Publication
Research Article
International Journal of MS Care
Effect of Cognitive Demand on Functional Mobility in Ambulatory Individuals with Multiple Sclerosis
Author(s):
In older adults and people with neurologic diseases, cognitive demands from everyday tasks might interfere with their functional mobility. People frequently perform functional mobility (eg, walking, standing, and turning) while simultaneously engaging in other tasks (eg, talking or listening). A key focus of rehabilitation interventions is to improve safety in ambulation and to reduce the likelihood of falls, both of which may be affected by reduced ability to perform dual tasks.
In people with multiple sclerosis (MS), assessing the impact of cognitive demands on functional ability is an important aspect of physical rehabilitation. It is known that individuals with MS often develop cognitive impairments,1–7 and estimates of the prevalence of those impairments vary from 43% to 70% depending on the progression of the disease.3–5 Although many domains of cognition may be affected, one of the most affected in people with MS is the ability to rapidly process information.6–9 This ability has been linked to working memory, another cognitive construct that is also frequently affected in MS.10–12 Much of the research on working memory in people with MS has compared the ability to perform rapidly or accurately under a high working memory load.10–12 The prevailing hypothesis is that people with MS have deficits in processing speed and working memory, both of which negatively affect performance as task difficulty increases. However, the implications for functional mobility (eg, ambulation and balance) in a person with MS are overlooked by assessments that focus predominantly on cognitive processes.
One model for assessing the effects of increased demands on cognitive resources is to measure a person's performance on a dual-task activity (ie, performing two or more tasks concurrently). If performance scores on one or both tasks are lower when performed simultaneously than when performed individually, the two tasks are assumed to interfere with each other. A useful aspect of this model is that it can be related to functional motor abilities. Research has demonstrated that there is reduced dual-tasking ability in people with central nervous system neurologic disorders, such as Alzheimer's disease13 and traumatic brain injury.14 Using a dual-task paradigm, Hamilton et al.15 demonstrated that performing a cognitive task while walking resulted in decreased walking speed and reduced the accuracy of the cognitive task in 18 people with MS compared with controls. A systematic review including 21 studies encompassing more than 800 participants concluded that individuals with MS reduce their gait speed while engaged in a concurrent cognitive task.16 It was also noted that most of these investigations focused on steady-state straight-line walking. Everyday living requires movements that go beyond solely walking for functional mobility, including turning, standing up, and sitting down, for example. Therefore, functional mobility tests should incorporate such tasks. In addition, the results of those tests may be affected by the severity of MS.
We sought to further understand the impact of cognitive demands on functional mobility of people with MS having different levels of disability. To achieve this objective, we used the Timed Up and Go (TUG) test,17 a functional measure that incorporates standing up, walking, turning, and sitting down. The TUG test has been used with older adults and individuals with neurologic disorders (see the review in the Rehabilitation Measures Database, for example).18 The TUG test was applied in single- and dual-task paradigms in individuals with MS at different levels of the Expanded Disability Status Scale (EDSS).19 In addition, we compared people with MS with controls to assess whether and how the two groups differ on functional mobility when performing tasks of increasing cognitive demand.
Methods
Participants
Two groups of individuals were recruited. Participants in the MS group (n = 52) came from a pool of community-dwelling individuals with MS who were recruited through multiple sources, including the University of Washington Multiple Sclerosis Clinic (UWMSC, previously known as Western MS Center), MS newsletter, MS Society website, and flyers (placed throughout the university, including the health clinics). To be included, participants had to have a confirmed diagnosis of MS at the UWMSC using either the McDonald or revised McDonald criteria, be 18 years or older, be able to ambulate at least 300 feet, and have an EDSS score of 6 or less. Potential participants were excluded if they could not read and complete forms in English. Participants in the control group (n = 57) were recruited from friends and families of the participants with MS, including individuals who accompanied patients to the MS clinics, and through flyers placed throughout the university. Individuals were considered eligible if they were aged 18 years or older and responded “yes” to the question: “Do you believe you are healthy?” We also sought to have controls that would make the age and sex distributions of the patient and control groups similar to each other, although we did not match them in pairs.
A research assistant conducted a brief interview (by telephone or in person) to determine the eligibility of each individual, and those who consented to participate came to the UWMSC for the procedures. All testing was performed at a single visit that lasted approximately 1 hour, and individuals were compensated for their participation. Study procedures were approved by the University of Washington institutional review board.
No previous sample size was calculated, and we collected data from a convenience sample recruited between December 1, 2005, and May 31, 2006.
Measures and Procedures
Demographic information collected included age, sex, race/ethnicity, and education. MS-related information was collected on MS subtype (relapsing-remitting, secondary progressive, primary progressive, progressive relapsing, and unknown), date of MS symptom onset, date of MS diagnosis, relevant laboratory diagnostic confirmation (magnetic resonance imaging, lumbar puncture, somatosensory evoked potential, or visual evoked potential), and level of MS impairment (EDSS score). Demographic and clinical data were collected via self-report and medical records.
The EDSS score is widely used as a measure of impairment/ambulation disability in people with MS and ranges from 0 (normal neurologic examination findings) to 10 (death due to MS).19 In this study, three subgroups were established for the analyses: people with EDSS scores of 0 to 3.5 (no ambulation problems), scores of 4 (fully ambulatory without aid, able to walk without aid or rest some 500 m) to 5.5 (ambulatory without aid or rest for about 100 m), and scores of 6 (intermittent or unilateral constant assistance [cane, crutch, or brace] required to walk approximately 100 m with or without rest).19 20
We used the TUG test to evaluate the functional mobility of the participants. The TUG has been validated in both the geriatric21 and MS22 23 populations and is recommended by the American Physical Therapy Association MS Evidence Database to Guide Effectiveness task force as a measure of mobility for people with MS.24 In the TUG test, a person is asked to rise from a standard-height chair, walk 10 feet, turn around, walk back to the chair, and sit down as fast as they safely can, and the time taken to perform the test is measured. Participants are able to use an assistive device when performing the TUG test. In the present study, this version of the test is referred to as TUG-alone.
Two different cognitive tasks were added to test the effect of increasing cognitive demand while performing the TUG test. In the first, the TUG test was performed while the person recited the alphabet continuously (referred to as TUG-alpha). Reciting the alphabet has been used with “walking while talking” tests, showing an increased performance time under the additional task.25 In the second condition, participants performed the TUG test while subtracting by 3s backward (referred to as TUG-3s),17 starting at either 46 or 71 (chosen at random).
All participants in both groups were asked to complete two trials of the three different versions of the TUG test (a total of six trials). The order of each participant's six TUG test trials was randomized. After completion of the trials, participants answered questionnaires on demographics and MS-related information. The EDSS scores were collected from the medical records.
For the participants' safety, all individuals were asked before testing whether they felt that they were at risk for falling. If they answered yes, they were required to wear a gait belt, and a trained research assistant walked alongside the participants during the TUG tests to guard them. In addition, the TUG-alpha and TUG 3s were audio-recorded to capture the participants' responses to the cognitive tasks (reciting the alphabet, counting backward), and the number of errors was counted.
Statistical Analysis
Descriptive analysis was performed for all demographic and clinical variables. Demographic characteristics between the MS and control groups were compared using the t test for age and χ2 tests for all other categorical variables. There were three main concerns before we analyzed the data on the TUG test: whether the sequence in which the conditions were applied influenced a subsequent test, whether there was a fatigue effect (later tests would have longer times), and whether learning effects were present (results of the second TUG trial of each type would be faster times due to a learning process). If we could assume that there was no effect of the sequence, that fatigue was not an issue, and that the learning effect was small or nonexistent, we could ignore the sequence of the trials and average the two values for each type of TUG test. We performed graphical analysis of the data by randomization order (data not shown but available on request) and found little influence of randomization order. Therefore, under those circumstances, we assumed that the averages of the two TUG tasks of each type were good estimators of the person's performance and analyzed the data using an analysis of variance for split-plot design (three conditions per person in each group), and the Greenhouse-Geisser adjustment for the degrees of freedom was used.
Results
Table 1 shows the demographic and clinical characteristics of the participants by group. None of the demographic variables were statistically significantly different between the two groups. Mean age was similar in both groups, approximately 47 to 48 years, and most participants were women. Controls had a slightly larger proportion of individuals with high school or less education but were less likely to be married. Most individuals were white non-Hispanic. Approximately 25% were living alone in both groups; 40% and 56% of individuals in the MS and control groups, respectively, were employed.
Demographic and clinical characteristics of the study participants
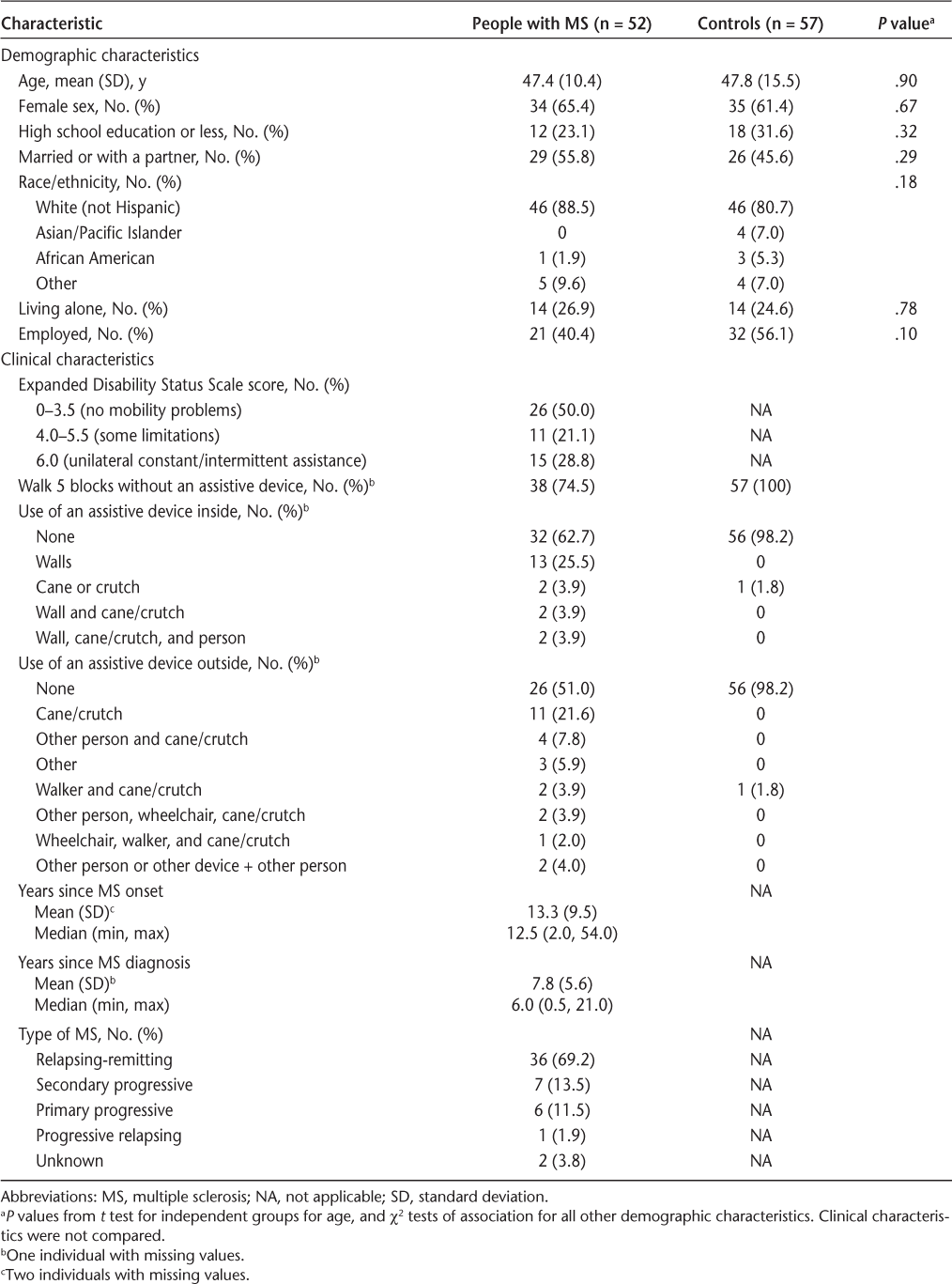
Of people with MS, 50% had no ambulation problems (EDSS score = 0–3.5), 21% had some limitations but did not need assistance to ambulate (EDSS score = 4.0–5.5), and 29% needed unilateral constant or intermittent assistance from a device to ambulate (EDSS score = 6.0). Patients with MS were a mean (SD) of 13.3 (9.5) years post–symptom onset for MS and 7.8 (5.6) years postdiagnosis. Most of the participants with MS had relapsing-remitting MS (69.2%), followed by secondary progressive MS (13.7%), primary progressive MS (11.5%), and progressive relapsing MS (1.9%). Type of MS was unknown for two participants.
Table 2 shows the descriptive results for the three TUG tests for each group. In each group, there is an increase in mean and median times from TUG-alone to TUG-alpha to TUG-3s. In addition, within each type of test, the time increased from controls to increasing EDSS levels in the MS group.
Descriptive analysis for performance times by TUG type and EDSS category for people with MS and controls
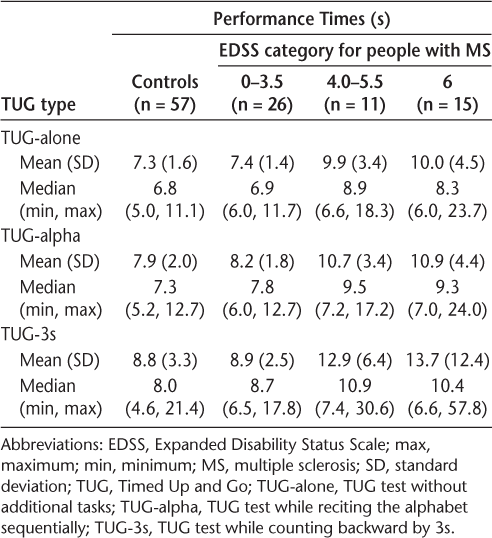
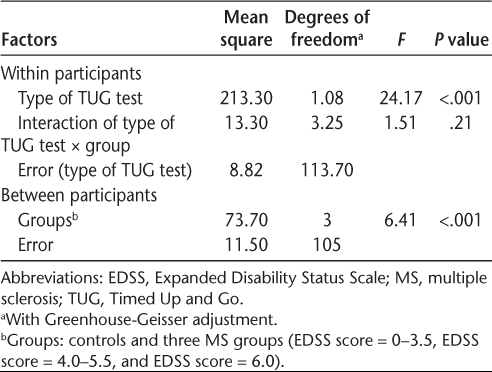
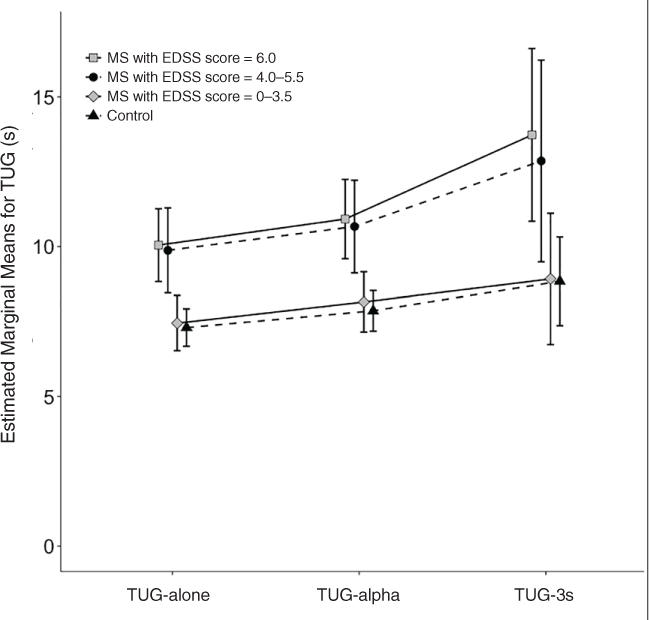
Table 3 shows the statistical comparisons between groups and TUG types, which were tested by a split-plot analysis of variance. There was no effect of the interaction of group × TUG type (F 3.25, 113.70 = 1.51, P = .21), but there was an effect of group and TUG type (F 3, 105 = 6.41 and F 1.08, 113.70 = 24.17, P < .001 for both). Figure 1 shows the estimated means from the analysis of variance by TUG type and group. A post hoc analysis using Bonferroni adjustment showed that all pairwise comparisons for TUG type and for groups were significant (P < .001 for all). Comparing TUG types, there was an increase in the mean of 8.7 seconds to perform the TUG-alone to 9.4 seconds for the TUG-alpha (8% increase) and 11.1 seconds for the TUG-3s (28% increase). Comparing the groups, controls had an overall mean time of 8.0 seconds, followed by individuals with MS and no mobility problems with 8.2 seconds (a slight increase), those with MS and some mobility limitation with 11.1 seconds (39% increase), and those with constant/intermittent need for assistance with 11.6 seconds (45% increase). Post hoc analysis showed that controls did not differ from people with MS and no mobility problems (P > .99) but did differ from the other two groups (P = .04 and .003); people with MS and no mobility problems did not differ from people with MS and some limitations (P = .10) but did differ from the more severe MS group (P = .02); the two more severe groups did not differ from each other (P > .99). Figure 1 makes it clear that individuals with MS and no mobility problems have a TUG test performance that is similar to that of controls, with very little increase in time due to the addition of cognitive tasks to the TUG test. The two more severe groups perform similarly to each other, with a steeper increase in time to perform the test when the cognitive demand increases.
Analysis of variance for TUG times by type of TUG test and group (controls, and 3 EDSS categories for MS group)
Estimated marginal mean scores and 95% confidence intervals for the Timed Up and Go (TUG) tests plotted against the TUG type for each Expanded Disability Status Scale (EDSS) group
We counted the number of errors in the two trials of the TUG-alpha and TUG-3s. For TUG-alpha, among controls there were no errors for 90% of individuals in the first trial and 95% in the second trial (maximum number of errors for both trials = 7), and among participants with MS there were no errors for 93% in the first trial and 90% in the second (maximum number of errors in both trials = 8). For the TUG-3s, among controls there were no errors for 63% of individuals in the first trial and 68% in the second trial (maximum number of errors for both trials = 6), and among participants with MS there were no errors for 68% in the first trial and 76% in the second (maximum number of errors in both trials = 9). Plots of the number of errors by TUG time did not show any pattern (data not shown but available on request). Counting data such as the number of errors would require a larger sample size to perform a meaningful Poisson regression, for example; therefore, we chose to present descriptive data only.
Discussion
Using the TUG test under the dual-task paradigm in people with and without MS, this study provided evidence that engaging simultaneously in cognitive and motor tasks can negatively affect performance on a task of functional mobility. All participants, with and without MS, increased their TUG time with the addition of a secondary cognitive task. This is similar to the findings in the older adult literature17 and in people with Parkinson's disease.26 27 However, individuals with MS and no mobility problems (EDSS score = 0–3.5) were similar to controls in their increase of time to perform the more demanding TUG tests. Individuals in the MS groups with mobility limitations paralleled the pattern from the other two groups but had a consistently greater time to perform on all three TUG tests. These observations are similar to previous reports of increased performance deficits in dual-task scenarios in individuals with MS who had greater disability.28 29
The results of this study suggest that the addition of even a simple cognitive task might cause a relatively large reduction in performance on the TUG test in people with MS, especially for individuals who are still ambulating but have EDSS scores greater than 3.5. Although we cannot automatically generalize the results to more complex everyday activities, such as walking or driving a car while talking on a cell phone, the reduction in performance is an important issue that should be discussed with the patient and his or her caregiver. Monjezi and colleagues30 also showed an increase in time from the single- to the dual-task TUG test (mean [SD] 10.5 [12.0] seconds vs. 12.6 [2.6] seconds, respectively). However, their changes were smaller than we observed in the more impaired groups, probably because they included only individuals with relapsing-remitting disease.
The mechanisms underlying impaired mobility during dual-task situations in people with MS who have gait impairment are not clear, but several possibilities exist. Based on the theoretical tenets of the attentional capacity model,31 it is suggested that individuals with MS who have mobility impairment require greater attentional resources during mobility tasks16 compared with those without impairment. Consequently, an additional cognitive load further influences their mobility performance. It is important to note that direct empirical evidence of this model is lacking in individuals with MS. It is also likely that in more advanced MS, where greater neural loss resulting in compensatory neuroplasticity has occurred, cognitive and motor tasks may share neuronal pathways.32
One limitation of the study is that although the total group was larger than most previous studies, the sample size for each EDSS subgroup was relatively small. A second limitation was that we did not specifically test the participants for cognitive impairment, although the primary objective was not to look at the results as related to level of cognitive impairment but level of EDSS. A third limitation was that the cognitive tasks added to the TUG test might not have been challenging enough to elicit the same type of response that another cognitive task would in the person's daily life. Finally, although we could not find evidence of learning effects or fatigue in this sample, it would be premature to assume that they might not exist.
With the present results, we can design studies to better answer the questions on the effect of cognitive demand on functional mobility in individuals with MS. This study is important because it expands on previous work with a larger sample size, focused on tasks other than simply walking, and included a broad range of MS severity in ambulating people with MS. Moreover, there is some emerging research suggesting that targeted rehabilitation can be beneficial for cognitive-motor interaction in individuals with MS.33 These results suggest that additional research is needed to understand the mechanisms by which dual tasking or multitasking is affected by MS. It is important to note that the present data were collected in a single point in time and it is not clear how dual-tasking abilities change over time or whether they are sensitive to disease progression. Future goals should focus on providing measures applicable in a clinical setting that are sensitive and specific to MS-related impairment in multitasking and should be translated into the person's participation in everyday activities.
PracticePoints
The Timed Up and Go (TUG) test is a commonly used measure of functional mobility that encompasses walking, turning, standing up, and sitting down. A cognitive task can be added to the TUG test, forming a dual-task paradigm.
The dual-task paradigm is often used to assess the impact of cognitive demand on postural control, balance, and gait in older adults and people with neurologic diagnoses.
While ambulating, individuals with MS might have a negative response to additional cognitive demands, and it is important to assess how that addition might affect their safety and functional mobility. The TUG test plus a cognitive task may be a useful tool for that assessment.
Financial Disclosures
Dr. Khurana has received consulting fees from Mallinckrodt and has received research support from Mallinckrodt and Medtronics. Dr. Sosnoff has received research support from MC10, Inc, and Perimobile, Inc, and has an ownership interest in Intelliwheels, Inc. Dr. Kraft is a member of the Axon Council, Acorda Therapeutics. The other authors have no conflicts of interest to disclose.
References
Rao SM. Neuropsychology of multiple sclerosis: a critical review. J Clin Exp Neuropsychol. 1986; 8:503–542.
Ron MA. Multiple sclerosis: psychiatric and psychometric abnormalities. J Psychosom Res. 1986; 30:3–11.
Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, I: frequency, patterns, and prediction. Neurol Neurol. 1991; 41:685–691.
Peyser JM, Rao SM, LaRocca NG, Kaplan E. Guidelines for neuropsychological research in multiple sclerosis. Arch Neurol. 1990; 47:94–97.
Benedict RHB, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2006; 12:549–558.
Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008; 8:1585–1596.
Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008; 7:1139–1151.
Bergendal G, Fredrikson S, Almkvist O. Selective decline in information processing in subgroups of multiple sclerosis: an 8-year longitudinal study. Eur Neurol. 2007; 57:193–202.
Rao SM, St Aubin-Faubert P, Leo GJ. Information processing speed in patients with multiple sclerosis. J Clin Exp Neuropsychol. 1989; 11:471–477.
Lengenfelder J, Bryant D, Diamond BJ, Kalmar JH, Moore NB, DeLuca J. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch Clin Neuropsychol. 2006; 21:229–238.
Parmenter BA, Shucard JL, Shucard DW. Information processing deficits in multiple sclerosis: a matter of complexity. J Int Neuropsychol Soc. 2007; 13:417–423.
Drew MA, Starkey NJ, Isler RB. Examining the link between information processing speed and executive functioning in multiple sclerosis. Arch Clin Neuropsychol. 2009; 24:47–58.
Lonie JA, Tierney KM, Herrmann LL, et al. Dual task performance in early Alzheimer's disease, amnestic mild cognitive impairment and depression. Psychol Med. 2009; 39:23–31.
McDowell S, Whyte J, D'Esposito M. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. NSY Neuropsychol. 1997; 35:1341–1353.
Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler. 2009; 15:1215–1227.
Wajda DA, Sosnoff JJ. Cognitive-motor interference in multiple sclerosis: a systematic review of evidence, correlates, and consequences. Biomed Res Int. 2015; 2015:1–8.
Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000; 80:896–903.
Rehabilitation Measures Database. http://www.rehabmeasures.org/default.aspx. Accessed March 14, 2017.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33:1444–1452.
Kurtzke JF. On the evaluation of disability in multiple sclerosis. Neurology. 1961; 11:686–694.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39:142–148.
Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006; 28:789–795.
Sebastião E, Sandroff BM, Learmonth YC, Motl RW. Validity of the Timed Up and Go Test as a measure of functional mobility in persons with multiple sclerosis. Arch Phys Med Rehabil. 2016; 97:1072–1077.
Potter K, Cohen ET, Allen DD, et al. Outcome measures for individuals with multiple sclerosis: recommendations from the American Physical Therapy Association Neurology Section Task Force. Phys Ther. 2014; 94:593–608.
Maranhão-Filho PA, Maranhão ET, Lima MA, Silva MM. Rethinking the neurological examination II: dynamic balance assessment. Arq Neuropsiquiatr. 2011; 69:959–963.
Campbell CM, Rowse JL, Ciol MA, Shumway-Cook A. The effect of cognitive demand on Timed Up and Go performance in older adults with and without Parkinson disease. J Neurol Phys Ther. 2003;27:2–7. http://journals.lww.com/jnpt/Fulltext/2003/27010/The_Effect_of_Cognitive_Demand_on_Timed_Up_and_Go.2.aspx.
Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neurol Sci. 2013; 335:160–163.
Sosnoff JJ, Boes MK, Sandroff BM, Socie MJ, Pula JH, Motl RW. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch Phys Med Rehabil. 2011; 92:2028–2033.
Sosnoff JJ, Socie MJ, Sandroff BM, et al. Mobility and cognitive correlates of dual task cost of walking in persons with multiple sclerosis. Disabil Rehabil. 2014; 36:205–209.
Monjezi S, Negahban H, Tajali S, Yadollahpour N, Majdinasab N. Effects of dual-task balance training on postural performance in patients with multiple sclerosis: a double-blind, randomized controlled pilot trial. Clin Rehabil. 2017; 31:234–241.
Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall; 1973.
Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011; 35:715–728.
Wajda DA, Mirelman A, Hausdorff JM, Sosnoff JJ. Intervention modalities for targeting cognitive-motor interference in individuals with neurodegenerative disease: a systematic review. Expert Rev Neurother. 2017; 17:251–261.







