Publication
Research Article
International Journal of MS Care
Effect of a 2-Week Trial of Functional Electrical Stimulation on Gait Function and Quality of Life in People with Multiple Sclerosis
Background: Footdrop is a common gait deviation in people with multiple sclerosis (MS) leading to impaired gait and balance as well as decreased functional mobility. Functional electrical stimulation (FES) provides an alternative to the current standard of care for footdrop, an ankle-foot orthosis (AFO). FES stimulates the peroneal nerve and activates the dorsiflexor muscles, producing an active toe clearance and a more normal gait. This study was undertaken to determine the effects of a 2-week FES Home Assessment Program on gait speed, perceived walking ability, and quality of life (QOL) among people with MS-related footdrop.
Methods: Participants completed the Timed 25-Foot Walk test (T25FW) and two self-report measures: 12-item Multiple Sclerosis Walking Scale (MSWS-12) and 29-item Multiple Sclerosis Impact Scale (MSIS-29). Measures were taken without FES before and with FES after 2 weeks of full-time FES wear.
Results: A total of 19 participants (10 female, 9 male) completed the study; mean age and duration of disease were 51.77 ± 10.16 and 9.01 ± 7.90 years, respectively. Use of FES for 2 weeks resulted in a significant decrease in time to complete the T25FW (P < .0001), the MSWS-12 standardized score (P < .0001), and the MSIS-29 total (P < .0001), Physical subscale (P < .0001), and Psychological subscale (P = .0006) scores.
Conclusions: These results suggest that use of FES can significantly improve gait speed, decrease the impact of MS on walking ability, and improve QOL in people with MS-related footdrop even over a short period of time.
Multiple sclerosis (MS) is an autoimmune disorder that affects the central nervous system (CNS). The disease has an unpredictable course, is usually progressive in nature, and affects 2.5 million people worldwide.1 About 50% of people with MS require assistance with gait, 10% will require a wheelchair within 15 years of disease onset, and 90% will have significant functional limitation 25 years after onset.2 Gait disturbances are reported as among the most disabling effects of the disease, and maintaining mobility is one of the highest priorities for people with MS.3 One of the more common gait disturbances is footdrop. Footdrop is an inability to dorsiflex the foot during the swing phase of gait, due to either dorsiflexor weakness, increased tone in the plantarflexor muscles, or disordered neural control causing co-contraction of agonist and antagonist muscles.4 This inappropriate neuromuscular control around the ankle creates a very unnatural gait and limits aspects of walking such as speed, endurance, energy expenditure, and balance.4 The loss of dorsiflexion also results in the patient dragging the foot, leading to an increased risk of trips and falls.5
The current standard of care for treatment of footdrop is an ankle-foot orthosis (AFO).6 An AFO is a rigid or hinged plastic brace that keeps the foot in a neutral position during the swing phase, thus increasing toe clearance. The disadvantages of AFOs include restriction of movement, muscle atrophy, limited choice of footwear, poor cosmetic appearance, and user discomfort.7 8 Neuroprosthetic devices using functional electrical stimulation (FES) have been developed as an alternative approach to the treatment of footdrop.9 FES electrically stimulates the dorsiflexor muscles of the foot during the swing phase of walking to provide an active and effective toe clearance. The advantages of FES are that it facilitates a more natural gait pattern, provides muscle activation, increases blood circulation, and reduces the occurrence of muscle atrophy.7 10 FES has also been shown to improve voluntary muscle control and to facilitate a therapeutic effect (improved function after the device is removed), which suggests positive neuroplastic changes.11–13 In previous studies, FES of the peroneal nerve has been shown to increase gait velocity,7 13 14 decrease energy expenditure with gait,11–13 improve voluntary muscle control,11 and improve gait symmetry.8 15
Gait dysfunction in people with MS limits independence, decreases community mobility, and affects social participation and quality of life (QOL). It is important to assess the effectiveness of devices designed to improve gait and alleviate disability. This study was designed to assess the effectiveness of the WalkAide FES device (WA) (Innovative Neurotronics, Austin, TX) in alleviating footdrop caused by MS. The purpose of the study was to determine whether using the WA for 2 weeks would improve walking ability, gait speed, and QOL in people with MS-related footdrop.
Methods
Participants
The participants in this study were individuals presenting to nine Hanger Orthopedic and Prosthetic Clinics across the United States for the purpose of participating in a WA Home Assessment Program (WA-HAP). The WA-HAP is a 2-week device trial period during which a patient gradually works up to full-time wear of the WA FES device and uses it for all walking activities. Patients entering into this program were asked to participate in this study. If the patient agreed to become a participant in the study, he or she signed a Western Institutional Review Board (WIRB) approved informed consent form and was screened for eligibility.
The primary inclusion criteria were 1) ability to walk at least 25 feet with or without an assistive device at a speed of between 8 and 45 seconds, 2) demonstration of inadequate dorsiflexion during the swing phase of gait (defined as −5° plantarflexion), 3) demonstration of adequate dorsiflexion of the ankle in response to peroneal nerve stimulation, 4) no current use of FES for treatment of footdrop, and 5) cognitive function adequate to permit reliable completion of evaluation instruments and to correctly use the WA. The primary exclusion criteria were 1) an MS exacerbation within the preceding 60 days, 2) need for an AFO for stance control of the foot, ankle, and/or knee, 3) a seizure disorder, 4) use of an existing electrical stimulation device (implantable cardioverter defibrillator, pacemaker, spinal stimulation), 5) receipt of botulinum toxin (Botox) injections in the lower extremity within the preceding 6 months, 6) use of a baclofen pump with unstable dosing in the last 3 months, 7) a diagnosis of peripheral nerve injury in the involved lower extremity with symptoms that limited participation in study activities, or 8) receipt of dalfampridine for the treatment of their MS symptoms. A complete list of inclusion and exclusion criteria appears in Table 1.
Inclusion and exclusion criteria

Procedures
Once confirmed as eligible, participants had a baseline visit during which demographic information was collected and they were fitted with the study device. The WA is a battery-operated, single-channel electrical stimulator used for treatment of footdrop of CNS origin. The device consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. The WA uses a tilt sensor and an accelerometer to trigger ankle dorsiflexion and control the timing and duration of peroneal nerve stimulation during the swing phase of the gait cycle. After initial fitting, programming, and patient education performed by a trained clinician, patients were able to use the WA to facilitate walking in daily activities. At each study site, the same WA-certified orthotist fit the device and collected all study data. All measures were taken at baseline before the patients were fitted with the WA (baseline without WA). After the patients were fitted with the WA, an additional measure of gait function with the device was taken (baseline with WA). All measures were repeated with the WA at a follow-up visit 2 weeks later.
Outcome Measures
Measures were chosen to assess gait function as well as the impact of gait disability on the participants' perception of their own walking ability and QOL.
Gait Speed
The Timed 25-Foot Walk test (T25FW) was used as a measure of gait speed and function. The T25FW is considered a reliable, objective measure of walking disability16 and is one of the most frequently used measures to assess gait function in people with MS.17 Patients are instructed to walk 25 feet at their fastest safe speed; assistive devices may be used. The time begins at a command of “start” and ends when the person crosses the 25-foot mark; the acceleration phase is included in the scoring. The person completes two trials, and the average of the trials is considered the final measure.
Perceived Walking Ability
The impact of MS on the participants' perceived walking ability was assessed using the 12-item Multiple Sclerosis Walking Scale (MSWS-12). The MSWS-12, considered more responsive than other walking-based scales, is a reliable and valid patient-based measure of the impact of MS on walking.18 19 For this measure the patient completes a questionnaire and rates the degree of limitation in walking due to MS experienced in the prior 2 weeks for each of 12 activities. Individual item responses are summed and the total score standardized to a scale with a range of 0 to 100. Higher scores reflect a greater perceived limitation on walking abilities due to MS.
Perceived Quality of Life
The impact of MS on perceived QOL was measured using the 29-item Multiple Sclerosis Impact Scale (MSIS-29), a patient-completed questionnaire that asks the patient to rate the degree of impact that MS has on the quality of his or her day-to-day life. Of the 29 questions on the MSIS-29, 20 address the physical impact component and 9 assess the psychological impact; a combined score can be generated, or both components can be reported separately. The MSIS-29 is a reliable and valid method of recording QOL that is well accepted for use both clinically and as a means of monitoring progress during clinical research trials.20 Higher scores reflect a greater perceived impact of MS on physical and psychological aspects of QOL.
Analysis
The data collected during this study were analyzed using paired t tests. All measures were analyzed for changes between the baseline without WA and the 2-week follow-up visit. The T25FW measure at baseline with the WA was also compared to baseline without the WA and to the 2-week follow-up with the WA. Only participants with complete data were included in the analysis.
Results
Twenty participants were enrolled, and 19 (10 female, 9 male) completed the study. One participant was removed from the study after it was discovered that he was taking dalfampridine, which was an exclusion criterion. A summary of the demographic characteristics of study participants appears in Table 2. The participants demonstrated statistically significant improvement in all outcome measures (Table 3).
Demographic characteristics
Pre- and post-WA-HAP measures
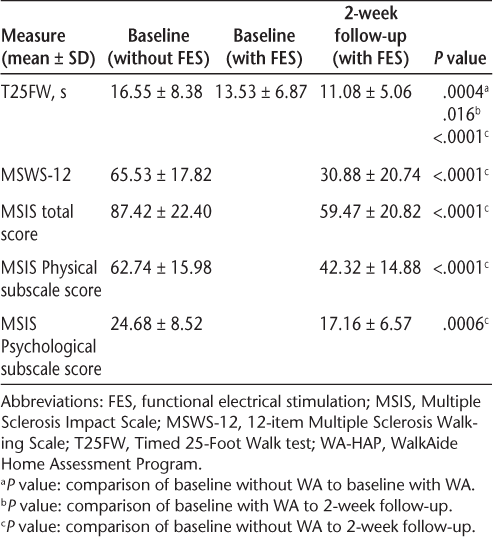
Participants demonstrated a statistically significant decrease in the amount of time taken to complete the T25FW, from an average of 16.55 ± 8.38 seconds at baseline without the WA to 11.08 ± 5.06 seconds at the 2-week follow-up with the WA (P < .0001), which is a 33.1% decrease. There was also a significant decrease in time to complete the T25FW between the measure at baseline without the WA, 16.55 ± 8.38, and the measure at baseline with the WA, 13.53 ± 6.87 (P = .0004), which is an 18.3% decrease. The percent change between the measure of T25FW at baseline with the WA (13.53 ± 6.87) and the 2-week follow-up (11.08 ± 5.06) was also significant (P = .016) and equal to 18.1%. All participants showed a decrease in T25FW score during the course of the 2-week WA-HAP. With the exception of two participants (participants 15 and 16) who demonstrated especially dramatic changes in time to complete the T25FW, the magnitude of changes in individual scores was relatively similar (Figure 1).
Timed 25-Foot Walk test scores by participant

The scores on the MSWS-12 decreased significantly as well, from a baseline without the WA of 65.53 ± 17.82 to 30.88 ± 20.74 at 2 weeks (P < .0001) (percent change of 52.9%), suggesting that the participants perceived the impact of MS on their walking ability to be lessened after FES.
The MSIS-29 total, Physical, and Psychological scores all demonstrated a significant decrease, indicating that the participants perceived a decrease in the impact of their MS on their QOL. The total score decreased from 87.42 ± 22.40 at baseline without the WA to 59.47 ± 20.82 after 2 weeks (P < .0001), a reduction of 32%. The MSIS-29 Physical subscale score decreased from 62.74 ± 15.98 at baseline without the WA to 42.32 ± 14.88 (32.6% decrease, P < .0001), and the Psychological subscale score decreased from 24.68 ± 8.52 to 17.16 ± 6.57 (30.5% decrease, P = .0006).
Discussion
The results of this study suggest that the WA FES device had a positive effect on gait function, with a significant decrease in time to complete the T25FW. The WA also positively affected the participants' perception of their own walking ability and their QOL, with statistically significant improvement on both the MSWS-12 and the MSIS-29.
Although the AFO is clinically accepted as the standard of care for MS-related footdrop, few studies have specifically described the effect of an AFO on gait speed and function for people with MS.21 Sheffler et al.21 looked at timed tests of functional ambulation, the T25FW and the Modified Emory Functional Ambulation Profile (mEFAP), and found no significant difference in performance between measures taken with and without an AFO. In contrast, the results of this study demonstrate not only a statistically significant improvement on the T25FW but also a change that is clinically significant. The minimum clinically important difference (MCID) for the T25FW has been noted in the literature to be a change ranging from 17% to 20% in time to complete the measure.22 23 The change noted between the baseline and 2-week follow-up measures in this study was 33.1%, suggesting that the change is quite relevant clinically.
Another important consideration for the use of the WA is the amount of time it takes for FES to have a beneficial effect on walking ability once turned on. In addition to the significant change over the 2-week study period, the results of this study also show a positive immediate “on-off” or “orthotic effect.” The orthotic effect in this study was defined by the difference between the T25FW measure taken at baseline without the WA and that taken at baseline with the WA. This difference was 18.3%, which was statistically as well as clinically significant, falling within the range recognized as the MCID.22 23 The percent change between the T25FW measure at baseline with the WA and that at 2 weeks was virtually equal to the immediate “on-off” effect at 18.1%. These results suggest that application of the WA facilitates a significant improvement in gait function immediately and that it continues to facilitate cumulative improvement over time. The fact that the use of this device can produce both statistically and clinically significant improvement over such a short period of time suggests that the WA has good potential to improve function and mobility for people with MS and is a viable option to reduce the disability imposed by footdrop.
In this study, participants perceived the impact of their MS on their walking ability and QOL to be lessened after wearing the WA. There is no MCID established for this measure, but the minimal detectable change (MDC) for the MSWS-12 score has been estimated in the literature to be a change of at least 22 points.24 The participants in this study demonstrated a mean difference between baseline and 2-week follow-up of 34.65 points, suggesting that the change in subject perception was likely to represent a true change in their walking ability. The percent change in this score from baseline to 2 weeks was 52.9%; a change of this magnitude likely reflects a significant decrease in the perception of the disability imposed by MS on walking. This perceived lessening of the impact of MS on the participants' walking may well result in increased activity and community mobility. The participant's perception of the impact of their MS on their QOL also decreased with the use of FES. In this study, total MSIS-29 scores as well as Physical and Psychological subscale scores showed statistically significant improvement. No MCID values have been established for this measure, but an MDC for the Physical subscale score has been reported to be 8 points.25 The participants in this study demonstrated a mean change in the MSIS-29 Physical subscale score of 20.42 points, suggesting that the participants' perception was representative of a true improvement in the physical domain of QOL.
Maintaining mobility is crucial; 70% of people with gait difficulty imposed by MS rank that impairment as the greatest challenge imposed by the disease.26 The MSWS-12 and the MSIS-29 are responsive measures of the impact of MS on walking ability and MS-related QOL. The T25FW, in addition to being a valid measure of gait function and speed, also reflects the impact of disability on other aspects of life. Increased T25FW scores have been shown to be associated with increased caregiver burden, decreased work productivity, and decreased health-related QOL.27 28 The results of this study demonstrate both statistically and clinically significant decreases in objective measures of gait function as well as self-report measures of the impact of MS on walking ability and QOL.
Limitations
This was a small study using a sample of convenience; the results may not be generalizable to a larger population of people with MS. The study was designed to assess the effectiveness of the WA within the parameters of the WA-HAP; therefore, participants served as their own controls. Larger studies with randomization of participants to both treatment and control groups are needed to further assess the effect of the WA on this population. Only the WA was used in this study. Other neuroprosthetic FES devices are currently available for the treatment of footdrop; these devices were not tested but might produce similar results.
Conclusion
Use of the WA FES device in a home assessment program can improve gait function and decrease the perceived impact of MS on walking ability and the physical and psychological domains of QOL. Significant changes with the WA were noted immediately after fitting and continued to increase over the 2-week WA-HAP, suggesting that use of FES, even over short periods of time, can have a significant positive effect on the function and QOL of individuals with MS-related footdrop.
PracticePoints
Functional electrical stimulation can be an effective treatment for MS-related footdrop by promoting active dorsiflexor muscle contraction during the swing phase of gait.
Functional electrical stimulation can increase gait speed and improve both perceived walking ability and quality of life. Changes can be seen in a short period of time, making a short assessment program an effective way to trial this technology.
References
World Health Organization. Atlas: Country Resources for Neurological Disorders. Results of a collaborative study of World Health Organization and World Federation of Neurology. Programme for Neurological Diseases and Neurosciences Department of Mental Health and Substance Abuse. http://www.who.int/mental_health/neurology/epidemiology/en/. Accessed April 23, 2013.
Frohman EM, Racke M, van Den Noort S. To treat, or not to treat: the therapeutic dilemma of idiopathic monosymptomatic demyelinating syndromes. Arch Neurol. 2000; 57: 930–932.
Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010; 26: 109–119.
Barrett CL, Mann GE, Taylor PN, et al. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009; 15: 493–504.
Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002; 83: 165–170.
Sheffler LR, Hennessey MT, Knutson JS, Naples GG, Chae J. Functional effect of an ankle foot orthosis on gait in multiple sclerosis. Arch Phys Med Rehabil. 2008; 87: 26–31.
Paul L, Rafferty D, Young S, et al. The effect of functional electrical stimulation on the physiological cost of gait in people with multiple sclerosis. Mult Scler. 2008; 14: 954–961.
Ring H, Treger I, Gruendlinger L, et al. Neuroprosthesis for footdrop compared with ankle-foot orthosis: effects on postural control during walking. J Stroke Cerebrovasc Dis. 2009; 18: 41–47.
Sheffler LR, Hennessey MT, Knutson JS, Chae J. Neuroprosthetic effect of peroneal nerve stimulation in multiple sclerosis: a preliminary study. Arch Phys Med Rehabil. 2009; 90: 362–365.
Stein RB, Chong SL, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006; 20: 371–379.
Everaert DG, Thompson AK, Chong SL, et al. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–177.
Kottink AI, Hermens HJ, Nene AV, et al. Therapeutic effects of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized clinical trial. Phys Ther. 2008; 88: 437–448.
Stein RB, Everaert DG, Thompson AK, et al. Long term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010; 24: 152–167.
Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Oddstock Dropped Foot Stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999; 80: 1577–1583.
Hausdorff JM, Ring H. Effects of a new radio frequency-controlled neuroprosthesis on gait symmetry and rhythmicity in patients with chronic hemiparesis. Am J Phys Med Rehabil. 2008; 87: 4–13.
Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. 2012; 18: 914–924.
Rasova K, Martinkova P, Vyskotova J, et al. Assessment set for evaluation of clinical outcomes in multiple sclerosis: psychometric properties. Patient Relat Outcome Meas. 2012; 3: 59–70.
Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31–36.
Motl RW, Dlugonski D, Suh Y, et al. Multiple Sclerosis Walking Scale-12 and oxygen cost of walking. Gait Posture. 2010; 31: 506–510.
Gray O, McDonnell G, Hawkins S. Tried and tested: the psychometric properties of the multiple sclerosis impact scale (MSIS-29) in a population-based study. Mult Scler. 2009; 15: 75–80.
Sheffler LR, Hennessey MT, Knutson JS, Naples GG, Chae J. Functional effect of an ankle foot orthosis on gait in multiple sclerosis. Arch Phys Med Rehabil. 2008; 87: 26–31.
Coleman CI, Sobieraj DM, Marinucci LN. Minimally important clinical difference of the Timed 25-Foot Walk Test: results from a randomized controlled trial in patients with multiple sclerosis. Curr Med Res Opin. 2012; 28: 49–56.
Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-Foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000; 6: 286–290.
Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013; 19: 1784–1791.
Costelloe L, O'Rourke K, Kearney H, et al. The patient knows best: significant change in the physical component of the Multiple Sclerosis Impact Scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry. 2007; 78: 841–844.
Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011; 4: 189–201.
Cohen JT. Walking speed and economic outcomes for walking-impaired patients with multiple sclerosis. Expert Rev Pharmacoeconom Outcomes Res. 2010; 10: 595–603.
Pike J, Jones E, Rajagopalan K, et al. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol. 2012; 12:94.
Financial Disclosures: Dr. Rogers is employed by Innovative Neurotronics (IN Inc.) as Director of Clinical Research and Training. All the other authors were employees of Hanger Prosthetics and Orthotic Clinics at the time of this study and were compensated by IN Inc. for the time taken to enroll participants and collect data. Ms. Sawers now works for Children's Healthcare of Atlanta in Atlanta, GA.
Funding/Support: Innovative Neurotronics provided partial funding for this study; this funding covered the institutional review board application fees, compensation to the orthotist authors for data collection, and travel expenses for study participants.







