Publication
Research Article
International Journal of MS Care
A Pooled Analysis of Two Phase 3 Clinical Trials of Dalfampridine in Patients with Multiple Sclerosis
Author(s):
Background: Two phase 3 clinical trials demonstrated that dalfampridine extended-release 10-mg tablets (D-ER), twice daily, significantly improved walking relative to placebo in patients with multiple sclerosis (MS). The objective of this study was to evaluate the efficacy and safety of D-ER in patients with MS using pooled data from the two phase 3 trials.
Methods: Data were pooled from the two trials, and D-ER was compared with placebo for timed-walk responder rate, changes in walking speed, and the 12-item Multiple Sclerosis Walking Scale (MSWS-12). Response rates were evaluated with respect to demographic and clinical characteristics.
Results: D-ER had a significantly higher proportion of timed-walk responders relative to placebo (37.6% vs. 8.9%; P < .0001). The responder rate was independent of age, gender, race, body-mass index, type of MS, duration of MS, baseline Expanded Disability Status Scale score, baseline walking speed, and concomitant use of immunomodulatory therapies. Significant improvements were observed in walking speed and in MSWS-12 score for the pooled D-ER group compared with placebo. The safety profile was consistent with the individual studies; no new safety or tolerability concerns were identified.
Conclusions: D-ER demonstrated efficacy for the improvement of walking in patients with MS; response was independent of demographic and clinical characteristics.
Treatment of individual symptoms of multiple sclerosis (MS) is recommended as part of an integrated approach to patient management.1–4 Although a variety of drugs have been used for some of the common symptoms of MS—spasticity, neuropathic pain, fatigue, neurogenic bowel and bladder, depression—a pharmacologic therapy has been recently approved for patients with walking impairment.
Dalfampridine extended-release 10-mg tablets (D-ER), twice daily, were approved in the United States in 2010 for improvement of walking in patients with MS, as demonstrated by an increase in walking speed.5 D-ER is also approved in a number of countries outside the United States, where it is known as prolonged-release fampridine in Europe and as fampridine modified or sustained release elsewhere.6 7 Dalfampridine is the nonproprietary drug name in the United States for the chemical 4-aminopyridine, a potassium channel blocker. Based on the role of axonal potassium channels in impaired impulse conduction, this compound was identified as having the potential to improve impaired neurologic function. Based on prior clinical studies, walking and mobility impairment in patients with MS appeared to be responsive to the drug, and improvement was readily measurable.8 The extended-release formulation was developed to meet the challenges associated with a narrow therapeutic range.9 10
Two phase 3 clinical trials (MS-F203 and MS-F204)11 12 demonstrated improvement of walking with D-ER compared with placebo. The trials used the proportion of patients who met a prospectively defined consistent responder criterion derived from a post hoc analysis of an earlier phase 2 trial as the primary efficacy endpoint.13 Responders were defined as patients who had a faster walking speed on the Timed 25-Foot Walk (T25FW) for at least three of the four visits during the double-blind treatment period compared with their maximum speed at any of the five off-drug visits. In both phase 3 trials, the proportion of responders was significantly higher with D-ER relative to placebo: 34.8% versus 8.3% (P < .0001) in MS-F203 and 42.9% versus 9.3% (P < .0001) in MS-F204.11 12 Among D-ER responders, walking speed improved during treatment in the two trials by an average of 25.2% and 24.7%, compared with 4.7% and 7.7% for the placebo-treated groups. Additionally, the clinical meaningfulness of the timed-walk response criterion was validated by the greater improvements observed on a patient-reported measure of walking, the 12-item Multiple Sclerosis Walking Scale (MSWS-12).14 Regardless of treatment assignment, the average MSWS-12 improvement from baseline was indicated by a change of −6.84 points for timed-walk responders versus a change of 0.05 point for nonresponders (nominal P = .0002) in MS-F203, and −6.04 points for timed-walk responders versus 0.85 point (worsening) for nonresponders (nominal P < .001) in MS-F204.
The purpose of the current pooled analysis was to increase the discriminative power of the data, enabling better characterization of the response, and, in particular, to allow a more powerful analysis of patient subsets. There is considerable scientific and practical interest in the question of whether any patient characteristics may be associated with and enable prediction of therapeutic response.
Methods
Phase 3 Trials
Both phase 3 trials had a randomized, double-blind, placebo-controlled design, and the methodology has previously been described in detail, including inclusion and exclusion criteria.11 12 In brief, following a screening assessment, patients aged 18 to 70 years with clinically definite MS and a T25FW time between 8 and 45 seconds entered a 2-week, single-blind, placebo run-in period during which they took 1 blinded tablet approximately every 12 hours. After the run-in, patients were randomized to either D-ER 10 mg twice daily or placebo. Patients returned at intervals of 2 or 4 weeks for evaluation, and a safety follow-up was performed 2 weeks after the final dose. The two studies differed in duration; MS-F203 involved 14 weeks of double-blind treatment, versus 9 weeks in MS-F204. The visit following the ninth week of treatment in MS-F204 was used to explore the maintenance of walking effect over the 12-hour interdosing period.
The primary efficacy outcome was based on walking speed, which was assessed using the T25FW. All clinical study sites were trained on the correct conduct of the T25FW to ensure uniformity. The T25FW is a clinically relevant measure that shows a strong correlation with the Expanded Disability Status Scale (EDSS) across MS types and level of walking impairment.15 16 The test is valid and reliable with negligible practice effects17–19 and requires a minimum of equipment, time, and space; it is therefore of practical use in clinical research settings.20 21 A change of 20% has been considered both reliable and clinically meaningful.22–26
A key secondary outcome in both trials was evaluation of walking from the patient's perspective using the MSWS-12,14 a clinically validated, patient-reported measure that assesses the impact of MS on walking ability, with a higher score indicating greater difficulty walking. Additional secondary outcomes in both trials were the Ashworth score for spasticity27 and the Lower Extremity Manual Muscle Test (LEMMT) for leg strength using the modified British Medical Research Council scale.28
Pooled Analyses
There was sufficient homogeneity of the populations and methods to enable pooling of the results from the two trials. Outcomes evaluated in the pooled analyses included those that were part of the individual trials such as responder rate, percent change in walking speed, and change in the MSWS-12, Ashworth, and LEMMT scores. The definition of a responder was the same as that used in the prospective analysis of the individual trials: a patient with a faster walking speed for at least three of the four on-treatment visits compared with the maximum speed during any of the five off-treatment visits. The proportions of participants achieving incremental thresholds of walking speed improvement—that is, improvements of 10% to 60% relative to baseline—were also determined to provide a set of alternative responder criteria to test the robustness of the primary result. Responses were also evaluated based on stratification of patients with respect to demographic and clinical characteristics including age, gender, race, body-mass index, MS phenotype, disease duration, baseline EDSS score, baseline walking speed, and concomitant use of immunomodulatory therapies.
The safety profile in the pooled population was characterized based on the incidence of adverse events (AEs; including serious AEs [SAEs] and withdrawals due to AEs), vital signs, clinical laboratory tests, and electrocardiogram measurements.
Statistics
Efficacy assessment was based on a modified intent-to-treat population defined as all randomized patients who received double-blind investigational drug and had at least one subsequent primary efficacy assessment. For demographic characteristics, P values were obtained from logistic regression or analysis of variance (ANOVA) models using the covariates of treatment, study, and center. Percent change from baseline was compared between D-ER and placebo using ANOVA with treatment group, center, and study as the explanatory variables. For proportion of responders, P values were obtained using logistic regression model with covariates of treatment, study, and center; and Fisher exact test was used for evaluation of proportion of patients achieving the various thresholds of increase in walking speed. Responder rates stratified by demographic and clinical characteristics were evaluated using logistic regression models, with covariates of treatment, study, center, characteristic, and interaction between treatment and the characteristic. Changes in MSWS-12, Ashworth, and LEMMT scores were evaluated using ANOVA with treatment group, center, and study as the explanatory variables. A P value of ≤.100 was considered significant for all interactions, and an alpha level of .05 was considered statistically significant for all other comparisons.
Results
Disposition and Demographics
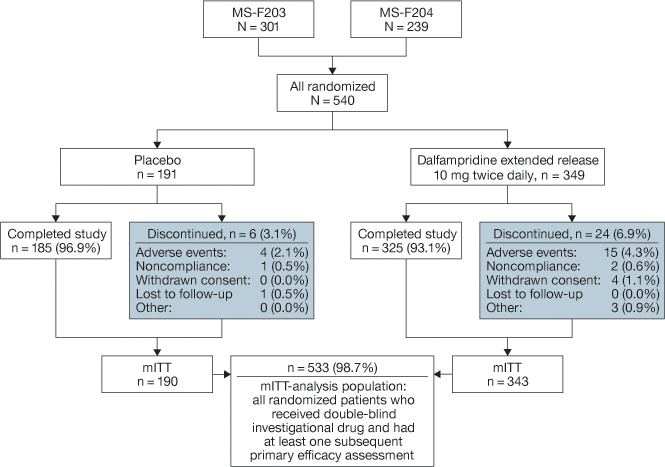
A total of 540 patients in the two studies were randomized to treatment with placebo (n = 191) or D-ER (n = 349), and 185 (96.9%) and 325 (93.1%) of the patients in the two groups, respectively, completed the study (Figure 1). Discontinuations were mainly for AEs: 2.1% in the placebo and 4.3% in the D-ER group.
Disposition of patients in the population pooled from studies MS-F203 and MS-F204. mITT, modified intent-to-treat (population).
Demographic and clinical characteristics were generally similar between treatment groups except for gender, for which there was a higher proportion of males in the placebo group relative to D-ER (38.7% vs. 28.1%; P = .0086) (Table 1). The predominant form of MS was secondary progressive, followed by relapsing remitting, and the mean EDSS scores were 5.6 (SD = 1.2; range 2–7) and 5.8 (SD = 1.0; range 3–7) for placebo and D-ER, respectively, indicating walking disability severe enough to prevent full daily activities and on the threshold of requiring unilateral assistance to walk 100 m (Table 1).
Demographic and clinical characteristics of the pooled population

Efficacy
Relative to placebo, patients treated with D-ER had a significantly greater overall percent improvement from baseline in walking speed, averaged over the treatment period: 13.8% versus 6.5% (P < .0001). The D-ER group was further characterized by a significantly higher proportion of T25FW responders: 37.6% versus 8.9% (P < .0001). Among the D-ER responders, the percent improvement from baseline in walking speed averaged over the treatment period was 25.0%, as compared with 7.0% for D-ER–treated nonresponders and 6.5% for the placebo group.
When the proportions of patients achieving incremental thresholds of walking speed improvement over baseline were evaluated, significantly (P < .05) greater proportions of D-ER–treated patients had increases of at least 10%, 20%, 30%, and 40% relative to placebo (Figure 2A). In particular, approximately 33% of the patients receiving dalfampridine had an increase of at least 20% in walking speed, compared with 13.7% of patients receiving placebo (P < .0001). Stratifying the D-ER treatment group by responder and nonresponder status revealed that the proportions of D-ER responders who achieved threshold levels of walking speed improvement were consistently higher than for D-ER nonresponders and those receiving placebo (Figure 2B). For the threshold of ≥20% increase in walking speed, the proportion of D-ER responders was 58.9%, which was more than 4-fold greater than for the placebo group (13.7%).
Proportions of pooled patients with increase in average walking speed during treatment at a range of threshold improvements. A, Dalfampridine extended release (dalfampridine-ER) versus placebo; B, stratified by dalfampridine-ER responders and nonresponders.

Among the patients in the D-ER treatment group, the responder rate was independent of demographic variables, disease characteristics, and level of disability as assessed by EDSS, with responder rates that were generally consistent across all variables. In all cases, the estimated interaction P values exceeded .100, providing no evidence for a treatment interaction between responder rate and the following: any of the demographic characteristics, including gender (P = .5373), age (<45 years, 45–64 years, ≥65 years; P = .7736), race (white, nonwhite; P = .9774), and body-mass index (<25 kg/m2, 25–30 kg/m2, >30 kg/m2; P = .8591); disease characteristics of MS type (P = .7762) or disease duration stratified by quartiles (P = .7239); disability stratified by baseline EDSS score (≤5.5, 6, ≥6.5; P = .2591) or baseline walking speed stratified by quartile (P = .7239). The responder rate in the D-ER group was consistently and substantially higher than in the placebo group across the strata of all variables. Similar results were obtained in an analysis stratified by whether or not patients used immunomodulatory therapies; responder rates were 36.5% and 6.8% in the D-ER and placebo groups, respectively, among users of these therapies, and 39.8% (D-ER) and 14.0% (placebo) among nonusers, with an interaction P value of .1230.
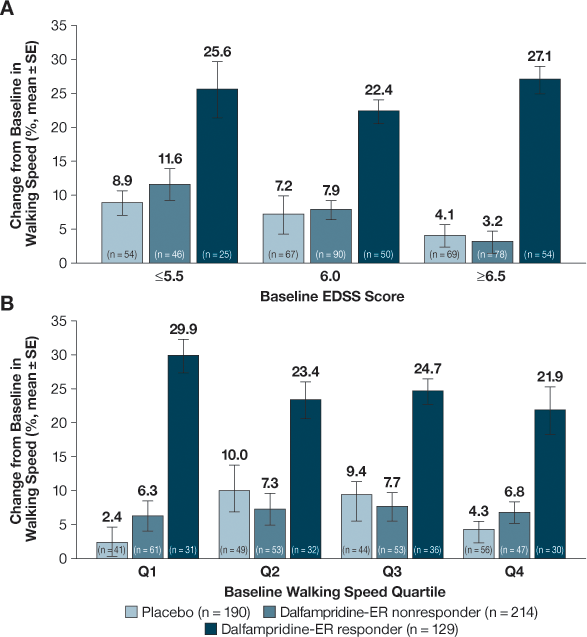
Evaluation of the percent change from baseline in walking speed among D-ER responders also demonstrated that the improvements were independent of baseline EDSS scores (Figure 3A) or walking speed (Figure 3B). D-ER responders demonstrated improvements in walking speed of 22.4% to 27.1% across EDSS scores and 21.9% to 29.9% across walking speed quartiles. These increases were substantially higher than for placebo and D-ER nonresponders and were consistent with the 25.0% observed for all D-ER responders.
Percent change in walking speed from baseline (averaged over the treatment period, intent-to-treat population). A, Baseline Expanded Disability Status Scale (EDSS) score; B, baseline walking speed quartile Q1 (0.48–1.54 ft/s), Q2 (1.54–2.17 ft/s), Q3 (2.17–2.70 ft/s), and Q4 (2.70–3.55 ft/s). Dalfampridine-ER, dalfampridine extended release.
Greater improvement on both the Ashworth score and the LEMMT was noted in the D-ER group compared with placebo as evaluated by the average change from baseline on both scales. For the Ashworth score, the average change with D-ER was −0.16 versus −0.07 for placebo (P < .001), and on the LEMMT the average changes in scores were 0.12 and 0.04 for D-ER and placebo, respectively (P = .001). Improvement on the LEMMT in the D-ER group was driven by the change of 0.17 among responders relative to 0.09 for nonresponders. In contrast, both responders and nonresponders showed improvement in the Ashworth: −0.16 and −0.17, respectively.
For the patient's subjective responses, the mean change in MSWS-12 score in the D-ER group, −2.68 points, indicated a significant improvement relative to the placebo group, which experienced a slight worsening in score (change of 0.69 point; P =.0042). Furthermore, the mean improvement in MSWS-12 score among D-ER responders, −6.61, was substantially greater than for D-ER nonresponders (−0.32) and placebo (0.69).
Safety
Adverse events were reported in 84.5% and 71.7% of D-ER– and placebo-treated patients, respectively, with the majority of these events classified as mild or moderate in severity (Table 2). The incidence of SAEs 6.3% in the D-ER group and 1.6% for placebo; none of the SAEs were reported in more than one patient in either treatment group except for pneumonia, syncope, and urinary tract infection (UTI), each of which was reported by two patients (0.6%) in the larger D-ER group. The incidence of treatment-related AEs was higher in the D-ER group (25.6%) than with placebo (17.8%), and few patients in either group discontinued due to AEs (3.2% D-ER and 2.1% placebo). No notable patterns were observed between treatment groups with respect to laboratory values, vital signs, or electrocardiogram results.
Adverse events (AEs) in the pooled analysis
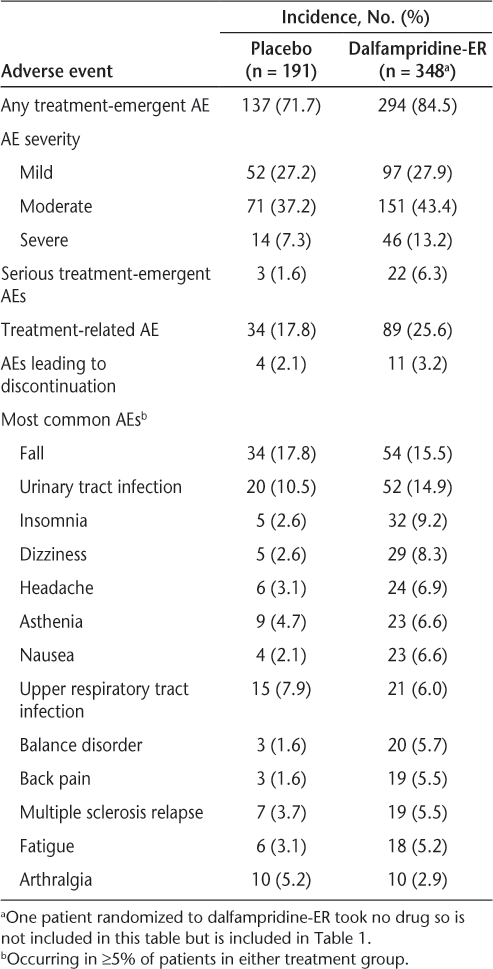
The most common AEs in both the groups were falls and UTIs, and several common AEs, including UTIs, insomnia, dizziness, headache, asthenia, and nausea, were observed with a higher frequency in the D-ER group relative to placebo (Table 2).
Two seizures were reported in total. One seizure, in the placebo group, was a presumed complex partial seizure, and the other, in a patient treated with D-ER, was a focal seizure involving the right extremity in the context of severe sepsis associated with community-acquired pneumonia. Both events were judged by the site investigators as possibly related to treatment.
Discussion
Results of this analysis, using pooled populations from two phase 3 trials, further support the efficacy and tolerability of D-ER for the improvement of walking that was observed in the individual studies. Improvements in walking associated with D-ER treatment encompassed both objective and patient-reported measures of impairment, and the results of the patient-reported outcomes supported the clinical impact of changes in the objective measures. Additionally, significant improvements with D-ER relative to placebo were observed in spasticity and lower-leg strength, although the clinical relevance of these changes was not determined.
The proportion of responders in the pooled D-ER group, 37.6%, was significantly higher than for placebo (8.9%; P < .0001) and was consistent with the findings of the individual trials.11 12 There was similar concordance between the pooled analysis and individual trials for improvement in walking outcomes among D-ER responders; these responders demonstrated a higher percent increase in walking speed and greater patient-reported improvements in walking on the MSWS-12 relative to placebo and D-ER nonresponders.
Methodological concerns have been raised regarding the post hoc responder analysis for the earlier phase 2 study.29 The consistency of results in phase 3, across trials, and pooled analysis confirms the underlying rationale for its use. Importantly, more than half of the D-ER responders (59%) had an increase in walking speed of ≥20%, which is considered a clinically meaningful change.22–25 30 The average change in the patient-reported MSWS-12 score among D-ER responders also exceeded the range of estimated values of the minimal clinically important difference (4.0–5.7 points),31 indicating that the improvements in walking are clinically relevant from the patient's perspective.
Analyses of the interactions between response and various demographic and clinical characteristics failed to determine any response predictors. Since response was independent of the evaluated characteristics, the data suggest that benefits may be achieved across the spectrum of these characteristics, including the various clinical phenotypes of MS, and regardless of disease duration or degree of walking impairment at baseline speed. Interestingly, not only was there no effect of these variables on the responder rate, but the relative magnitude of the improvement in walking speed also appeared to be independent of baseline level of disability, assessed using EDSS and walking speed. In this context, it is important to note that the recruitment criteria for the studies included baseline walking speeds of 8 to 45 seconds in order to reduce the potential for ceiling effects from patients who walked too quickly on the T25FW and the wide variability associated with more severe deficits. Nevertheless, the data do not suggest a relationship between deficit severity and treatment benefits, and therefore do not lead to the expectation that therapeutic benefit could not be obtained in patients outside this range of baseline walking speed. However, it should also be noted that the efficacy of dalfampridine was not tested in subjects outside of the 8- to 45-second range on the T25FW, and there are likely to be thresholds above and below for which benefits are unlikely to be obtained, and such thresholds require further characterization. Of additional clinical relevance, the response was independent of the use of immunomodulatory therapies, indicating that D-ER can be used whether or not patients are using these medications.32–34 It should also be noted that D-ER as a treatment option does not preclude use of exercise, physical therapy, assistive devices, or other symptomatic therapies that may improve the ease or safety of walking, although D-ER in combination with other modalities has not been formally evaluated.
The observed safety profile was similar to what was reported in the individual trials, and no new safety signals were identified. Many of the most common AEs were related to the central nervous system and, consistent with the mechanism of action of D-ER,35 may be related to potential stimulatory effects of the drug on the nervous system. No definite drug-related seizures were reported among D-ER–treated patients during the course of the phase 3 studies, and just one seizure event was reported in each treatment group (<1%). It should also be noted that a history of seizure was an exclusion criterion in both of the studies on which this pooled analysis was based and is a contraindication for use of the drug.
Although a possible limitation of the current analysis is the pooling of data from two studies with different double-blind treatment durations, the responder criterion used in these analyses is conceptually independent of study duration. Additionally, the robustness of the results supports the validity of pooling the two studies.
Conclusion
Results of a pooled analysis were consistent with individual clinical trials in demonstrating the efficacy and tolerability of D-ER for the improvement of walking impairment in patients with MS. The use of a responder analysis as the primary outcome, as prospectively defined and used in the studies reported here, is supported by the consistency of the response with alternative efficacy outcomes, thus demonstrating that the responder analysis provides a robust and useful outcome assessment in these patients.
Although walking improvements were observed in a subset of patients, these improvements were independent of any identified predictors of response. Further research is needed to identify and characterize predictors of responsiveness and to evaluate other potential functional outcomes.
PracticePoints
Dalfampridine, a potassium channel blocker, has been identified as having the potential to improve impaired neurologic function—that is, walking impairment—in patients with MS.
Pooled data from two trials showed that dalfampridine extended-release 10-mg tablets (D-ER) were safe and effective when compared with placebo.
Compared with placebo, D-ER was effective in improving walking in patients with MS—that is, it had a higher proportion of timed-walk responders and was associated with significant improvement in walking speed and 12-item Multiple Sclerosis Walking Scale score.
Acknowledgments
The authors thank E. Jay Bienen, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY, for editorial assistance. Editorial assistance was supported by Acorda Therapeutics, Inc., Ardsley, NY.
References
Crayton HJ, Rossman HS. Managing the symptoms of multiple sclerosis: a multimodal approach. Clin Ther. 2006; 28: 445–460.
Thompson AJ, Toosy AT, Ciccarelli O. Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol. 2010; 9: 1182–1199.
Samkoff LM, Goodman AD. Symptomatic management in multiple sclerosis. Neurol Clin. 2011; 29: 449–463.
de Sa JC, Airas L, Bartholome E, et al. Symptomatic therapy in multiple sclerosis: a review for a multimodal approach in clinical practice. Ther Adv Neurol Disord. 2011; 4: 139–168.
Ampyra [package insert]. Ardsley, NY: Acorda Therapeutics, Inc; 2013.
Australian Government, Department of Health and Aging, Therapeutic Goods Administration. Australian Public Assessment Report for Fampridine. http://www.tga.gov.au/pdf/auspar/auspar-fampyra.pdf. Accessed August 30, 2012.
European Medicines Agency Committee for Medicinal Products for Human Use. Summary of Opinion (Initial Authorisation) for Fampyra (Fampridine). http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002097/WC500106531.pdf. Accessed September 18, 2012.
Schwid SR, Petrie MD, McDermott MP, Tierney DS, Mason DH, Goodman AD. Quantitative assessment of sustained-release 4-aminopyridine for symptomatic treatment of multiple sclerosis. Neurology. 1997; 48: 817–821.
Vollmer T, Blight AR, Henney HR. Steady-state pharmacokinetics and tolerability of orally administered fampridine sustained release 10-mg tablets in patients with multiple sclerosis: a 2-week, open-label, follow-up study. Clin Ther. 2009; 31: 2215–2223.
Vollmer T, Henney HR. Pharmacokinetics and tolerability of single escalating doses of fampridine sustained-release tablets in patients with multiple sclerosis: a phase I-II, open-label trial. Clin Ther. 2009; 31: 2206–2214.
Goodman AD, Brown TR, Krupp L, et al. Sustained release of oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009; 373: 732–738.
Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010; 68: 494–502.
Goodman AD, Brown TR, Cohen JA, et al. Dose-comparison trial of sustained-release fampridine in multiple sclerosis. Neurology. 2008; 71: 1134–1141.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31–36.
Kalkers NF, de Groot V, Lazeron RH, et al. MS functional composite: relation to disease phenotype and disability strata. Neurology. 2000; 54: 1233–1239.
Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite; a new clinical outcome measure for multiple sclerosis clinical trials. Mult Scler. 2002; 8: 359–365.
Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999; 122: 871–882.
Cohen JA, Fischer JS, Bolibrush DM, et al. Intrarater and interrater reliability of the MS functional composite outcome measure. Neurology. 2000; 54: 802–806.
Schwid SR, Goodman AD, Mattson DH, et al. The measurement of ambulatory impairment in multiple sclerosis. Neurology. 1997; 49: 1419–1424.
Bethoux FA, Bennett SE. Evaluating walking mobility in patients with multiple sclerosis: utility of instruments in clinical trials and clinical practice. Int J MS Care. 2011; 13: 4–14.
Gijbels D, Dalgas U, Romberg A, et al. Which walking capacity tests to use in multiple sclerosis? a multicentre study providing the basis for a core set. Mult Scler. 2011; 18: 364–371.
Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000; 6: 286–290.
Schwid SR, Goodman AD, McDermott MP, Bever CF, Cook SD. Quantitative functional measures in MS: what is a reliable change? Neurology. 2002;58:1294–1296.
Kragt JJ, van der Linden FA, Nielsen JM, Uitdehaag BM, Polman CH. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Mult Scler. 2006; 12: 594–598.
van Winsen LM, Kragt JJ, Hoogervorst EL, Polman CH, Uitdehaag BM. Outcome measurement in multiple sclerosis: detection of clinically relevant improvement. Mult Scler. 2010; 16: 604–610.
Bosma LV, Kragt JJ, Brieva L, et al. Progression on the Multiple Sclerosis Functional Composite in multiple sclerosis: what is the optimal cut-off for the three components? Mult Scler. 2010;16:862–867.
Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964; 192: 540–542.
Medical Research Council (MRC). Aids to the Investigation of Peripheral Nerve Injuries. London, United Kingdom: Her Majesty's Stationery Office; 1976.
Kryscio RJ. Fampridine for MS responders: clinically relevant or hypothesis generating? [comment]. Neurology. 2008; 71: 1130–1131.
Coleman CI, Sobieraj DM, Marinucci LN. Minimally important clinical difference of the Timed 25-Foot Walk Test: results from a randomized controlled trial in patients with multiple sclerosis. Curr Med Res Opin. 2012; 28: 49–56.
Hobart J. Prolonged-release fampridine for multiple sclerosis: was the effect on walking ability clinically significant? [abstract]. Mult Scler. 2010;(suppl 16):S72.
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009; 256: 568–576.
Rinon A, Buch M, Holley D, Verdun E. The MS Choices Survey: findings of a study assessing physician and patient perspectives on living with and managing multiple sclerosis. Patient Prefer Adherence. 2011; 5: 629–643.
Margolis JM, Fowler R, Johnson BH, Kassed CA, Kahler K. Disease-modifying drug initiation patterns in commercially insured multiple sclerosis patients: a retrospective cohort study. BMC Neurol. 2011; 11:122.
Dunn J, Blight A. Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr Med Res Opin. 2011; 27: 1415–1423.
Financial Disclosures: Dr. Goodman has served as a paid consultant for Acorda, Biogen Idec, Alexion, Genzyme, and Teva. Dr. Brown has served as a consultant for Acorda, Bayer, Biogen, Genzyme, Pfizer, and Teva; has received honoraria from Acorda, Pfizer, and Teva; and has received research funding/grants from Acorda, Biogen, Lilly, and Teva. Mr. Klingler, Dr. Cohen, and Dr. Blight are employees and stockholders of Acorda Therapeutics, Inc., Ardsley, NY. Dr. Schapiro has no conflicts of interest to disclose.
Funding/Support: This research was supported by Acorda Therapeutics, Inc.







