Publication
Research Article
International Journal of MS Care
Effects of Treadmill Training on Muscle Oxidative Capacity and Endurance in People with Multiple Sclerosis with Significant Walking Limitations
Author(s):
Abstract
Background:
Exercise can improve muscle function and mobility in people with multiple sclerosis (MS). However, the effects of exercise training on skeletal muscle oxidative capacity and endurance in people with MS remain unclear, and few studies have evaluated muscle plasticity in people with MS who have moderate-to-severe disability. The present study evaluated the effects of treadmill training on muscle oxidative capacity and muscle endurance and examined the relationship to walking function in people with MS who have moderate-to-severe disability.
Methods:
Six adults (mean ± SD age, 50 ± 4.9 years) with MS (Expanded Disability Status Scale score, 6.0–6.5) performed treadmill training for 24 minutes approximately twice per week for approximately 8 weeks (16 sessions total) using an antigravity treadmill system. The following measures were taken before and after the intervention phase: muscle oxidative capacity in the medial gastrocnemius using near-infrared spectroscopy after 15 to 20 seconds of electrical stimulation; muscle endurance in the medial gastrocnemius using accelerometer-based mechanomyography during 9 minutes of twitch electrical stimulation in three stages (3 minutes per stage) of increasing frequency (2, 4, and 6 Hz); and walking function using the 2-Minute Walk Test.
Results:
Mean ± SD muscle oxidative capacity increased from 0.64 ± 0.19 min−1 to 1.08 ± 0.52 min−1 (68.2%). Mean ± SD muscle endurance increased from 80.9% ± 15.2% to 91.5% ± 4.8% at 2 Hz, from 56.3% ± 20.1% to 76.6% ± 15.8% at 4 Hz, and from 29.2% ± 13.1% to 53.9% ± 19.4% at 6 Hz of stimulation in the gastrocnemius. There were no significant improvements in walking function.
Conclusions:
Treadmill training can improve muscle oxidative capacity and endurance in people with MS who have moderate-to-severe levels of disability.
Multiple sclerosis (MS) is an autoimmune disease that causes demyelination of axons in the central nervous system. Multiple sclerosis is associated with various cognitive and physical impairments, with declines in mobility being reported as one of the most common symptoms of the disease.1,2 Moreover, reduced mobility is accompanied by decreases in physical activity and physiological deconditioning. which may contribute to the progression of physical disability in people with MS.3–5
Physiological deconditioning in MS is characterized by declines in exercise capacity, alterations in muscle phenotype, and reduced muscle function.6–10 Indeed, previous studies have shown that moderate-to-severe levels of disability are associated with a 15% to 30% reduction in aerobic capacity (VO2peak) and a 30% to 50% decrease in muscle strength compared with mild levels of disability in people with MS.6 Furthermore, VO2peak, strength, and muscle oxidative capacity are related to walking impairments in MS, suggesting that these aspects of deconditioning may be important targets for rehabilitation interventions.11–15 While exercise training has been shown to increase VO2peak in people with MS, measures of whole-body oxygen consumption are influenced by central and peripheral factors and do not directly evaluate changes in muscle-specific oxidative capacity.16–18 Muscle oxidative capacity, or the capacity to produce energy through aerobic pathways, is directly related to muscle endurance, and, therefore, may be important in interventions aiming to improve endurance in people with MS. A recent study demonstrated improved muscle metabolism with electrical stimulation training in persons with MS,19 but there is little evidence to support the use of voluntary exercise in interventions aimed at improving muscle oxidative capacity.
Few studies have evaluated muscle plasticity in individuals with MS who have moderate-to-severe levels of disability.20,21 People with MS who have moderate-to-severe levels of disability have substantial ambulatory limitations and require the use of assistive devices. Therefore, people with moderate-to-severe levels of disability may not easily or safely participate in traditional endurance exercise training interventions (walking over ground, running, cycling, etc). Previous studies evaluating endurance exercise training in populations with limited mobility have used specialized equipment such as recumbent cycles and body weight–supported treadmill systems to create adaptive exercise training programs.21–23 Body weight–supported treadmill training (BWSTT) has been shown to improve muscle strength in people with MS who have moderate-to-severe disability,21 but the effect of BWSTT on muscle oxidative capacity and endurance has not been evaluated. A novel form of BWSTT using an antigravity treadmill provides a way for people with significant disability to exercise safely without the potential discomfort of the overhead harness typically used with BWSTT. The present study aimed to evaluate the effects of antigravity treadmill training on muscle oxidative capacity, muscle endurance, and walking function in people with MS who have moderate-to-severe levels of disability. We hypothesized that antigravity treadmill training will result in improvements in muscle oxidative capacity and muscle endurance and that changes in muscle endurance will be associated with improvements in walking function.
Methods
Participants
Criteria for enrollment included a diagnosis of MS, approval for participation in exercise training by a physician, an Expanded Disability Status Scale (EDSS) score of at least 6.0, and older than 18 years. Demographic information and type of MS were assessed using a self-report questionnaire, and level of disability was evaluated using the self-administered EDSS.24 The EDSS is commonly used to characterize disability in people with MS, and, by definition, those with EDSS scores greater than 6.0 have substantial ambulatory limitations and require the use of assistive devices. This study was approved by the Research Review Committee at Shepherd Center (Atlanta, GA), and all the participants gave written informed consent before participation.
Exercise Training
The antigravity treadmill training was performed using the AlterG Anti-Gravity Treadmill system (AlterG Inc, Fremont, CA) and consisted of 16 sessions over approximately 8 weeks. Sessions were held on nonconsecutive days, two per week. Training sessions were facilitated by physical therapists and trained research personnel. Sessions included a 2-minute warm-up, a 20-minute exercise training period, and a 2-minute cool down. A rest break (3–5 minutes) was provided halfway through the exercise training period. Initial body weight support and treadmill speed were set to 50% and 0.5 mph, respectively. Heart rate and rating of perceived exertion (RPE) were measured at minutes 10 and 20 of the exercise training period. The RPE was assessed using a modified Borg scale (scoring range, 0–10).25,26 Body weight support (35%–70%) and treadmill speed (0.2–2.5 mph) were adjusted throughout the training program to maintain effort without exceeding an RPE of 8.0.
Experimental Protocols
Muscle oxidative capacity, muscle endurance, and walking function were measured before the start of the training program and within 10 days of completing the last exercise training session.
Muscle Oxidative Capacity
Muscle oxidative capacity of the medial gastrocnemius was measured using near-infrared spectroscopy (NIRS) as previously described elsewhere.27,28 In brief, NIRS signals can be used to measure changes in muscle oxygen saturation, and, during periods of ischemia, the rate of change in NIRS signals reflects the rate of oxygen metabolism.27,28 The NIRS measures of muscle oxygen metabolism can be used after a short bout of exercise to quantify muscle metabolic capacity. Immediately after exercise, oxygen metabolism is increased to restore intramuscular phosphocreatine stores, which are depleted during exercise, and the rate at which the metabolic rate returns to basal levels (phosphocreatine restored) can be used as an index of muscle oxidative capacity.29,30 Thus, NIRS can be used to evaluate the recovery of muscle metabolic rate using a series of ischemic periods after exercise.28 The rates of oxygen metabolism during a series of postexercise ischemic periods can be fitted to the exponential function: y(t) = End − Δ × e −kt. In this equation, End is the end exercise metabolic rate, and the recovery rate constant, k, is used as a measure of muscle oxidative capacity.28 The NIRS measures of oxidative capacity reflect the capacity of muscle mitochondria to produce cellular-free energy (adenosine triphosphate) using oxygen (oxidative phosphorylation), and this technique has been cross-validated with established in vivo (31P magnetic resonance spectroscopy)30 and in situ (high-resolution respirometry)31 assessments of mitochondrial oxidative phosphorylation.
The NIRS optical probe (PortaMon, Artinis Medical Systems, Zetten, the Netherlands) was placed over the medial gastrocnemius muscle with the participant in the supine position. Exercise was performed by applying 10 to 30 seconds of electrical stimulation to the gastrocnemius muscle. Intermittent ischemia was created by rapidly inflating a blood pressure cuff just proximal to the knee joint to approximately 100 mm Hg above systolic blood pressure. A series of 18 to 22 blood pressure cuff inflations were performed (5–20 seconds for each cuff) immediately after exercise to measure the recovery of oxygen consumption. Muscle oxidative capacity was quantified as the average rate constant from two recovery tests.28
Muscle Endurance
Muscle endurance was evaluated using accelerometer-based mechanomyography twitch electrical stimulation32,33 to assess skeletal muscle–specific (peripheral) endurance. Muscle contraction intensity was measured using an accelerometer (WAX9, Axivity Ltd, Newcastle upon Tyne, UK) placed on the skin over the gastrocnemius muscle using double-sided tape. Electrical stimulation was applied at three low-stimulation (twitch) frequencies (2, 4, and 6 Hz) for 3 minutes each (9 minutes total). Muscle endurance was defined as the preservation of muscle contraction intensity during repeated muscle contractions and quantified using an endurance index. The endurance index was calculated as the percentage acceleration measured at the end of each stage of frequency (2, 4, and 6 Hz) relative to peak acceleration. The reference peak acceleration for each frequency was defined as the peak acceleration measured before the end of each stage.
Walking Function
Walking function was measured using the 2-Minute Walk Test (2MWT) as previously described elsewhere.34 The 2MWT is a standard measure of the NIH Toolbox for Assessment of Neurological and Behavioral Function, and the reliability and validity of this test as a measure of walking function has been evaluated in several studies.35–37 The 2MWT measures the distance walked during a 2-minute period. Participants were permitted to use an assistive device during the 2MWT.
Data Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp, Armonk, NY). Measures of muscle oxidative capacity and walking function before and after antigravity treadmill training were compared using the paired t test. Changes in muscle endurance (endurance index) measured at each frequency of electrical stimulation before and after antigravity treadmill training were evaluated using a two-way repeated-measures analysis of variance (stimulation frequency*training). Data were evaluated for normality violations using the Shapiro-Wilk test. Significance was assumed at P < .05. All values are reported as mean ± SD unless otherwise indicated.
Results
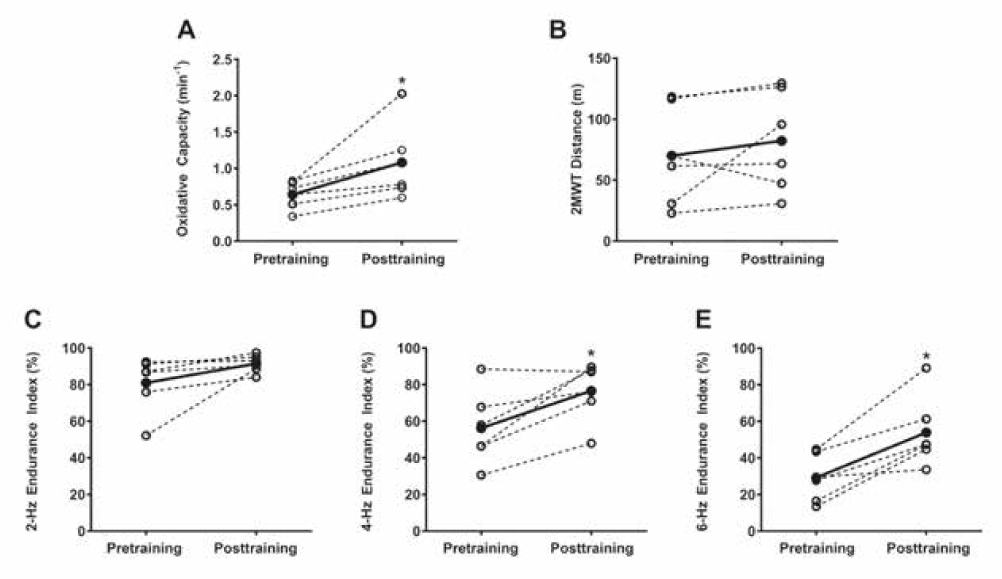
Participant characteristics are listed in Table 1. Nine people with MS were enrolled in the study: seven completed the 16 training sessions, and six participants completed all the posttraining testing sessions (Table 1). One participant performed two sessions a week for 9 weeks except for attending only one training session in weeks 5 and 9. Two participants dropped out of the study: one was injured in a car accident, causing a lapse in training, and the other experienced changes in resting blood pressure outside of appropriate study parameters. This participant was evaluated and cleared for training by a managing neurologist and a primary care physician after missing multiple sessions but did not meet the training criteria due to missed sessions. Across all antigravity treadmill training sessions, participants exercised at a mean ± SD of 55.2% ± 17.4% of their maximal heart rate (age-predicted) and reported an RPE of 5.0 ± 1.9. Treadmill walking speed ranged from 0.6 ± 0.2 mph to 1.0 ± 0.5 mph, and body weight support ranged from 36.3% ± 6.8% to 48.6% ± 3.5%. Posttraining measurements were obtained 5 to 9 days after the last day of training. Muscle oxidative capacity increased from 0.64 ± 0.19 min−1 to 1.08 ± 0.52 min−1 (68.2%; P < .05) (Figure 1A). No significant changes in walking function were found (Figure 1B). There was a main effect of treatment on muscle endurance of the gastrocnemius (P < .05), and endurance index increased from 80.9% ± 15.2% to 91.5% ± 4.8% at 2 Hz, from 56.3% ± 20.1% to 76.6% ± 15.8% at 4 Hz, and from 29.2% ± 13.1% to 53.9% ± 19.4% at 6 Hz of stimulation (Figure 1C–E).
Participant characteristics
Antigravity treadmill training
Discussion
The primary finding of the present study was that antigravity treadmill training improved muscle oxidative capacity in the gastrocnemius muscles of people with MS. Consistent with previous studies, we found that increases in muscle oxidative capacity were accompanied by increases in muscle endurance.38 The present results suggest that endurance exercise training can induce muscle plasticity in people with MS, even in the presence of moderate-to-severe disability.
The antigravity treadmill training used in the present study provided a partial weightbearing aerobic exercise stimulus to the lower-extremity muscles, and we found an approximately 68% increase in muscle oxidative capacity of the gastrocnemius after 8 weeks of training. Indeed, this increase is comparable in magnitude with previous studies reporting approximately 50% improvements in NIRS measures of muscle oxidative capacity in nondiseased individuals with 4 weeks (five times per week) of voluntary wrist flexion exercise training.39 Interestingly, we found a wide range of improvements in muscle oxidative capacity after training (22%–150%), and two participants improved their muscle oxidative capacity to values similar to those previously reported for controls (~1.7 min−1).40 Indeed, previous studies have reported that people with MS have greater variability in gait mechanics compared with controls,41–43 and, therefore, the variability in the magnitude of adaptations measured in the present study may reflect differences in lower limb muscle activation and gait mechanics among participants. Although studies using electromyography have demonstrated reduced gastrocnemius activation during push-off compared with controls,41,44 the improvement in oxidative capacity observed in the present study suggests that the voluntary activation of the gastrocnemius muscle during the antigravity treadmill training was sufficient to initiate the biochemical pathways required for mitochondrial biogenesis.45 Reductions in muscle oxidative capacity may be related to walking dysfunction in people with MS,7,13,40 and the present findings lend support to the use of antigravity treadmill training in interventions aiming to improve muscle oxidative metabolism in people with MS who have significant walking impairments.
Previous studies have reported reduced oxidative enzyme activity and declines in type I muscle fiber cross-sectional area in people with MS,46,47 suggesting a shift in muscle characteristics to favor a more glycolytic, fatigable phenotype. Yet, few studies have evaluated exercise-mediated improvements in muscle-specific endurance in this population.48 We found that antigravity treadmill training improved muscle endurance by approximately 56% on average in the medial gastrocnemius. The present findings are consistent with previous work reporting a 30% increases in cross-sectional area and a 27% increase in the proportion of type I (fatigue-resistant) muscle fibers with lower-extremity endurance training in people with MS.49 Although changes in fiber type composition were not evaluated in the present study, the observed improvements in muscle oxidative capacity and muscle endurance indicate that exercise training resulted in a more oxidative, fatigue-resistant muscle profile in these participants. These results demonstrate the plasticity of skeletal muscle in people with MS who have significant walking impairments and establish a physiological link between metabolic and functional muscle adaptations in this population.
We did not find improvements in muscle endurance to be associated with significant improvements in walking function. These findings suggest that the improvements in gastrocnemius muscle oxidative capacity and endurance observed in the present study were not sufficient to improve walking function. Notably, five of six participants improved their 2MWT distance (3.3%–212%), and, although not statistically significant, the calculated overall effect size for the 2MWT was d = 0.31. This effect size is similar in magnitude to values reported in previous studies evaluating the effect of various exercise interventions on walking function in MS (d = 0.2).50 The exercise protocol in the present study consisted of a training frequency at the lower end of recommended exercise guidelines for persons with MS (~two sessions per week),51,52 and future studies should evaluate changes in muscle and walking function associated with antigravity treadmill training paradigms of higher frequency.
There are several limitations to consider in interpreting the findings of the present study. Primarily, only six participants were tested, and a larger sample size could improve the strength of the findings. However, the present study evaluated a relatively homogenous group of participants and was sufficiently powered to evaluate changes in muscle oxidative capacity (d = 1.1) and muscle endurance (d = 1.5). Based on the effect size calculated for walking function, an additional 65 participants would be needed to achieve the adequate power for this outcome. Considering the degenerative nature of MS, it may also be difficult to interpret the magnitude of change in the outcome measures without comparison with a follow-up (detraining) measurement or a nontraining control group.39 Posttraining measurements were also obtained at variable lengths of time after the last bout of exercise (5–9 days), and the participants with the longest periods between the last training session and testing (8 and 9 days) were among the lowest responders regarding muscle oxidative capacity. Thus, the present results may have underreported the magnitude of improvements in muscle oxidative capacity and endurance in these participants.39 Although we found robust improvements in oxidative capacity and endurance in the medial gastrocnemius muscle, it should also be considered that these findings may not reflect changes in other lower-extremity muscles. Moreover, previous studies have reported asymmetrical lower-limb muscle function in people with MS,13,53 and the present study did not evaluate bilateral differences, which may have provided more insight when comparing exercise-mediated adaptations in muscle endurance and walking function.
In conclusion, antigravity treadmill training can improve muscle oxidative capacity and muscle endurance in adults with MS who have moderate-to-severe disability. Further investigation is warranted to establish the role of muscle oxidative capacity and endurance in the rehabilitation of people with MS.
PRACTICE POINTS
Antigravity treadmill systems use lower-body positive pressure to deliver body weight support, providing an opportunity for people with significant walking impairments to participate in treadmill exercise training.
Antigravity treadmill training can improve muscle oxidative capacity and muscle endurance in people with MS.
Endurance exercise training can induce muscle plasticity in people with MS, even in the presence of moderate-to-severe disability.
Financial Disclosures
Dr. McCully is the president of Infrared RX. The other authors declare no conflicts of interest.
References
Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991.
Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189–201.
Sandroff BM, Klaren RE, Motl RW. Relationships among physical inactivity, deconditioning, and walking impairment in persons with multiple sclerosis. J Neurol Phys Ther. 2015;39:103–110.
Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:2342–2348.
Motl RW, Sandroff BM, Pilutti LA, Klaren RE, Baynard T, Fernhall B. Physical activity, sedentary behavior, and aerobic capacity in persons with multiple sclerosis. J Neurol Sci. 2017;372:342–346.
Pilutti LA, Sandroff BM, Klaren RE, et al. Physical fitness assessment across the disability spectrum in persons with multiple sclerosis: a comparison of testing modalities. J Neurol Phys Ther. 2015;39:241–249.
Kent-Braun JA, Sharma KR, Miller RG, Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve. 1994;17:835–841.
Romberg A, Virtanen A, Aunola S, Karppi SL, Karanko H, Ruutiainen J. Exercise capacity, disability and leisure physical activity of subjects with multiple sclerosis. Mult Scler. 2004;10:212–218.
Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve. 2004;29:843–852.
Klaren RE, Sandroff BM, Fernhall B, Motl RW. Comprehensive profile of cardiopulmonary exercise testing in ambulatory persons with multiple sclerosis. Sports Med. 2016;46:1365–1379.
Broekmans T, Gijbels D, Eijnde BO, et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler. 2013;19:112–119.
Wetzel JL, Fry DK, Pfalzer LA. Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: performance and explanatory factors. Physiother Can. 2011;63:166–180.
Hansen D, Feys P, Wens I, Eijnde BO. Is walking capacity in subjects with multiple sclerosis primarily related to muscle oxidative capacity or maximal muscle strength? a pilot study. Mult Scler Int. 2014;2014:759030.
Agiovlasitis S, Sandroff BM, Motl RW. Prediction of oxygen uptake during walking in ambulatory persons with multiple sclerosis. J Rehabil Res Dev. 2016;53:199–206.
Harp MA, McCully KK, Moldavskiy M, Backus D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult Scler J Exp Transl Clin. 2016;2:2055217316678020.
Kerling A, Keweloh K, Tegtbur U, et al. Effects of a short physical exercise intervention on patients with multiple sclerosis (MS). Int J Mol Sci. 2015;16:15761–15775.
Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94:1800–1828.e3.
Zaenker P, Favret F, Lonsdorfer E, Muff G, de Seze J, Isner-Horobeti ME. High-intensity interval training combined with resistance training improves physiological capacities, strength and quality of life in multiple sclerosis patients: a pilot study. Eur J Phys Rehabil Med. 2018;54:58–67.
Reynolds MA, McCully K, Burdett B, Manella C, Hawkins L, Backus D. Pilot study: evaluation of the effect of functional electrical stimulation cycling on muscle metabolism in nonambulatory people with multiple sclerosis. Arch Phys Med Rehabil. 2015;96:627–632.
Filipi ML, Kucera DL, Filipi EO, Ridpath AC, Leuschen MP. Improvement in strength following resistance training in MS patients despite varied disability levels. NeuroRehabilitation. 2011;28:373–382.
Beer S, Aschbacher B, Manoglou D, Gamper E, Kool J, Kesselring J. Robot-assisted gait training in multiple sclerosis: a pilot randomized trial. Mult Scler. 2008;14:231–236.
Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18:221–229.
Skjerbaek AG, Naesby M, Lutzen K, et al. Endurance training is feasible in severely disabled patients with progressive multiple sclerosis. Mult Scler. 2014;20:627–630.
Collins CD, Ivry B, Bowen JD, et al. A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult Scler. 2016;22:1349–1358.
Morrison EH, Cooper DM, White LJ, et al. Ratings of perceived exertion during aerobic exercise in multiple sclerosis. Arch Phys Med Rehabil. 2008;89:1570–1574.
Ng AV, Dao HT, Miller RG, Gelinas DF, Kent-Braun JA. Blunted pressor and intramuscular metabolic responses to voluntary isometric exercise in multiple sclerosis. J Appl Physiol (1985). 2000;88:871–880.
Hamaoka T, Iwane H, Shimomitsu T, et al. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol (1985). 1996;81:1410–1417.
Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985). 2012;113:175–183.
Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(pt 1):C501–C510.
Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985). 2013;115:1757–1766.
Ryan TE, Brophy P, Lin CT, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592:3231–3241.
Bossie HM, Willingham TB, Van Schoick RA, O'Connor PJ, McCully KK. Mitochondrial capacity, muscle endurance and low energy in Friedreich ataxia. Muscle Nerve. 2017;56:773–779.
Willingham TB, McCully KK. Assessment of skeletal muscle endurance using twitch electrical stimulation and accelerometer-based mechanomyography. Adv Skeletal Muscle Function Assessment. 2017;1:9–15.
Gijbels D, Alders G, Van Hoof E, et al. Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler. 2010;16:618–626.
Carlozzi NE, Goodnight S, Casaletto KB, et al. Validation of the NIH Toolbox in individuals with neurologic disorders. Arch Clin Neuropsychol. 2017;32:555–573.
Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, Wagster MV. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 2010;9:138–139.
Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(suppl 3):S65–S75.
Southern WM, Ryan TE, Kepple K, Murrow JR, Nilsson KR, McCully KK. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol Rep. 2015;3:e12353.
Ryan TE, Southern WM, Brizendine JT, McCully KK. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc. 2013;45:2346–2352.
Harp MA, McCully KK, Moldavskiy M, Backus D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult Scler. 2016;2:1–7.
Lencioni T, Jonsdottir J, Cattaneo D, et al. Are modular activations altered in lower limb muscles of persons with multiple sclerosis during walking? evidence from muscle synergies and biomechanical analysis. Front Hum Neurosci. 2016;10:620.
Kalron A. Gait variability across the disability spectrum in people with multiple sclerosis. J Neurol Sci. 2016;361:1–6.
Socie MJ, Motl RW, Pula JH, Sandroff BM, Sosnoff JJ. Gait variability and disability in multiple sclerosis. Gait Posture. 2013;38:51–55.
Ravera EP, Crespo MJ, Braidot AA. Estimation of muscle forces in gait using a simulation of the electromyographic activity and numerical optimization. Comput Methods Biomech Biomed Engin. 2016;19:1–12.
Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472.
Kent-Braun JA, Ng AV, Castro M, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985). 1997;83:1998–2004.
de Haan A, de Ruiter CJ, van Der Woude LH, Jongen PJ. Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve. 2000;23:1534–1541.
Surakka J, Romberg A, Ruutiainen J, et al. Effects of aerobic and strength exercise on motor fatigue in men and women with multiple sclerosis: a randomized controlled trial. Clin Rehabil. 2004;18:737–746.
Wens I, Dalgas U, Vandenabeele F, et al. High intensity exercise in multiple sclerosis: effects on muscle contractile characteristics and exercise capacity, a randomised controlled trial. PLoS One. 2015;10:e0133697.
Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. 2009;23:108–116.
Dalgas U, Ingemann-Hansen T, Stenager E. Physical exercise and MS recommendations. Int MS J. 2009;16:5–11.
Latimer-Cheung AE, Martin Ginis KA, Hicks AL, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:1829–1836.e7.
Larson RD, McCully KK, Larson DJ, Pryor WM, White LJ. Lower-limb performance disparities: implications for exercise prescription in multiple sclerosis. J Rehabil Res Dev. 2014;51:1537–1544.







