Publication
Research Article
International Journal of MS Care
Case Report: Osmotic Demyelination Syndrome in an Adolescent with Neuromyelitis Optica
A 17-year-old girl presented with intractable vomiting due to area postrema involvement in the first presentation of seronegative neuromyelitis optica (NMO). During the course of her illness, she developed mild hyponatremia, and magnetic resonance imaging revealed abnormalities consistent with the co-occurrence of osmotic demyelination syndrome (ODS). This combination of imaging features is novel, and this case expands the spectrum of brain abnormalities seen in NMO and NMO spectrum disorders. It was suspected that NMO may predispose to ODS by causing astrocyte dysfunction involving aquaporin 4 water channels, which are implicated in both conditions.
Osmotic demyelination syndrome (ODS) predominantly occurs in the setting of chronic illness. Herein we present an unusual case of neuromyelitis optica (NMO) complicated by concomitant ODS.
Case Report
A 17-year-old female patient with no significant medical history developed persistent, daily vomiting that lasted 1 month. She received outpatient investigation, but no cause was uncovered. Four weeks later, she was admitted to the hospital with a subacute onset over days of weakness of her limbs and bilateral visual loss. Neurologic examination revealed dilated pupils with no response to light and markedly impaired visual acuity (hand movements), in keeping with bilateral optic neuritis, and a spastic quadriparesis with a cervical sensory level. Within days she deteriorated to reach a nadir of 0/5 Medical Research Council grading of power in the left arm, left leg, and right arm and 2/5 in the right leg, with no perception of light in either eye and features of a pseudobulbar palsy (dysarthria, labile mood). She required intubation for ventilatory support in the intensive care unit. Throughout her intensive care unit admission, she maintained a normal level of consciousness apart from short periods of sedation.
On initial laboratory investigations, mild hyponatremia was present (sodium level, 132 mmol/L). Full blood cell count, international normalized ratio, prothrombin time, renal and liver functions, vitamin B12 level, and potassium level were normal. Syphilis and HIV serologic test results were negative. Results of an autoimmune screen (antinuclear factor, anti–double-stranded DNA, antimyeloperoxidase, anticardiolipin antibodies, rheumatoid factor) were negative apart from a weakly positive lupus anticoagulant. Cerebrospinal fluid showed a raised protein level of 0.86 g/L, a glucose level of 2.4 mmol/L, and a lymphocyte count of 1/mm3. Of note, testing for the aquaporin 4 immunoglobulin G antibody had negative findings (visual fluorescence-observation cell-based assay; Euroimmun Medizinische Labordiagnostika AG, Luebeck, Germany).
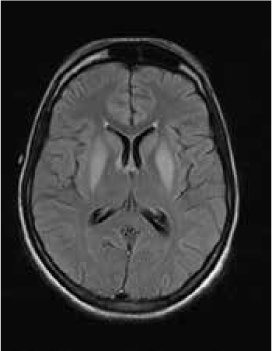
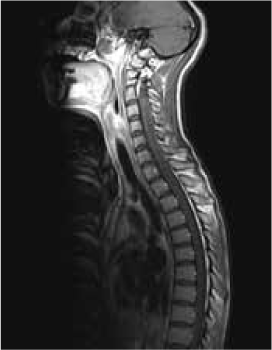
Magnetic resonance imaging (MRI) of the brain showed symmetrically increased T2-weighted and fluid-attenuated inversion recovery signal in the central pons (Figure 1) and in the striatum of the basal ganglia (Figure 2), with no contrast enhancement seen on corresponding T1-weighted postgadolinium images (Figures 3 and 4). Spinal MRI (Figure 5) demonstrated high signal on T2-weighted images from the area postrema in the medulla extending caudally to the T9 thoracic segment, with marked cord swelling and mild enhancement on T1-weighted postcontrast studies (Figure 6), in keeping with a longitudinally extensive inflammatory myelitis.
Magnetic resonance image of brain shows symmetrical, central high signal in pons on axial fluid-attenuated inversion recovery sequences
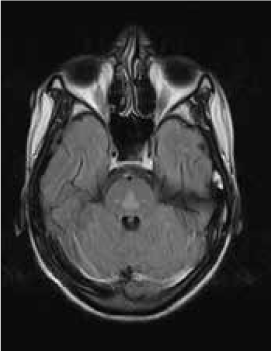
Magnetic resonance image of brain shows bilateral symmetrical high signal in caudate and lentiform nuclei on axial fluid-attenuated inversion recovery sequences
Postcontrast magnetic resonance image of brain show nonenhancing changes in pons
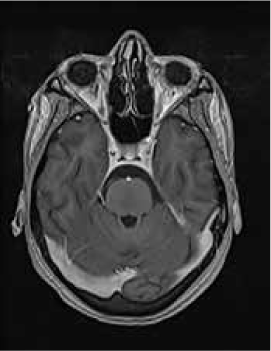
Postcontrast magnetic resonance image of brain show nonenhancing changes in basal ganglia

Magnetic resonance image of spine shows longitudinally extensive intrinsic increased cord signal on sagittal T2-weighted sequences
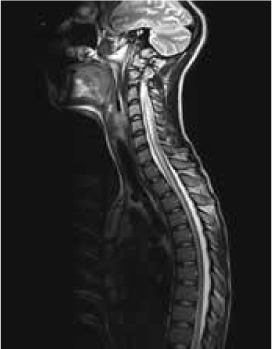
Magnetic resonance imaging shows longitudinally extensive cord signal abnormality with peripheral enhancement on sagittal T1-weighted postcontrast sequence
The clinical and radiologic features supported the diagnosis of NMO. However, the symmetrical pontine and basal ganglia high signal without contrast enhancement were considered to be strongly suggestive of concomitant ODS (pontine and extrapontine myelinolysis).
The patient was treated promptly with 5 days of intravenous methylprednisolone followed by oral corticosteroids and plasmapheresis. Urinary electrolytes were not tested. However, the patient was treated for euvolemic hyponatremia with fluid restriction until her serum sodium level normalized after 5 days. A chest radiograph revealed patchy airway consolidation. Real-time polymerase chain reaction for Mycobacterium tuberculosis (GeneXpert; Cepheid, Sunnyvale, CA) on tracheal aspirate had positive results, and she commenced a three-drug treatment regimen for tuberculosis consisting of rifampicin, isoniazid, and pyrazinamide. Ethambutol was not given due to concerns that it would exacerbate visual impairment.
During the course of plasmapheresis, her vision improved to counting fingers, and after 4 weeks she was able to identify colors correctly. Limb power also improved gradually. Azathioprine therapy was started in an attempt to prevent further relapses. She was referred to an inpatient rehabilitation facility for intensive physiotherapy and occupational therapy. At the 6-month follow-up visit, she had made a modest recovery and was able to perform some activities of daily living (eating, washing) unaided and to walk using only one crutch. However, she required supervision when leaving her home, and her visual acuity remained static. At the follow-up visit at 14 months, she was able to ambulate with the assistance of one crutch, and had residual bilateral severe visual impairment being able to count fingers only.
The University of Cape Town Human Research Ethics Committee granted permission for the research, and the patient provided informed consent after her 18th birthday.
Discussion
As mentioned, ODS mainly occurs in the setting of chronic illness. It occurs exclusively in patients admitted to the hospital and was not described before the advent of intravenous fluids, supporting an iatrogenic etiology.1 Rapid intravenous correction of hyponatremia is the main cause, and in an ill patient, hyponatremia need not be severe2 and the correction need not be rapid3 to give rise to ODS.
Neuromyelitis optica may lead to hyponatremia via at least two mechanisms. Area postrema involvement frequently causes intractable vomiting, and this may result in a low serum sodium level. Moreover, the syndrome of inappropriate antidiuretic hormone may develop in patients with NMO and hypothalamic lesions. In the present patient, the lowest recorded sodium level was 132 mmol/L. However, MRI demonstrated features of both central pontine and extrapontine myelinolysis, which, when occurring together, are considered virtually pathognomonic of ODS.4 Typical features include the symmetrical nature of the changes, the involvement of areas typically affected in ODS (pons, basal ganglia), and the sparing of peripheral pontine fibers. However, it remains a possibility that these radiologic changes were due to NMO primarily rather than osmotic demyelination. In a recent review, Kim et al.5 expanded on the range of MRI abnormalities consistent with NMO and NMO spectrum disorders. Gray matter demyelination of the thalamus and bilateral involvement of the corticospinal tracts have been described, although published examples are not as strikingly symmetrical as in this case and do not show the combination of central pontine demyelination with symmetrical striatal involvement.5 To our knowledge, these typical ODS changes in the context of NMO have been described in only one other case, in the Japanese literature.6
In both NMO and ODS, there is aquaporin 4 channel dysfunction in astrocytes. In seropositive NMO, autoantibodies target aquaporin 4 channels. In seronegative NMO, T cells reacting to aquaporin 4 occur in similar frequencies to seropositive NMO and in significantly greater numbers than in controls.7 8 The loss of aquaporin 4 channels has been demonstrated in astrocytes of ODS lesions in autopsy specimens.9 The occurrence of ODS in the context of NMO is intriguing and raises the question of whether immune-mediated astrocyte dysfunction due to humoral targeting of the aquaporin 4 water channel in NMO increases the susceptibility to osmotic demyelination.
PRACTICE POINTS
We present an unusual case of neuromyelitis optica (NMO) complicated by concomitant osmotic demyelination syndrome (ODS). Astrocyte dysfunction may underlie the susceptibility to the co-occurrence of NMO and ODS.
Intractable vomiting with or without hyponatremia should alert the clinician to consider a central cause such as an area postrema syndrome secondary to NMO; in such a setting, judicious correction of hyponatremia is necessary to prevent ODS.
Symmetrical imaging abnormalities consistent with ODS broaden the spectrum of brain magnetic resonance imaging lesions seen in NMO and NMO spectrum disorders.
Financial Disclosures:
The authors have no conflicts of interest to disclose.
References
Alleman AM. Osmotic demyelination syndrome: central pontine myelinolysis and extrapontine myelinolysis. Semin Ultrasound CT MR. 2014;35:153–159.
Ruiz-Sandoval JL, Chiquete E, Álvarez-Palazuelos LE, et al. Atypical forms of the osmotic demyelination syndrome. Acta Neurol Belg. 2013;113:19–23.
Qayyum A, Afshan R. Can central pontine myelinolysis be prevented through non-rapid serum sodium correction? Acta Neurol Belg. 2013;113:341–342.
Osborn AG. Diagnostic Imaging. Salt Lake City, UT: Amirsys; 2004.
Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–1173.
Sakai W, Matsui N, Fujita K, et al. A case of neuromyelitis optica spectrum disorder with central pontine and extrapontine myelinolysis preceded by syndrome of inappropriate antidiuretic hormone secretion [in Japanese]. Clin Neurol. 2014;54:556–560.
Bernard-Valnet R, Liblau RS, Vukusic S, et al. Neuromyelitis optica: a positive appraisal of seronegative cases. Eur J Neurol. 2015;11:1511–1518.
Vaknin-Dembinsky A, Brill L, Kassis I, et al. T-cell reactivity against AQP-4 in neuromyelitis optica. Neurology. 2012;79:945–947.
Popescu BF, Bunyan RF, Guo Y, et al. Evidence of aquaporin involvement in human central pontine myelinolysis. Acta Neuropathol Commun. 2013;1:40.







