Publication
Research Article
International Journal of MS Care
Assessing Relapses and Response to Relapse Treatment in Patients with Multiple Sclerosis
Author(s):
There are currently no assessment tools that focus on evaluating patients with multiple sclerosis (MS) who are experiencing a relapse or that evaluate patients' response to acute relapse treatment. In practice, assessments are often subjective, potentially resulting in overlooked symptoms, unaddressed patient concerns, unnoticed or underrecognized side effects of therapies (both disease modifying and symptomatic), and suboptimal therapeutic response. Systematic evaluation of specific symptoms and potential side effects can minimize the likelihood of overlooking important information. However, given the number of potential symptoms and adverse events that patients may experience, an exhaustive evaluation can be time-consuming. Clinicians are thus challenged to balance thoroughness with brevity. A need exists for a brief but comprehensive objective assessment tool that can be used in practice to 1) help clinicians assess patients when they present with symptoms of a relapse, and 2) evaluate outcomes of acute management. A working group of expert nurses convened to discuss recognition and management of relapses. In this article, we review data related to recognition and management of relapses, discuss practical challenges, and describe the development of an assessment questionnaire that evaluates relapse symptoms, the impact of symptoms on the patient, and the effectiveness and tolerability of acute treatment. The questionnaire is designed to be appropriate for use in MS specialty clinics, general neurology practices, or other practice settings and can be administered by nurses, physicians, other clinicians, or patients (self-evaluation). The relapse assessment questionnaire is currently being piloted in a number of practice settings.
Relapses can be a defining feature of multiple sclerosis (MS) for patients. They involve acute physical and cognitive symptoms and present psychosocial and emotional challenges that can significantly disrupt a patient's life.1 2 Relapses may contribute to disease progression and disability, although some debate exists regarding whether and to what extent they may affect long-term outcomes.3–7 They may be indicative of a suboptimal response to chronic treatment with disease-modifying drugs (DMDs)8 or a sign of increased disease activity.9
Despite awareness of the impact of relapses on patients with MS, relatively little practical guidance is available with respect to the determination of the relapse severity, acute treatment response, and the extent of recovery. In the clinical setting, most clinicians recognize the importance of considering patients' perceptions. This perspective is often overlooked in research studies that shape clinical practice. Data from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, for example, indicate that many patients are dissatisfied with corticosteroid treatment, the most common form of relapse management.10 A more systematic approach to relapse evaluation that takes into account the patient experience may lead to improved management and clinical outcomes. Although several objective assessment scales for MS-associated symptoms and disability are available, the field lacks an assessment tool designed specifically to evaluate relapses. In particular, the need exists for an instrument that can be used to evaluate not only the acute changes in symptoms and the impact on daily functioning associated with relapse, but also the patient's perception of the degree of symptom resolution, restoration of function, and satisfaction with treatment and outcomes. Such an instrument will provide critical information regarding past and present symptoms and functional activities. It will also examine outcomes that include patient perceptions and satisfaction, and ultimately will contribute to the more optimal, patient-based approaches for resolving future relapses.
A working group of MS nurse experts from the United States and Canada convened in February 2011 to discuss the impact, evaluation, and treatment patterns of MS relapses in clinical practice. The purpose of the meeting was to arrive at a consensus on 1) criteria to consider when assessing an MS relapse, 2) how to evaluate treatment outcomes, and 3) the appropriate timing of such assessments. Consensus reached in these three areas led to the development of an instrument that can be widely used in the MS community for the assessment of relapses and response to treatment. This article was developed based on the meeting, and was intended to provide a nursing perspective on best practices in relapse management. It will briefly review data related to identifying, assessing, and managing MS relapses; describe challenges associated with assessing and managing relapses; and outline the creation of a new tool designed to facilitate assessment and response to the treatment of acute relapse.
Defining an MS Relapse
Optimal management of MS relapses begins with determining what a relapse is. Generally accepted principles exist, but specific definitions vary considerably in the MS community. There are limited objective diagnostic criteria that can be used in clinical practice. The recently updated McDonald criteria11 define an attack (relapse, exacerbation) as “patient-reported or objectively observed events typical of an acute inflammatory demyelinating event in the CNS [central nervous system], current or historical, with duration of at least 24 hours, in the absence of fever or infection.” This definition is appropriate for use as a standard criterion for the purposes of documenting the occurrence of relapses in a uniform way, such as in clinical trials. However, the definition is somewhat ambiguous and, as such, may not be interpreted consistently by practicing clinicians.12 Moreover, this definition may not necessarily capture the reality faced by MS clinicians. Additional considerations to verify a relapse or to determine the necessity of treatment may be required in certain circumstances.13 For example, subjective findings may be necessary in order to identify a sensory relapse. A key consideration, noted in the definition of relapse above, is the need to rule out fever, metabolic issues, infections, or other physiological processes, as these all may be indicators of pseudorelapse.7 9
A pseudorelapse is defined as an acute worsening of symptoms that is typically associated with an increase in body temperature, which can be due to infection, exercise, or heat exposure.7 9 In contrast to true relapses, which represent the manifestation of a demyelinating event, pseudorelapses produce symptoms that are associated with other physiological processes.7 9 14 Additional characteristics of true relapses are that they generally last for at least 24 hours, can last up to weeks or months, and frequently require treatment. Pseudorelapses, on the other hand, are more transient and typically resolve when the causative physiological stress has been removed or resolved.7 9 14 In clinical practice, however, the distinction between a true relapse and a pseudorelapse may not be as clear. For instance, in some cases, a true exacerbation may be the underlying cause of an infection (eg, an exacerbation associated with urinary symptoms/bladder retention can lead to a urinary tract infection), or an exacerbation can occur concomitantly with an infection. Although a comprehensive discussion of these issues is beyond the scope of this article, education of both patients and clinicians with regard to distinguishing between relapse and pseudorelapse would likely prove beneficial.
One reason for the difficulty in providing a more precise definition of relapse is that the presentation of an MS relapse is highly variable. Specific symptoms of MS relapses can differ widely among patients, often depending on the body system(s) affected. Symptoms can also differ between relapses within a single patient, although some evidence suggests that subsequent relapses tend to occur in the same CNS location as the initial event.15 In the London, Ontario, natural history cohort, sensory system involvement was reported most frequently (54%), followed by optic (22%), brainstem (21%), motor (18%), cerebellar (6%), and bowel/bladder (3%) involvement.3 The London, Ontario, data also illustrate that a single relapse can affect multiple body systems. Changes in cognition and increased fatigue are also common and should not be overlooked, as they are likely to impair patients' activities of daily living (ADLs) and work productivity.13 16
The severity of an MS relapse is one factor to consider when determining an appropriate management strategy. As with other aspects of relapse, little guidance is available in the literature with respect to the definition of severity. Freedman and colleagues17 developed a system that categorizes relapse severity as mild, moderate, or severe, depending on such issues as the number and type of body systems involved, effects on ADLs, whether treatment and/or hospitalization is required, and time to recovery. These suggested guidelines were developed to evaluate relapses retrospectively, however, not as guidance for the clinician who needs to determine whether treatment is necessary. Generally, a relapse that affects a patient's function, regardless of his or her particular symptoms, would be considered severe enough to recommend treatment.7
It is difficult to predict whether and when a particular patient will experience a severe relapse. One study showed that the risk for severe relapse was lower among patients receiving treatment with DMDs and higher among younger individuals.18 Another study found that greater severity of an initial demyelinating event was predicted by younger age and brainstem/cerebellum or cerebrum involvement; this study also demonstrated that occurrence of a prior severe event was associated with an increased odds ratio for a subsequent severe event.19 A relapse can be interpreted as an outward manifestation of damage in the CNS.9 Although no data are available that identify a clear correlation between physical or cognitive findings and damage to the CNS, lesions may ultimately affect functioning. Conversely, even if a patient does not perceive severe symptoms, a significant lesion may nonetheless be present.20
Another important aspect of defining an MS relapse is patients' understanding of what relapses are and their perception of relapse symptoms, which can differ considerably from clinicians' perceptions. Some patients may not spontaneously report relapses, whereas others may report having experienced multiple “relapses” over a short period of time, particularly if they perceive fluctuations in symptoms as relapses. Further, patients have different thresholds for symptom tolerability and degree of disability that they find manageable. Therefore, patient education is an important component of MS management, particularly with regard to relapses. For example, patients should be given clear instructions regarding when new symptoms or changes in the severity or frequency of chronic symptoms warrant professional contact. In addition, it is advisable that the clinician develop a collaborative patient relationship in order to identify changes from the patient's normal baseline physical and cognitive functioning.
Assessing MS Relapse and Recovery: Standardized Scales and Practical Challenges
Standardized assessments used in clinical trials of MS include the Expanded Disability Status Scale (EDSS)21; the Multiple Sclerosis Functional Composite (MSFC, which comprises the Nine-Hole Peg Test [NHPT], the Timed 25-Foot Walk [T25FW], and the Paced Auditory Serial Addition Test [PASAT])22; the Symbol Digit Modalities Test (SDMT)23; and the Fatigue Impact Scale (FIS).24 Characteristics and considerations regarding the use of each scale are summarized in Table 1.21 23–32 Although use of these scales is common in clinical trials, their use in clinical practice varies, and their utility in evaluating relapse and response to relapse treatment is somewhat limited. The EDSS and MSFC are not used routinely in the context of evaluating relapses, but may be required for clinical trials, insurance authorization for coverage of DMDs, or, in some clinical settings, as standard documentation for monitoring patients over time.
Selected standardized assessment scales used in clinical trials of MS
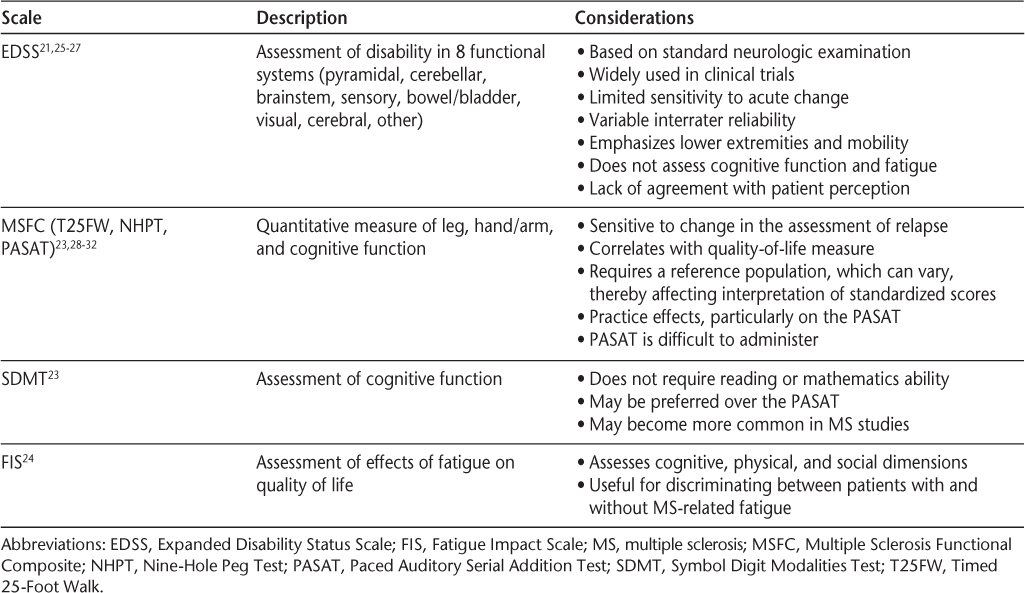
Several other assessment instruments have been developed and may be used as alternatives to the EDSS or MSFC in clinical trials or clinical practice to quantify disease severity. For example, the Scripps Neurological Rating Scale (SNRS) is an assessment based on a neurologic examination33; it has been used in some studies (eg, Sipe et al.34 and Lublin et al.35) but currently is not widely used in clinical trials or in clinical practice. An assessment tool for use in clinical practice was developed by Ross and colleagues.36 This instrument was designed to be completed by patients prior to an office visit. Although not formally validated, it provided a relatively easy way for clinicians to evaluate patient perception and quickly identify issues on which to focus. All of these scales were designed to help clinicians assess disease severity but may be of limited usefulness in capturing the important nuances necessary for evaluating relapse recovery.
Magnetic resonance imaging (MRI) can be used in the context of evaluating MS relapse; its value depends on the individual patient history and presentation. It is used more often to monitor disease progression or to evaluate whether chronic treatment needs to be altered.17 It can prove useful, however, in patients with vague, persistent symptoms, in order to determine if an increase in disease activity is present.
Practical challenges are often encountered in the initial assessment to determine whether a patient is experiencing a relapse and whether treatment is needed. A clinician may be unable to see a patient in the office for evaluation of an acute attack for a number of reasons. Relapses may occur outside of normal office hours, or a clinician may be unavailable for a patient to schedule an appointment with. Patients may be physically unable to get to a physician's office because of lack of transportation, bad weather, or severe symptoms, or they may be deterred by insurance limitations on the number of covered visits or co-payment requirements. Alternatives to an in-office assessment can include a telephone consultation, referral to a primary-care physician or to a hospital emergency department, or telemedicine (eg, via an Internet video service like Skype). The acceptability and frequency of use of these alternatives vary by practice. The relapse evaluation tool that was developed as a result of this nursing consensus initiative was designed to address the aforementioned limitations of the existing models, as a versatile option for acquisition of information.
In the context of evaluating recovery from relapse, establishing a baseline prior to relapse is necessary for later determination of the extent of recovery.35 For example, it is not sufficient to simply calculate the change in EDSS score from the assessment during the relapse to the assessment after the relapse. This change in score can indicate significant improvement even though the patient may have experienced an incomplete or partial recovery with residual disability based on the change from the pre-relapse baseline.14 37 Because the presence of residual symptoms or disability may have implications for the overall disease management strategy, it is important that clinicians accurately assess recovery in terms of a return to baseline.
Differences in the timing of post-relapse follow-up assessments can affect the degree of symptom improvement or residual disability observed.14 35 38 For example, in studies by Lublin et al.35 and Hirst et al.,14 mean EDSS scores were higher during the second month after relapse (days 30 to 59) and decreased during the third month (days 60 to 89), suggesting that disability may be largely stabilized by 3 months post-relapse. However, mean EDSS scores increased again at assessments conducted after 3 months. A practical consideration with regard to timing is that the post-relapse follow-up should be conducted within a reasonable amount of time; patients may be unable to accurately recall their response to treatment and/or side effects several months after an MS relapse, and a patient's clinical status can change during that time.
An important aspect of the assessment of relapse and treatment response is the patient's perception of symptoms and disability. As previously noted, the effect of symptoms varies according to the patient and is a key component in determining whether treatment is necessary. Similarly, it is important to ascertain how patients feel about their response to treatment. They may note a change in the severity of their symptoms but still experience considerable disability or side effects from the treatment. Some evidence suggests that a lack of agreement exists between scores on standardized assessments and patients' perceptions. For example, data from a 2010 study by van Winsen et al.26 indicated that EDSS scores do not accurately reflect patients' perceptions of improvement, with 42% of participants who were identified as experiencing significant improvement on the EDSS reporting that they felt little or no improvement. In addition, data from the NARCOMS Registry indicate that a substantial percentage of patients treated with intravenous (IV; 30%) or oral corticosteroids (39%) reported that their treatment had no effect or made their relapse symptoms worse.10 Considering previously reported evidence that many patients experience an incomplete recovery from relapses, even with treatment,14 18 35 these findings are not entirely surprising.
MS Relapse Management: Treatment Considerations
Relapses are generally considered self-limiting, tending to resolve over time even if not treated. However, as mentioned earlier, the relationship of unaddressed or poorly managed relapses to disease progression and disability is an area deserving of investigation. Importantly, MS is not only an inflammatory process but also a degenerative one.39 In addition to addressing the inflammation associated with relapses, it is possible that relapse treatments protect against neurodegeneration via a mechanism that is not yet well understood. For example, the anti-inflammatory actions of relapse treatments may provide indirect protection from neuronal damage,39 or there may be neuroprotective or neurotrophic effects mediated via factors such as brain-derived neurotrophic factor (BDNF) and cytokines or through direct actions on immune cells.40–46 Research on the treatment of MS relapses is incomplete in this respect. In addition, there remain many uncertainties around the relationship between relapse symptoms, lesions, and disability, as well as the effects of relapse treatment. As noted earlier, MRI can detect lesions indicative of inflammatory activity (ie, gadolinium-enhancing lesions) even in the absence of clinical relapse symptoms.20 Further, changes in MRI do not necessarily mirror the clinical course of symptom resolution associated with relapse treatment.47 48 Finally, there is currently no published evidence to demonstrate conclusively that treatment of relapses has an effect on the progression or long-term course of MS.49 50 Nonetheless, treatment has been shown to shorten the duration of a relapse and promote recovery,7 37 50 induces short-term immunologic effects,49 and may reduce residual disability in the short term.14 Based on this premise, treatment of relapses is part of MS standard practice.
Several treatment options for a relapse—including possible dosing regimens, adverse events, and corresponding references—are summarized in Table 2,50–73 with a brief description of each of these options noted below. Detailed descriptions of the clinical trials evaluating each relapse treatment are outside the scope of this assessment-focused review. We refer the reader to a recent review by Repovic and Lublin74 for a comprehensive summary of the literature supporting the efficacy and tolerability of various MS relapse treatments.
Treatment options for MS relapses
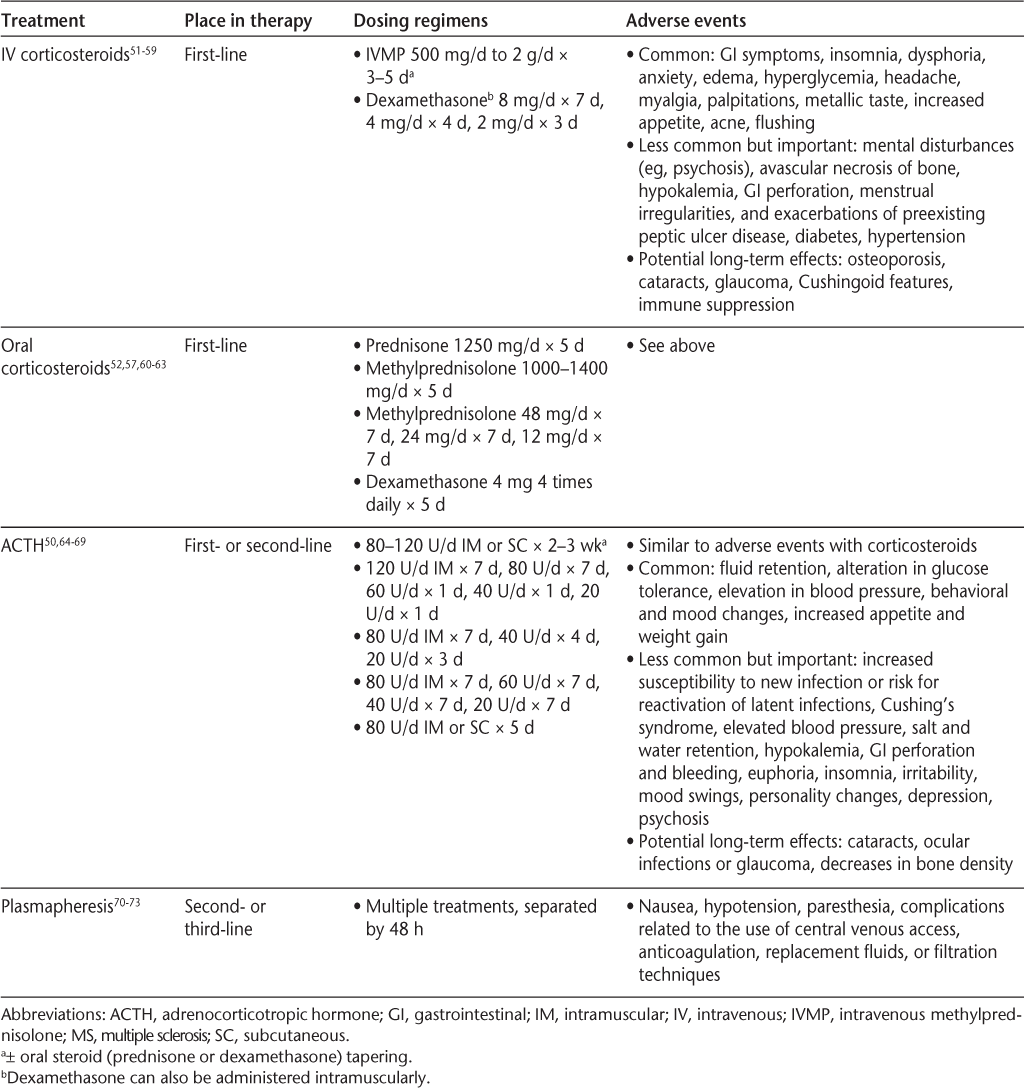
High-dose corticosteroid treatment is accepted as the standard of care for MS relapses.51 The efficacy of high-dose corticosteroids in speeding recovery from relapses has been demonstrated in several studies,52 56–58 75 including the Optic Neuritis Treatment Trial.54 The anti-inflammatory and immunosuppressive effects of corticosteroids are achieved through multiple mechanisms, including inhibition of inflammatory cytokine production, inhibiting activation of macrophages or microglia, reducing leukocyte chemotaxis, and increasing function of regulatory T cells; generally the actions of exogenous corticosteroids are mediated via binding to glucocorticoid receptors.76 Although IV administration of corticosteroids has been the most common approach, evidence of comparable efficacy and tolerability52 57 has led to increased use of orally administered high-dose steroids for the treatment of MS relapses.63 Potential benefits of high-dose oral corticosteroids include ease of initiating treatment, lack of need for home care or hospitalization, convenience, and low cost.61
Another US Food and Drug Administration (FDA)–approved therapy for the treatment of relapse is long-acting adrenocorticotropic hormone (ACTH) gel, a formulation of pro-opiomelanocortin peptides including ACTH(1–39).64 ACTH is a native melanocortin peptide that contains 39 amino acids.77 Historically, ACTH gel was thought to produce anti-inflammatory action indirectly, solely through physically increasing the release of cortisol from the adrenal gland (corticotropic effects) via a melanocortin receptor found in the adrenal cortex.78 However, the effects of ACTH gel are likely more than adrenocorticotropic in nature due to additional melanocortin receptor–mediated mechanisms.79 80 Melanocortin receptors as a class have been shown to exhibit various physiological actions in the body, including a variety of anti-inflammatory actions such as decreasing proinflammatory factors (cytokines and chemokines, nitric oxide, and adhesion molecules), inhibiting white blood cell migration, and increasing anti-inflammatory factors that may help to restore immune balance.79 80 According to the National Multiple Sclerosis Society (NMSS), ACTH gel may be appropriate in certain situations, such as when IV infusion is impractical, or in cases in which positive effects on bone via stimulation of 5-dehydroepiandrosterone (DHEA) and mineralocorticoids may be desirable.51 Other patient types who may benefit from ACTH gel treatment are those who do not tolerate or do not respond to steroids.
Other treatment options that are used less frequently include plasmapheresis and intravenous immunoglobulin (IVIG). Plasmapheresis (therapeutic plasma exchange) is typically reserved for those patients who experience an incomplete recovery with other treatments. Recently updated guidelines from the American Academy of Neurology suggest that plasmapheresis is probably effective as adjunctive therapy and possibly effective for exacerbations that fail to respond to high-dose corticosteroids.70 Intravenous immunoglobulin is generally considered to be second- or third-line therapy for patients in whom corticosteroids are contraindicated or ineffective, or for the treatment of relapses during pregnancy or the postpartum period.81 82
With regard to the timing of relapse therapy, the NMSS recommends treating major relapses as quickly as possible but notes that minor relapses may be monitored initially.51 However, some clinicians will begin treatment for minor relapses immediately if requested to do so by the patient. Usually this is done if symptoms have affected the patient's ADLs in ways that are significant to the patient. Treatment can be initiated several weeks into a relapse (eg, in the case of a patient who experiences a relapse but cannot obtain an appointment for 3 weeks); however, it may not be necessary to initiate treatment several months into a relapse or in those patients whose relapse symptoms appear to be improving.51
In practice, determination of the need for treatment is highly dependent on the individual patient. Certain symptoms that are not bothersome to some patients may cause significant impairment in others, depending on a person's occupation, home responsibilities, or leisure activities. For example, sensory relapses may require treatment, as these symptoms can have a significant impact on some patients' functional ability.13 Patient preference with regard to benefits versus risks associated with steroids and other treatments must be considered; relapses during pregnancy or breastfeeding can be particularly challenging in this respect. Other factors that contribute to the decision to initiate therapy and to the selection of appropriate treatment include cost, insurance coverage, and prior experience.
It is difficult to predict how well and how quickly a patient will recover from a relapse. For example, although some evidence suggests that patients with more severe relapses experience a greater response to IV methylprednisolone versus those with less severe relapses (based on the decrease in EDSS score from relapse to post-treatment),37 other studies have found that greater severity of relapse predicts worse overall recovery19 or residual disability.18 Incomplete recovery from a prior relapse, increased age, and location (spinal cord) also may be predictors of worse recovery.19 Therefore, managing patients' expectations is challenging and requires an understanding of patients' perceptions of their relapse symptoms and associated disability, as well as their expectations of treatment (or with respect to recovery if treatment is not initiated). As illustrated by the aforementioned NARCOMS Registry data,10 follow-up evaluations of patients' assessment of their recovery and/or treatment response are important for ascertaining whether a given relapse has been managed successfully. Moreover, these assessments are helpful and informative for determining the optimal management of future relapses.
Development of the Assessing Relapse in Multiple Sclerosis (ARMS) Questionnaire
The group of MS expert nurses agreed that there is a need for a relapse assessment tool that can be used clinically to evaluate short-term, relevant changes in symptoms and disability, as well as to assess treatment response in a way that more closely mirrors patients' perceptions. In creating this assessment tool, the aim was to develop questions that are easy to understand and that help patients identify how they are feeling relative to baseline when they arrive for treatment and following their treatment. It is important to determine patients' capabilities with respect to ADLs and to identify those symptoms that are experienced on a regular basis or at baseline, in order to assess both the impact of relapse symptoms and the degree of treatment response/recovery. Other considerations were that the assessment tool include an organized, systematic evaluation, with fatigue and cognitive function specifically addressed; that the tool determine whether patients have experienced changes in function; that the assessment of response gauge patients' status relative to how they were feeling during the relapse and also relative to “normal” (ie, prior to relapse), and inquire into treatment tolerability; and that the instrument be adaptable to a variety of clinical settings.
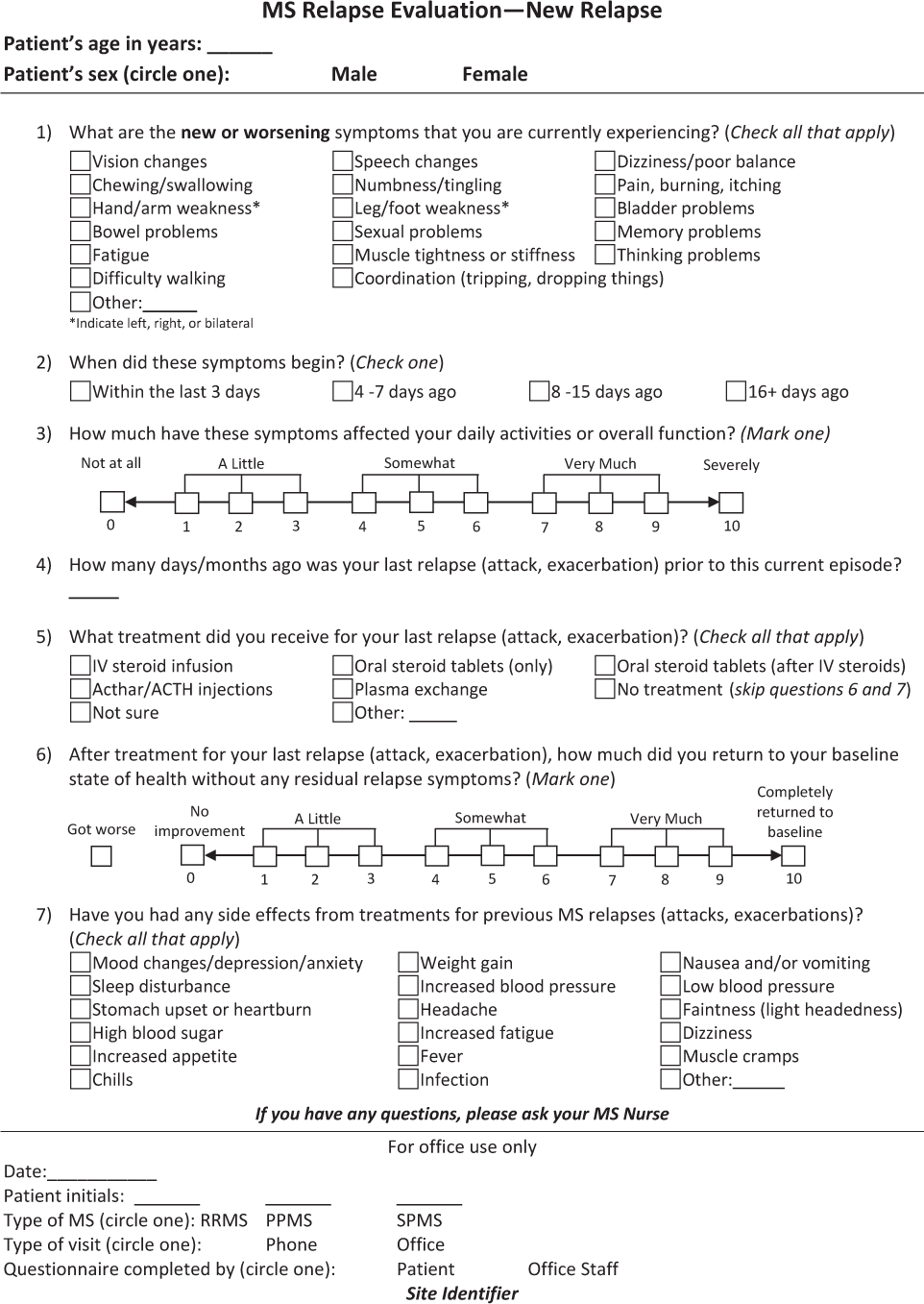
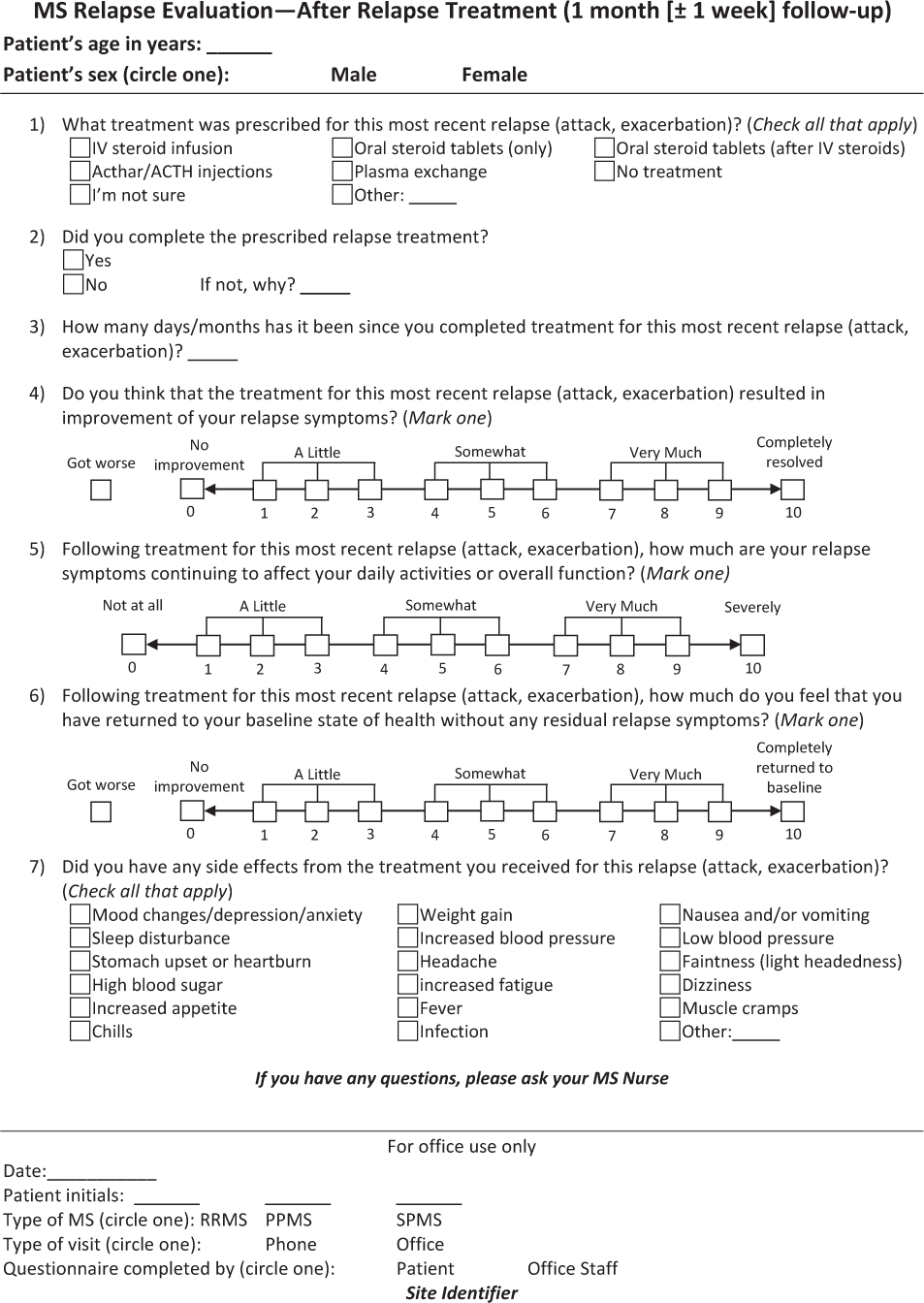
The Assessing Relapse in Multiple Sclerosis (ARMS) Questionnaire is shown in Figure 1. There are two parts to this questionnaire, with information collected at two time points. Figure 1A includes seven questions to evaluate a patient's relapse symptoms and how these symptoms affect his or her ADLs and overall function. This section also assesses the patient's response to past treatments of prior relapses and is thus a means of guiding treatment selection. Figure 1B includes seven questions to evaluate treatment response in terms of symptom relief and function, as well as treatment tolerability; this section is intended to be used approximately 3 to 6 weeks after treatment. The questionnaire can be used in MS specialty centers, general neurology practices, or other practice settings, and was designed with enough flexibility to be administered by nurses, other clinicians, or patients themselves. It lends itself to use for telephone interviews and ultimately could be incorporated into electronic health records.
Assessing Relapse in Multiple Sclerosis (ARMS) Questionnaire – New relapse
Assessing Relapse in Multiple Sclerosis (ARMS) Questionnaire – After relapse treatment
The assessment tool will be pilot-tested in the practices of the meeting participants in order to evaluate its utility in describing relapses and in evaluating how patients respond to relapse treatment. Following collection of data from the pilot program and feedback, the assessment tool will be further refined. There are plans to report on the findings, and future initiatives may be undertaken to develop algorithms that provide guidance on relapse assessment and management.
Summary and Conclusions
Diagnosis and treatment of MS relapses can be challenging because of the variable nature of the disease. Establishing long-term relationships with patients allows clinicians to more easily recognize and determine the appropriate course of treatment for those individuals experiencing relapses. Knowledge of an individual patient, his or her history, and the patient's life quality and satisfaction following treatment are key elements in evaluating relapses and treatment response, as well as selecting optimal treatment approaches for future relapses. The reality of clinical practice, however, is such that many clinicians are challenged to efficiently assess the nature, quality, and impact of relapses. The development of an easy-to-use MS relapse assessment tool that is adaptable to a variety of practice settings, facilitates communication with patients, and is reliable and valid would be of great value in the comprehensive management of MS.
PracticePoints
MS relapses are common and may be debilitating. However, the variability in each clinical picture from one case to another, and even from one relapse to another within the same patient, may lead to clinicians' having difficulty in identifying and managing relapses.
The MS professional community currently lacks a standardized assessment tool to assess symptoms of a relapse and their impact on daily functioning, response to and tolerability of prescribed treatments, and extent of resolution and functional recovery.
Better definition of patients' and professionals' perceptions of the effects of relapses and potential treatment response would enhance relapse management in clinical practice.
Acknowledgments
We would like to thank Sherri D. Jones, PharmD, of MedVal Scientific Information Services, LLC, for assistance with manuscript preparation, which was funded by Questcor Pharmaceuticals, Inc.
References
Halper J. The psychosocial effect of multiple sclerosis: the impact of relapses. J Neurol Sci. 2007;256(suppl 1):S34–S38.
Kalb R. The emotional and psychological impact of multiple sclerosis relapses. J Neurol Sci. 2007;256(suppl 1):S29–S33.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010; 133: 1914–1929.
Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009; 73: 1616–1623.
Bennetto L, Burrow J, Sakai H, Cobby J, Robertson N, Scolding N. The relationship between relapse, impairment and disability in multiple sclerosis. Mult Scler. 2011; 17: 1218–1224.
Scott TF, Schramke CJ. Poor recovery after the first two attacks of multiple sclerosis is associated with poor outcome five years later. J Neurol Sci. 2010; 292: 52–56.
Thrower BW. Relapse management in multiple sclerosis. Neurologist. 2009; 15: 1–5.
Freedman MS, Cohen B, Dhib-Jalbut S, et al. Recognizing and treating suboptimally controlled multiple sclerosis: steps toward regaining command. Curr Med Res Opin. 2009; 25: 2459–2470.
Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci. 2007;256(suppl 1):S5–S13.
Nickerson M, Marrie RM. Intravenous and oral steroids may be insufficient for treating relapses in a significant proportion of patients with multiple sclerosis: patient experiences collected by NARCOMS [abstract]. Mult Scler. 2011; 17(10 suppl):S446.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292–302.
Hawkes CH, Giovannoni G. The McDonald criteria for multiple sclerosis: time for clarification. Mult Scler. 2010; 16: 566–575.
Cohen BA, Khan O, Jeffery DR, et al. Identifying and treating patients with suboptimal responses. Neurology. 2004; 63: S33–S40.
Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol. 2008; 255: 280–287.
Mowry EM, Deen S, Malikova I, Pelletier J, Bacchetti P, Waubant E. The onset location of multiple sclerosis predicts the location of subsequent relapses. J Neurol Neurosurg Psychiatry. 2009; 80: 400–403.
Morrow SA, Jurgensen S, Forrestal F, Munchauer FE, Benedict RH. Effects of acute relapses on neuropsychological status in multiple sclerosis patients. J Neurol. 2011; 258: 1603–1608.
Freedman MS, Patry DG, Grand'Maison F, Myles ML, Paty DW, Selchen DH. Treatment optimization in multiple sclerosis. Can J Neurol Sci. 2004; 31: 157–168.
Vercellino M, Romagnolo A, Mattioda A, et al. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009; 119: 126–130.
Mowry EM, Pesic M, Grimes B, Deen S, Bacchetti P, Waubant E. Demyelinating events in early multiple sclerosis have inherent severity and recovery. Neurology. 2009; 72: 602–608.
Traboulsee A. MRI relapses have significant pathologic and clinical implications in multiple sclerosis. J Neurol Sci. 2007;256(suppl 1):S19–S22.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452.
Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite: a new clinical outcome measure for multiple sclerosis trials. Mult Scler. 2002; 8: 359–365.
Drake AS, Weinstock-Guttman B, Morrow SA, Hojnacki D, Munschauer FE, Benedict RH. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler. 2010; 16: 228–237.
Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83.
Amato MP, Portaccio E. Clinical outcome measures in multiple sclerosis. J Neurol Sci. 2007; 259: 118–122.
van Winsen LM, Kragt JJ, Hoogervorst EL, Polman CH, Uitdehaag BM. Outcome measurement in multiple sclerosis: detection of clinically relevant improvement. Mult Scler. 2010; 16: 604–610.
Sharrack B, Hughes RA, Soudain S, Dunn G. The psychometric properties of clinical rating scales used in multiple sclerosis. Brain. 1999;122(pt 1):141–159.
Patzold T, Schwengelbeck M, Ossege LM, Malin JP, Sindern E. Changes of the MS functional composite and EDSS during and after treatment of relapses with methylprednisolone in patients with multiple sclerosis. Acta Neurol Scand. 2002; 105: 164–168.
Pascual AM, Bosca I, Coret F, Escutia M, Bernat A, Casanova B. Evaluation of response of multiple sclerosis (MS) relapse to oral high-dose methylprednisolone: usefulness of MS functional composite and Expanded Disability Status Scale. Eur J Neurol. 2008; 15: 284–288.
Ozakbas S, Cagiran I, Ormeci B, Idiman E. Correlations between multiple sclerosis functional composite, expanded disability status scale and health-related quality of life during and after treatment of relapses in patients with multiple sclerosis. J Neurol Sci. 2004; 218: 3–7.
Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(suppl 3):S8–15.
Solari A, Radice D, Manneschi L, Motti L, Montanari E. The Multiple Sclerosis Functional Composite: different practice effects in the three test components. J Neurol Sci. 2005; 228: 71–74.
Sipe JC, Knobler RL, Braheny SL, Rice GP, Panitch HS, Oldstone MB. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984; 34: 1368–1372.
Sipe JC, Romine JS, Koziol JA, McMillan R, Zyroff J, Beutler E. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet. 1994; 344: 9–13.
Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003; 61: 1528–1532.
Ross AP, Hackbarth N, Rohl C, Whitmyre K. Effective multiple sclerosis management through improved patient assessment. J Neurosci Nurs. 2008; 40: 150–157.
Nos C, Sastre-Garriga J, Borras C, Rio J, Tintore M, Montalban X. Clinical impact of intravenous methylprednisolone in attacks of multiple sclerosis. Mult Scler. 2004; 10: 413–416.
Durelli L, Cocito D, Riccio A, et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology. 1986; 36: 238–243.
Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011; 24: 224–229.
Caruso C, Carniglia L, Durand D, Gonzalez PV, Scimonelli TN, Lasaga M. Melanocortin 4 receptor activation induces brain-derived neurotrophic factor expression in rat astrocytes through cyclic AMP-protein kinase A pathway. Mol Cell Endocrinol. 2012; 348: 47–54.
Leussink VI, Jung S, Merschdorf U, Toyka KV, Gold R. High-dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch Neurol. 2001; 58: 91–97.
Lindquist S, Hassinger S, Lindquist JA, Sailer M. The balance of pro-inflammatory and trophic factors in multiple sclerosis patients: effects of acute relapse and immunomodulatory treatment. Mult Scler. 2011; 17: 851–866.
Strand FL, Kung TT. ACTH accelerates recovery of neuromuscular function following crushing of peripheral nerve. Peptides. 1980; 1: 135–138.
Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007; 148: 4918–4926.
Delgado R, Carlin A, Airaghi L, et al. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J Leukoc Biol. 1998; 63: 740–745.
Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines a-melanocyte-stimulating hormone and transforming growth factor-b2. J Leukoc Biol. 2002; 72: 946–952.
Miller DH, Thompson AJ, Morrissey SP, et al. High dose steroids in acute relapses of multiple sclerosis: MRI evidence for a possible mechanism of therapeutic effect. J Neurol Neurosurg Psychiatry. 1992; 55: 450–453.
Gasperini C, Pozzilli C, Bastianello S, et al. The influence of clinical relapses and steroid therapy on the development of Gd-enhancing lesions: a longitudinal MRI study in relapsing-remitting multiple sclerosis patients. Acta Neurol Scand. 1997; 95: 201–207.
Martinez-Caceres EM, Barrau MA, Brieva L, Espejo C, Barbera N, Montalban X. Treatment with methylprednisolone in relapses of multiple sclerosis patients: immunological evidence of immediate and short-term but not long-lasting effects. Clin Exp Immunol. 2002; 127: 165–171.
Filippini G, Brusaferri F, Sibley WA, et al. Corticosteroids or ACTH for acute exacerbations in multiple sclerosis. Cochrane Database Syst Rev. 2000; 4:CD001331.
National Clinical Advisory Board of the National Multiple Sclerosis Society. Recommendations Regarding Corticosteroids in the Management of Multiple Sclerosis. New York, NY: National Multiple Sclerosis Society; 2008.
Burton JM, O'Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2009; 3:CD006921.
Sellebjerg F, Barnes D, Filippini G, et al. EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. 2005; 12: 939–946.
Beck RW, Cleary PA, Anderson MM Jr, et al.; The Optic Neuritis Study Group. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992; 326: 581–588.
Milanese C, La Mantia L, Salmaggi A, et al. Double-blind randomized trial of ACTH versus dexamethasone versus methylprednisolone in multiple sclerosis bouts: clinical, cerebrospinal fluid and neurophysiological results. Eur Neurol. 1989; 29: 10–14.
La Mantia L, Eoli M, Milanese C, Salmaggi A, Dufour A, Torri V. Double-blind trial of dexamethasone versus methylprednisolone in multiple sclerosis acute relapses. Eur Neurol. 1994; 34: 199–203.
Barnes D, Hughes RA, Morris RW, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997; 349: 902–906.
Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. clinical effects. J Neurol Neurosurg Psychiatry. 1987; 50: 511–516.
Oliveri RL, Valentino P, Russo C, et al. Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology. 1998; 50: 1833–1836.
Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology. 2004; 63: 1079–1080.
De Keyser J, Zwanikken CM, Zorgdrager A, Oenema D, Boon M. Treatment of acute relapses in multiple sclerosis at home with oral dexamethasone: a pilot study. J Clin Neurosci. 1999; 6: 382–384.
Martinelli V, Rocca MA, Annovazzi P, et al. A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology. 2009; 73: 1842–1848.
Morrow SA, Metz LM, Kremenchutzky M. High dose oral steroids commonly used to treat relapses in Canadian MS clinics. Can J Neurol Sci. 2009; 36: 213–215.
H.P. Acthar® Gel (repository corticotropin injection) [prescribing information]. Hayward, CA: Questcor Pharmaceuticals, Inc; June 2011.
Thompson AJ, Kennard C, Swash M, et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology. 1989; 39: 969–971.
Miller H, Newell DJ, Ridley A. Multiple sclerosis: treatment of acute exacerbations with corticotrophin (A.C.T.H.). Lancet. 1961; 2: 1120–1122.
Rose AS, Kuzma JW, Kurtzke JF, Namerow NS, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo—final report. Neurology. 1970; 20: 1–59.
Barnes MP, Bateman DE, Cleland PG, et al. Intravenous methylprednisolone for multiple sclerosis in relapse. J Neurol Neurosurg Psychiatry. 1985; 48: 157–159.
Simsarian JP, Saunders C, Smith DM. Five-day regimen of intramuscular or subcutaneous self-administered adrenocorticotropic hormone gel for acute exacerbations of multiple sclerosis: a prospective, randomized, open-label pilot trial. Drug Des Devel Ther. 2011; 5: 381–389.
Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011; 76: 294–300.
Weinshenker BG, O'Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999; 46: 878–886.
Weiner HL, Dau PC, Khatri BO, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989; 39: 1143–1149.
Magana SM, Keegan BM, Weinshenker BG, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011; 68: 870–878.
Repovic P, Lublin FD. Treatment of multiple sclerosis exacerbations. Neurol Clin. 2011; 29: 389–400.
Alam SM, Kyriakides T, Lawden M, Newman PK. Methylprednisolone in multiple sclerosis: a comparison of oral with intravenous therapy at equivalent high dose. J Neurol Neurosurg Psychiatry. 1993; 56: 1219–1220.
Andersson PB, Goodkin DE. Glucocorticosteroid therapy for multiple sclerosis: a critical review. J Neurol Sci. 1998; 160: 16–25.
Tatro JB. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996; 3: 259–284.
Sibley WA. Spotlight series: pivotal trials through today's knowledge—adrenocorticotrophic hormone. Int MS J. 2009; 16: 42–46.
Catania A. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 2008; 31: 353–360.
Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004; 56: 1–29.
Elovaara I, Kuusisto H, Wu X, Rinta S, Dastidar P, Reipert B. Intravenous immunoglobulins are a therapeutic option in the treatment of multiple sclerosis relapse. Clin Neuropharmacol. 2011; 34: 84–89.
Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008; 15: 893–908.
Consensus Conference Participants: Amy Perrin Ross, APN, MSN, CNRN, MSCN (Chair), Aliza Ben-Zacharia, DrNP, ANP-BC, Constance Easterling, RN, MSN, ANP, MSCN, June Halper, MSN, APN-C, MSCN, FAAN, Colleen J. Harris, MN, NP, MSCN, Patricia Kennedy, RN, CNP, MSCN, Beverly Layton, RN, CCRC, MSCN, Lynn McEwan, RN, MSN, CNNC, MSCN, Carol Saunders, BSN, BA, MSCN, Jennifer Smrtka, MSN, ANP-C, MSCN.
Financial Disclosures: Ms. Perrin Ross has received consulting fees or honoraria from Questcor Pharmaceuticals and has served on speakers' bureaus for Questcor. Ms. Halper has received consulting fees or honoraria from Questcor. Ms. Harris has received consulting fees or honoraria from Questcor.
Funding/Support: Funding for the working group meeting and development of this manuscript was provided by Questcor Pharmaceuticals, Inc.







