Scler. 2010;16:1141–1147.
Taylor PN, Barrett C, Mann G, Wareham W, Swain I. A feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis: FES for dropped foot and hip stability in MS. Neuromodulation. 2014;17:75–84.
Van der Linden M, Hooper J, Cowan P, et al. Habitual functional electrical stimulation therapy improves gait kinematics and walking performance, but not patient-reported functional outcomes, of people with multiple sclerosis who present with foot-drop. PLoS One. 2014;9:e103368.
Barrett C, Taylor P. The effects of the Odstock drop foot stimulator on perceived quality of life for people with stroke and multiple sclerosis. Neuromodulation. 2010;13:58–64.
Hausmann J, Sweeney-Reed CM, Sobieray U, et al. Functional electrical stimulation through direct 4-channel nerve stimulation to improve gait in multiple sclerosis: a feasibility study. J Neuroeng Rehabil. 2015;12:100.
Taylor PN, Wikinson-Hart IA, Khan MS, et al. The correction of dropped foot due to multiple sclerosis using the STIMuSTEP implanted dropped foot stimulator. Int J MS Care. 2016;18:239–247.
Fuhrer MJ. Assessing the efficacy, effectiveness, and cost-effectiveness of assistive technology interventions for enhancing mobility. Disabil Rehabil Assist Technol. 2007;2:149–158.
Taylor P, Burridge J, Dunkerly A, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–1583.
Gray O, McDonnell G, Hawkins S. Tried and tested: the psychometric properties of the Multiple Sclerosis Impact Scale (MSIS-29) in a population-based study. Mult Scler. 2009;15:75–80.
Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011;17:604–612.
Costello L, O'Rourke K, Kearney H, et al. The patient knows best: significant change in the physical component of the Multiple Sclerosis Scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry. 2007;78:841–844.
Miller L, Rafferty D, Paul L, Mattison P. The impact of walking speed on the effects of functional electrical stimulation for foot drop in people with multiple sclerosis. Disabil Rehabil Assist Technol. 2016;11:478–483.
Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473–483.
Riazi A, Hobart JC, Lamping DL, et al. Using the SF-36 measure to compare the health impact of multiple sclerosis and Parkinson's disease with normal population health profiles. J Neurol Neurosurg Psychiatry. 2003;74:710–714.
Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics. 2004;22:27–38.
Brambilla L, Rossi Sebastiano D, Aquino D, et al. Early effect of dalfampridine in patients with MS: a multi-instrumental approach to better investigate responsiveness. J Neurol Sci. 2016;368:402–407.
Demers L, Monette M, Descent M, et al. The Psychosocial Impact of Assistive Devices Scale (PIADS): translation and preliminary psychometric evaluation of a Canadian-French version. Qual Life Res. 2002;11:583–592.
Martins AC, Pinheiro J, Farias B, et al. Psychosocial impact of assistive technologies for mobility and their implications for active ageing. Technologies. 2016;4:1–9.
Jutai J, Day H. Psychological impact of assistive devices scale: the assessment of assistive technology outcomes, effects and costs. Technol Disabil. 2002;14:107–111.
Dedding CWM, Cardol M, Eyssen ICJM, et al. Validity of the Canadian occupational performance measure: a client-centred outcome measurement. Clin Rehabil. 2004;18:660–667.
Colquhoun HL, Letts LJ, Law MC, MacDermid JC, Missiuna CA. Administration of the Canadian Occupational Performance Measure: effect on practice. Can J Occup Ther. 2012;79:120–128.
Lexell ME, Flansbjer UB, Lexell J. Self-perceived performance and satisfaction with performance of daily activities in persons with multiple sclerosis following interdisciplinary rehabilitation. Disabil Rehabil. 2014;36:373–378.
Fanelli D. Do pressures to publish increase scientists' bias? an empirical support from US States data. PLoS One. 2010;5:e10271.
Street T, Singleton C. Five-year follow-up of a longitudinal cohort study of the effectiveness of functional electrical stimulation for people with multiple sclerosis. Int J MS Care. 2018;20:224–230.







