Publication
Research Article
International Journal of MS Care
Muscle Dysfunction and Walking Impairment in Women with Multiple Sclerosis
Author(s):
Abstract
Background:
Recent evidence suggests that skeletal muscle dysfunction is involved in disability progression in people with multiple sclerosis (MS). However, the relationship between muscle dysfunction and walking impairments in MS remains unclear. Thus, the cross-sectional relationships between muscle-specific oxidative capacity and walking endurance in women with MS were evaluated.
Methods:
Twenty women with MS (11 African American, 9 white) were tested. Muscle oxidative capacity of the medial gastrocnemius was measured using near-infrared spectroscopy after electrical stimulation. Muscle endurance was evaluated using accelerometer-based mechanomyography during electrical stimulation. Muscle strength was measured during maximal voluntary plantarflexion using handheld dynamometry. Walking function was measured using the Timed 25-Foot Walk test and the 6-Minute Walk Test (6MWT).
Results:
Reduced muscle oxidative capacity (R 2 = 0.68–0.71, P < .01) and muscle endurance (R 2 = 0.59–0.78, P < .01) were associated with lower Timed 25-Foot Walk time and 6MWT distance. Muscle strength was weakly correlated to 6MWT distance (R 2 = 0.21, P = .02). No differences in muscle function or clinical outcome measures were found between African American and white subgroups. Women with moderate-to-severe disability (Expanded Disability Status Scale [EDSS] score, 5.0–6.5) had significantly reduced muscle oxidative capacity, muscle endurance, and walking ability compared with women with mild disability (EDSS score, 2.5–4.5).
Conclusions:
Reductions in muscle function in people with MS are related to declines in walking function across all levels of disability. Muscle dysfunction is not differentially related to walking impairment in African American and white women with MS.
Multiple sclerosis (MS) is a degenerative autoimmune disease characterized by demyelination in the central nervous system. Multiple sclerosis is estimated to affect more than 2 million people worldwide, with women being three times more likely to develop MS than men.1 Historically, people of European descent were thought to be at higher risk for developing MS compared with other ethnic groups; however, contemporary studies have found that African American people may have higher incidence rates than white Americans.2 3 Moreover, some evidence suggests that African Americans with MS may experience a more rapid disease course,4–8 but the mechanisms are unknown.9 10 Despite increasing awareness surrounding the importance of diversity in clinical research, minority populations have been largely underrepresented in MS research, and the predominance of women in the MS population further advocates for studies focusing on underrepresented subgroups of women with MS.1 9
Although marked heterogeneity exists in the rate of disease progression and the nature of impairments associated with MS, reduced walking ability is among the most common complications reported by people with MS.11 Impairments in walking function occur early in disease onset and continue to decline with the progression of disability in people with MS.12 Furthermore, reductions in mobility in people with MS can result in decreased participation in physical activity and physiological deconditioning.13 Consequences of deconditioning, such as reduced exercise capacity and skeletal muscle dysfunction, are related to walking impairments in people with MS, suggesting that peripheral mechanisms may contribute to the development of walking impairments in this population.14–17
Skeletal muscle dysfunction associated with MS is characterized by declines in muscle strength,14 muscle mass,18 and muscle oxidative capacity.17 However, there is a lack of agreement with respect to which aspects of muscle dysfunction are most closely related to walking function in people with MS, and the relationship between muscle dysfunction and walking impairment remains unclear.14 16 Some studies have found lower extremity muscle strength to be a major predictor of walking function,14 whereas other studies have found that walking function may be more closely related to muscle oxidative capacity.16 Elucidating the relationships between skeletal muscle dysfunction and walking impairment in people with MS could potentially identify novel physiological targets for rehabilitation interventions. Moreover, few studies have evaluated skeletal muscle dysfunction in people with MS who have significant ambulatory limitations (Expanded Disability Status Scale [EDSS] score ≥ 5.0), which may be important in understanding the role of muscle dysfunction in disability progression in people with MS.
A limitation to previous studies evaluating muscle metabolic dysfunction in people with MS is that the methods of approach have not evaluated muscle-specific oxidative capacity. For example, measures of whole-body oxygen consumption (eg, peak oxygen consumption [VO2peak]) and onset kinetics have been shown to correlate with measures of walking function in people with MS, but these measures can be influenced by impairments in cardiovascular capacity or central muscle activation and may not reflect muscle-specific oxidative capacity in the presence of pathology.16 Near-infrared spectroscopy (NIRS) has been used to evaluate muscle-specific oxidative function in clinical populations, and studies using NIRS in people with MS suggest that muscle metabolism may be related to walking impairments.17 However, the applications of NIRS technology and assessments of walking function in these studies have varied, thus, the relationship between muscle-specific oxidative capacity and walking function is unclear and warrants further investigation.17
The purpose of the present study was to characterize the relationship between muscle dysfunction and walking impairment across the spectrum of disability in women with MS. In addition, the relationship between measures of muscle oxidative capacity and muscle endurance was evaluated to establish a link between muscle metabolism and muscle endurance in people with MS. As a secondary aim, the present study evaluated differences in muscle function between African American and white women with MS. It was hypothesized that measures of muscle oxidative capacity and muscle endurance would be correlated to each other and to measures of walking function and that people with moderate-to-severe disability (EDSS score = 5.0–6.5) would have reduced muscle oxidative capacity and muscle endurance compared with people with mild disability (EDSS score = 2.5–4.5).
Methods
Participants
Participants were recruited through the Shepherd Center in Atlanta, Georgia. Women older than 18 years with a diagnosis of MS were included in the study. Participants with acute deep vein thrombosis, open wounds, and unhealed fractures were excluded from the study. Time since diagnosis, type of MS, duration of trouble walking, race, and age were self-reported by participants. All tests were performed during a single visit, with assessments separated by a 60-minute rest break. This study was approved by the Shepherd Center research review committee and the institutional review board of the University of Georgia. All the participants provided written informed consent before participating in any study procedures. All the participants enrolled in the study completed all the outcome measures.
Muscle Oxidative Capacity
Muscle oxidative capacity was noninvasively evaluated using NIRS,19 which relies on the optical properties of hemoglobin to noninvasively assess muscle oxygen saturation using the reflectance of near-infrared light. Changes in the NIRS oxygen signals during limb ischemia can be used to evaluate the rate of oxygen metabolism,19 20 and recent applications have used NIRS to evaluate oxygen recovery kinetics after a bout of exercise. After brief exercise, oxygen consumption is increased to replenish intramuscular phosphocreatine, and the rate at which oxygen consumption returns to basal levels can be used as an index of muscle oxidative capacity.21 22 Herein, the NIRS device (PortaMan; Artinis Medical Systems, Einsteinweg, the Netherlands) was placed at the greatest circumference of the medial head of the gastrocnemius for each participant, and a cuff (20c/d; D. E. Hokanson Inc, Bellevue, WA) was placed proximal to the knee joint. Surface neuromuscular electrical stimulation (Theramini 2; Richmar, Chattanooga, TN) was applied to the gastrocnemius muscle by placing electrodes (SuperStim, Richmar) (7.62 × 12.7 cm) just proximal and distal to the NIRS optode for 15 to 20 seconds (4 Hz). After electrical stimulation, a brief arterial occlusion was performed by rapidly inflating the cuff (5–10 seconds) using a rapid cuff inflation system. Post–electrical stimulation oxygen metabolism (end) was calculated as the rate of change in the NIRS signal during this initial arterial occlusion. A series of brief arterial occlusions were performed to evaluate the recovery of muscle oxygen metabolism after electrical stimulation. The rate of recovery of muscle oxygen metabolism was quantified by fitting the oxygen metabolism rates to the exponential equation y(t) = End − Δ × e −kt. The rate constant, k, was used as an index of muscle oxidative capacity. The NIRS protocol was performed twice at the left and right medial gastrocnemius muscle, and the average rate constant was used to quantify muscle oxidative capacity.
Muscle Endurance
Muscle-specific endurance was quantified as the ability to sustain muscle contraction intensity during repeated muscle contractions. Muscle contraction intensity was measured using a wireless, triaxial accelerometer (WAX9; Axivity Ltd, Newcastle upon Tyne, UK) during twitch electrical stimulation as previously described.23 24 In brief, the accelerometer was placed on the surface of the skin at the greatest circumference of the medial head of the gastrocnemius using double-sided adhesive tape. Acceleration was measured during 9 minutes of electrical stimulation. The electrical stimulation included three stages (3 minutes per stage) of increasing frequency (2, 4, and 6 Hz), with each stage separated by 3 to 5 seconds of no stimulation. Muscle twitch acceleration was calculated as the peak-to-peak magnitude of resultant acceleration from all three axes of acceleration.24 Muscle endurance was quantified using a muscle endurance index (EI), calculated as the percentage of muscle twitch acceleration measured at the end of each stage of frequency relative to the previous peak acceleration (EI = [Aend /Apeak ] × 100). Therefore, greater EI values reflect greater muscle endurance.
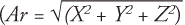
Muscle Strength
Muscle strength was quantified as the peak force produced during maximal voluntary contraction of ankle plantar flexors. Force was measured using handheld dynamometry (Baseline; Fabrication Enterprises, White Plains, NY).25 Left and right ankle plantarflexion maximal voluntary contraction was measured twice with the participant in the supine position and the ankle at 45°. The average of two trials was recorded.
Walking Function
Walking function was evaluated by quantifying walking speed, walking endurance, and perception of walking ability. Walking speed was measured using the Timed 25-Foot Walk (T25FW) test, and walking endurance was measured using the 6-Minute Walk Test (6MWT) as previously described.26 27 The T25FW test measures walking speed as the velocity calculated from the time elapsed during a 25-foot walk. The 6MWT evaluates the ability to maintain walking speed over time by measuring the distance walked during a 6-minute period. The participant was permitted the use of a walking aid during the T25FW test and the 6MWT. Perception of walking ability was measured using the 12-item Multiple Sclerosis Walking Scale,27 a self-report questionnaire about the perception of the impact of MS on walking ability.
Perceived Fatigue, Physical Activity, and Disability
Perceptions of fatigue were measured using the 5-item Modified Fatigue Impact Scale, a self-report questionnaire with questions pertaining to perceptions of the impact of MS on physical, cognitive, and psychosocial aspects of fatigue. Physical activity was measured using the International Physical Activity Questionnaire survey. Metabolic equivalent of task (MET)–minutes per week (MET min*week−1) were calculated by multiplying the previously established MET values for vigorous activity (MET = 8.0), moderate activity (MET = 4.0), and walking (MET = 3.3) by the minutes and days of reported participation for each activity.28–30 Disability level was measured using the self-administered EDSS, which has been shown to be highly correlated with the physician-administered EDSS.31 The EDSS scores level of disability from 0 (no disability) to 10 (death due to MS). The EDSS considers many domains of disability, including bowel/bladder function, speech, visual function, and mobility. With respect to ambulation, EDSS scores of 5.0 or greater imply that ambulation is impaired.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp, Armonk, NY). Differences between measurements at different levels of disability were identified using one-way analysis of variance. A two-way repeated-measures analysis of variance was performed to identify differences in EI at the different electrical stimulation frequencies during the endurance test. Simple linear regression analysis was performed to evaluate relationships between measures of muscle function, walking function, and self-reported outcomes. Differences in correlations among variables were compared between African American and white subgroups using Fisher z tests. Data are reported as mean ± SD unless otherwise specified. All the muscle measurements are reported as bilateral averages. Significance was accepted at P < .05 for all correlations and comparisons.
Results
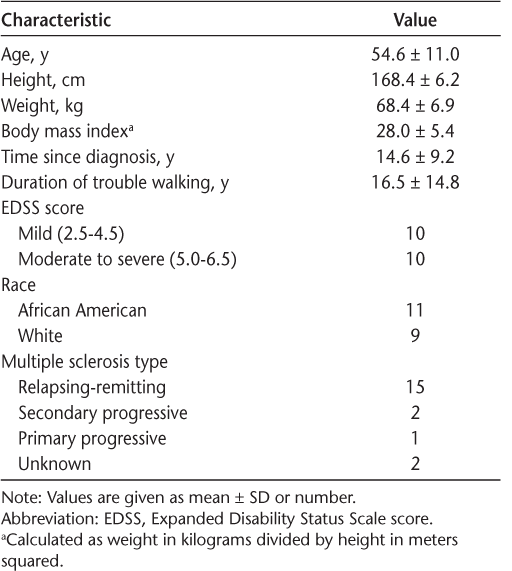
Twenty women with MS (age range, 34–71 years; 11 African American, 9 white) were tested. Other participant characteristics are listed in Table 1.
Characteristics of the 20 study participants
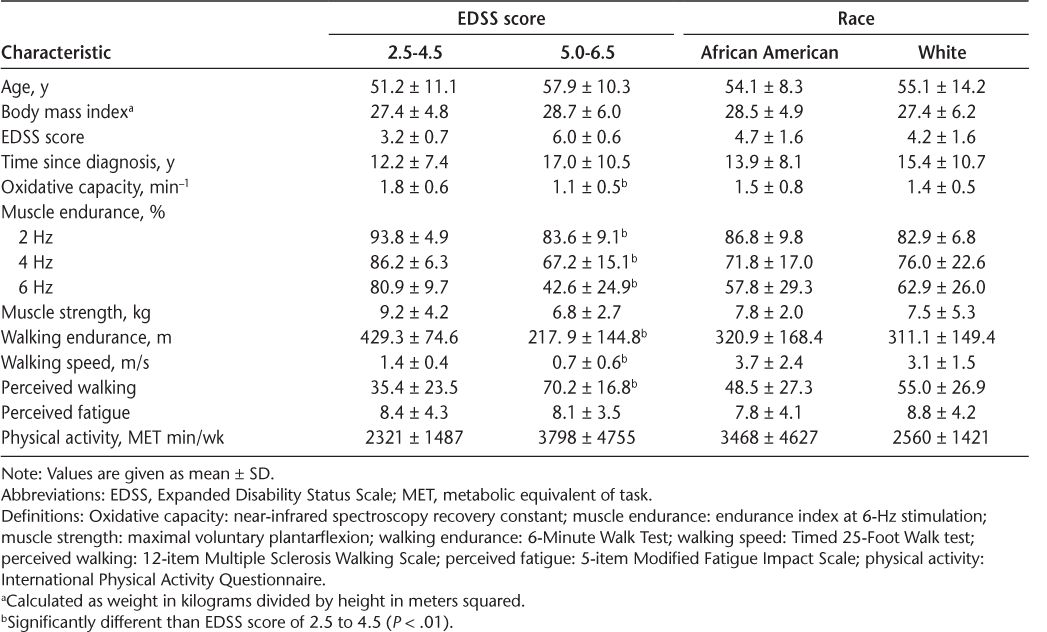
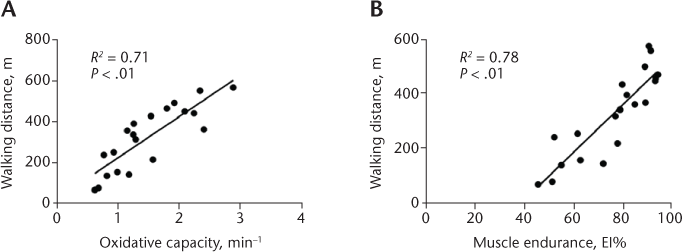
Walking endurance and walking speed were significantly lower in people with moderate-to-severe disability (EDSS score = 5.0–6.5) compared with people with mild disability (EDSS score = 2.5–4.5) (Table 2). Five participants with moderate-to-severe disability used an assistive device for walking. Muscle oxidative capacity and measures of muscle endurance measured at 2-, 4-, and 6-Hz electrical stimulation were all significantly lower in people with moderate-to-severe disability compared with people with mild disability (P < .01) (Table 2). Across all participants, reduced muscle oxidative capacity was associated with lower walking endurance (R 2 = 0.71, P < .01) (Figure 1) and slower walking speed (R 2 = 0.68, P < .01). Measures of muscle endurance at 2-, 4-, and 6-Hz electrical stimulation were all correlated with walking endurance (R 2 = 0.7–0.8, P < .01) and walking speed (R 2 = 0.6–0.7, P < .01). Muscle strength was weakly correlated to walking endurance as measured by the 6MWT (R 2 = 0.21, P = .02) (Table 3). In women of similar age, diagnosis, and body composition, no significant differences were found in muscle function or walking ability between African American and white women with MS (P > .05) (Table 2). Moreover, correlations between measures of muscle function and walking ability were not different between these groups (P > .05).
Differences between levels of disability and between racial subgroups
Relationships between muscle dysfunction and walking endurance in 20 people with multiple sclerosis (Expanded Disability Status Scale score = 2.5–6.5)
Correlations (R 2 ) between outcome measures
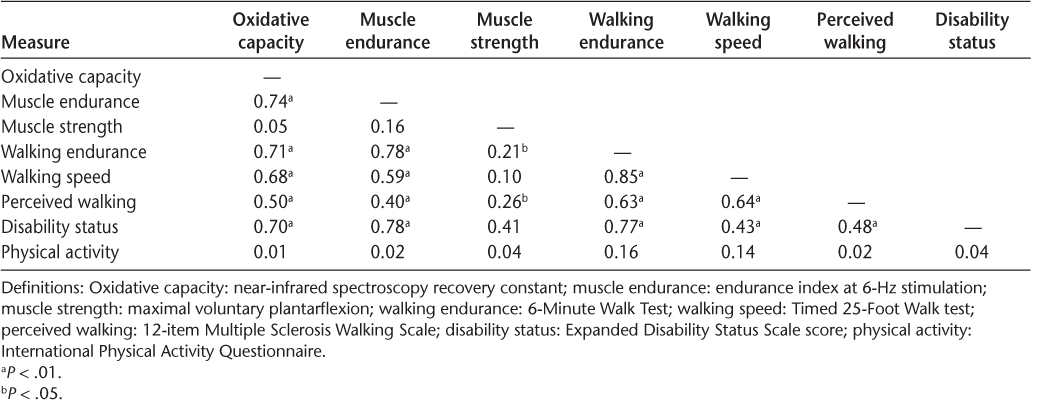
People with moderate-to-severe disability (EDSS score = 5.0–6.5) reported significantly lower perceptions of walking ability compared with people with mild disability (EDSS score = 2.5–4.5) (Table 2). However, no differences were found in perceptions of fatigue (Table 2). Notably, self-reported physical activity was not significantly different between people with moderate-to-severe disability and people with mild disability. Muscle oxidative capacity (R 2 = 0.50, P < .01), muscle strength (R 2 = 0.26, P < .01), and measures of endurance at 2-, 4-, and 6-Hz electrical stimulation (R 2 = 0.3–0.4, P < .01) were all correlated to perceptions of walking ability (Table 3). No differences in measures of perceived walking ability, perceived fatigue, or physical activity were found between African American and white women with MS.
Discussion
The primary finding of the present study was that muscle-specific oxidative capacity and endurance are related to walking function and perceptions of walking impairment in people with MS across a wide range of functional statuses. Likewise, people with more severe disability were found to have more severe muscle dysfunction. These results provide evidence to link reductions in muscle oxidative metabolism to muscle endurance, and ultimately physical function, in people with MS.
Declines in walking function in people with MS are associated with skeletal muscle dysfunction, reductions in motor control, and exercise capacity, but the exact mechanisms underlying walking impairments are unknown.14 16 32 To our knowledge, this is the first study to evaluate the relationship between muscle-specific oxidative capacity and walking function in people with MS, and the present results suggest that muscle oxidative capacity is closely related to walking endurance, walking speed, and perceived walking ability. Although these findings are in agreement with previous studies demonstrating the relationship between whole-body measures of oxidative capacity and walking endurance,16 33 the muscle-specific assessment of oxidative capacity used in the present study establishes a clear relationship between skeletal muscle metabolic dysfunction and walking impairment. Moreover, there is limited evidence characterizing oxidative capacity in people with MS who have higher levels of disability (EDSS score ≥ 5.0), and we believe the present study is the first to evaluate muscle-specific oxidative metabolic capacity in people with MS who have moderate-to-severe levels of disability (EDSS score = 5.0–6.5). We found that participants with moderate-to severe levels of disability had oxidative capacity values approximately 35% lower than previously reported control values and more similar to values previously reported in individuals with spinal cord injury (~0.7 min−1).34 Previous studies using 31P magnetic resonance spectroscopy to evaluate muscle-specific oxidative capacity have reported an approximately 50% reduction in people with MS (EDSS score = 2.5–8.0),15 35 but the relationship between muscle dysfunction and level of disability was not evaluated. Interestingly, participants in the present study with lower levels of disability (EDSS score = 2.5–4.5) had oxidative capacity values similar to those previously reported for controls (~1.7 min−1).17 These findings suggest that muscle oxidative capacity may be maintained at lower levels of disability (where ambulation is unaffected), despite the presence of deficits in bowel/bladder function, speech, and visual function. Furthermore, the strong relationship between muscle oxidative capacity and walking endurance lends support to the use of exercise interventions that target muscle oxidative capacity in people with MS who have walking impairments.
Reductions in lower extremity muscle endurance and strength have been reported in MS.15 36 37 However, findings from studies evaluating muscle endurance in people with MS have varied, and the peripheral mechanisms of muscle dysfunction in MS are still not completely understood.15 36 37 The relationship between measures of muscle endurance and muscle oxidative capacity in the present study suggests that alterations in muscle bioenergetics contribute to declines in muscle endurance in people with MS. Indeed, previous studies have reported that shifts in muscle fiber composition associated with MS favor a more glycolytic, fatigable phenotype, but these studies did not evaluate the relationship between changes in muscle fiber characteristics and muscle endurance.15 36 In addition, we found reductions in muscle endurance to be moderately correlated with declines in walking function, higher ratings of perceived walking impairment, and higher levels of disability. Comparably, Kalron et al38 found a moderate correlation between indexes of plantarflexion muscle endurance and gait parameters in people with clinically isolated syndrome. In another study of people with mild (EDSS score = 1.5–4.0) and moderate MS (EDSS score = 4.5–6.5), Broekmans et al14 also reported that knee extension muscle endurance was moderately correlated with measures of walking speed and endurance. Previous studies have also reported strong relationships between muscle strength and walking function in people with MS,14 38 and although plantarflexion strength was only weakly correlated to walking endurance in the present study, we found measures of strength to be moderately related to perceived walking ability. Taken together, these data suggest that both muscle endurance and strength are related to walking dysfunction in people with MS.
There is increasing interest surrounding variations in disease progression between different racial/ethnic populations with MS,9 and recent evidence indicates that African Americans have a more rapid rate of disease progression compared with white Americans.4–8 10 For example, Cree et al4 reported that African Americans with MS had an increased risk of developing walking dysfunction and an approximately l.7-fold increase in risk of requiring an assistive device to ambulate compared with white Americans with MS. Although muscle dysfunction has been shown to be associated with declines in mobility in people with MS, there are seemingly no studies to date evaluating differences in muscle function between African American and white subgroups in people with MS.14–16 An important finding of the present study was that no differences in muscle function were found between African American and white women with MS who had similar degrees of walking impairment. In addition, the relationships between measures of muscle function and walking ability across a wide range of functional statuses were consistent between African American and white women of similar age and diagnosis. These findings suggest that skeletal muscle (peripheral) dysfunction may not be differentially related to the progression of physical disability in African American and white women with MS. Future longitudinal studies evaluating muscular and neurologic dysfunction in people with MS will be important in identifying the physiological mechanisms related to the heterogeneity in disease progression rate in racial/ethnic subgroups with MS.
The limitations of the present study should be considered. Muscle function and walking ability were evaluated only in women participants to allow for comparison between levels of disability without the confounding influence of sex. Previous studies have reported differences in muscle function between males and females, and the lack of male participants should be considered in the interpretation of the findings.37 However, the participant pool represents a large range of walking functions and disability levels (EDSS score = 2.5–6.5), and measures of walking endurance and speed in the present study are similar to those previously reported for men and women with MS.26 27 The medial gastrocnemius muscle was evaluated in the present study because of the critical role of this muscle in ambulation and its association with gait parameters.38 39 Yet, note that many other lower extremity and trunk muscles contribute to ambulation,39 and relationships between muscle function and walking ability may vary if assessed in different lower extremity muscles. In addition, muscle strength was evaluated during voluntary contractions, which can be influenced by both central and peripheral factors, so the observations related to strength cannot be solely attributed to peripheral mechanisms associated with skeletal muscle dysfunction. Multiple raters were also used to evaluate muscle strength, and previous studies have found high interrater variably in measures of strength using handheld dynamometry in people with MS.40 Therefore, the relationships between muscle strength and measures of walking function reported in the present study may be influenced by interrater variability. Consistent with previous studies using the International Physical Activity Questionnaire to evaluate physical activity in people with MS,29 41 metabolic expenditure was calculated as MET min*week−1 using MET estimates for vigorous activity (MET = 8.0), moderate activity (MET = 4.0), and walking (MET = 3.3).28 However, studies have shown that people with MS may not achieve the high metabolic expenditure rates42 43 commonly associated with vigorous activity (8 METs) and that gait abnormalities in people with moderate disability can be associated with increased oxygen consumption during walking.44 Thus, estimations of metabolic expenditure using self-reported questionnaires in this population may overestimate and underestimate metabolic equivalents associated with vigorous activities and walking, respectively. Certainly, previous studies have reported relationships between markers of muscle oxidative metabolism and measures of physical activity in people with MS,15 and further investigation is warranted to determine the role of inactivity in the development of muscle dysfunction in this population.
In conclusion, skeletal muscle oxidative capacity and endurance are related to walking function in people with MS. Although people with moderate-to-severe disability (EDSS score = 5.0–6.5) had significantly reduced muscle function and walking ability compared with people with mild disability (EDSS score = 2.5–4.5), no differences in muscle function were found between African American and white women with MS of similar walking ability. The present findings suggest that muscle metabolic dysfunction is related to walking impairment in people with MS and identify muscle oxidative capacity and endurance as potential therapeutic targets for interventions aimed at improving walking function in this population.
PRACTICE POINTS
In 20 women with MS, we studied associations among muscle oxidative capacity (recovery of muscle oxygen metabolism), muscle endurance (ability to sustain muscle contraction intensity), and measures of walking.
Muscle oxidative capacity and muscle endurance are related to walking impairment and perceptions of walking impairment in people with MS across a wide range of functional statuses.
Although recent evidence indicates that African Americans have a more rapid rate of disease progression compared with white Americans, muscle oxidative capacity and muscle endurance were not different between African American and white women with MS with similar degrees of walking impairment, and the relationships between measures of muscle function and walking ability were consistent between African American and white women similar in age and diagnosis.
Financial Disclosures
Dr. McCully is the president of Infrared Rx. The other authors declare no conflicts of interest.
References
Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8:255–263.
Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013;80:1734–1739.
Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(pt 6):1778–1785.
Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63:2039–2045.
Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75:217–223.
Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler. 2006;12:775–781.
Kaufman MD, Johnson SK, Moyer D, Bivens J, Norton HJ. Multiple sclerosis: severity and progression rate in African Americans compared with whites. Am J Phys Med Rehabil. 2003;82:582–590.
Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult Scler. 2003;9:293–298.
Khan O, Williams MJ, Amezcua L, Javed A, Larsen KE, Smrtka JM. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract. 2015;5:132–142.
Weinstock-Guttman B, Ramanathan M, Hashmi K, et al. Increased tissue damage and lesion volumes in African Americans with multiple sclerosis. Neurology. 2010;74:538–544.
Motl RW, Learmonth YC. Neurological disability and its association with walking impairment in multiple sclerosis: brief review. Neurodegener Dis Manag. 2014;4:491–500.
Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006;12:620–628.
Backus D. Increasing physical activity and participation in people with multiple sclerosis: a review. Arch Phys Med Rehabil. 2016;97(suppl):S210–S217.
Broekmans T, Gijbels D, Eijnde BO, et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler. 2013;19:112–119.
Kent-Braun JA, Ng AV, Castro M, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985). 1997;83:1998–2004.
Hansen D, Feys P, Wens I, Eijnde BO. Is walking capacity in subjects with multiple sclerosis primarily related to muscle oxidative capacity or maximal muscle strength? a pilot study. Mult Scler Int. 2014;2014:759030.
Harp MA, McCully KK, Moldavskiy M, Backus D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult Scler. 2016;2:1–7.
Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve. 2003;27:456–464.
Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985). 2012;113:175–183.
Hamaoka T, Iwane H, Shimomitsu T, et al. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol (1985). 1996;81:1410–1417.
Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(pt 1):C501–C510.
Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985). 2013;115:1757–1766.
Bossie HM, Willingham TB, Schoick RAV, O'Connor PJ, McCully KK. Mitochondrial capacity, muscle endurance and low energy in Friedreich ataxia. Muscle Nerve. 2017;56:773–779.
Willingham TB, McCully KK. Assessment of skeletal muscle endurance using twitch electrical stimulation and accelerometer-based mechanomyography. Adv Skeletal Muscle Funct Assess. 2017;1:9–15.
Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10:e0140822.
Gijbels D, Eijnde BO, Feys P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult Scler. 2011;17:1269–1272.
Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390.
Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395.
Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Community Health. 2007;30:144–150.
Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 2006;28:1151–1156.
Collins CD, Ivry B, Bowen JD, et al. A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult Scler. 2016;22:1349–1358.
Thoumie P, Lamotte D, Cantalloube S, Faucher M, Amarenco G. Motor determinants of gait in 100 ambulatory patients with multiple sclerosis. Mult Scler. 2005;11:485–491.
Klaren RE, Sandroff BM, Fernhall B, Motl RW. Comprehensive profile of cardiopulmonary exercise testing in ambulatory persons with multiple sclerosis. Sports Med. 2016;46:1365–1379.
Willingham TB, McCully KK. In vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy. Front Physiol. 2017;8:689.
Hansen D, Wens I, Vandenabeele F, Verboven K, Eijnde BO. Altered signaling for mitochondrial and myofibrillar biogenesis in skeletal muscles of patients with multiple sclerosis. Transl Res. 2015;166:70–79.
Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil. 2005;86:224–229.
Skurvydas A, Brazaitis M, Andrejeva J, Mickeviciene D, Streckis V. The effect of multiple sclerosis and gender on central and peripheral fatigue during 2-min MVC. Clin Neurophysiol. 2011;122:767–776.
Kalron A, Achiron A, Dvir Z. Muscular and gait abnormalities in persons with early onset multiple sclerosis. J Neurol Phys Ther. 2011;35:164–169.
Lencioni T, Jonsdottir J, Cattaneo D, et al. Are modular activations altered in lower limb muscles of persons with multiple sclerosis during walking? evidence from muscle synergies and biomechanical analysis. Front Hum Neurosci. 2016;10:620.
Toomey E, Coote S. Between-rater reliability of the 6-minute walk test, Berg balance scale, and handheld dynamometry in people with multiple sclerosis. Int J MS Care. 2013;15:1–6.
Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012;126:256–262.
Langeskov-Christensen M, Heine M, Kwakkel G, Dalgas U. Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med. 2015;45:905–923.
Heine M, Hoogervorst EL, Hacking HG, Verschuren O, Kwakkel G. Validity of maximal exercise testing in people with multiple sclerosis and low to moderate levels of disability. Phys Ther. 2014;94: 1168–1175.
Sebastiao E, Bollaert RE, Hubbard EA, Motl RW. Gait variability and energy cost of oveground walking in persons with multiple sclerosis: a cross-sectional study. Am J Phys Med Rehabil. 2018;97:646–650.







