Publication
Research Article
International Journal of MS Care
A Randomized Crossover Trial of Dalfampridine Extended Release for Effect on Ambulatory Activity in People with Multiple Sclerosis
Background: Dalfampridine extended release (D-ER) is indicated to improve walking in people with multiple sclerosis (MS) as demonstrated by an increase in walking speed. This study assessed the effects of D-ER on accelerometer-based measures in people with MS, including intensity of walking and total amount of walking during daily activities.
Methods: In this double-blind placebo-controlled crossover study, people with MS-related walking difficulty were randomized (1:1) to receive 4 weeks of D-ER 10 mg twice daily and 4 weeks of placebo in either order separated by a 2-week washout. Participants wore accelerometers for 7 days at baseline and week 3 of each on-drug period. The primary outcome was the peak activity index (PAI), defined as the most intense 30 individual minutes of the day (strides per minute). Secondary outcomes included daily step count, 6-Minute Walk Test (6MWT), Timed Up and Go (TUG) test, and patient-reported outcomes. A mixed-effects repeated-measures statistical model was used.
Results: Forty-three participants were randomized (mean Expanded Disability Status Scale score, 5.17). Least squares mean (standard error) change from baseline on the PAI was 0.6 (0.54) strides/min on D-ER and 0.3 (0.55) strides/min on placebo and in daily step count was 148.7 (222.4) on D-ER and 128.0 (225.4) on placebo. Other accelerometer-based measures and the 6MWT showed no significant differences between D-ER and placebo. The TUG test (P = .042) favored D-ER. There were no serious adverse events.
Conclusions: Dalfampridine did not show an effect on accelerometer-measured ambulatory activity in people with MS-related walking difficulty. More work is needed to confirm these results.
Multiple sclerosis (MS) is a chronic neurologic disease characterized by demyelination and axonal degeneration. Overall, approximately 75% to 85% of individuals with MS have some degree of ambulatory impairment, and more than half need assistance both indoors and outdoors.1 2 More than one-third do not retain the ability to walk beyond 20 years after the diagnosis.3 La Rocca4 reported the results of a survey of 1011 people with MS in the United States. Of respondents who reported having walking difficulty, 70% considered it to be the most challenging aspect of MS, and most of these people with MS said that walking difficulty adversely affected their performance of daily tasks, self-esteem, work life, and ability to travel.4
Dalfampridine extended release (D-ER) (fampridine outside the United States) is a partial voltage-dependent potassium channel inhibitor that is approved for walking difficulty caused by MS. In two randomized, controlled, phase 3 trials, D-ER increased the frequency of patients with consistently faster Timed 25-Foot Walk (T25FW [7.62 m]) test values using responder analysis.5 6 Responders improved in a self-report measure of walking, the 12-item Multiple Sclerosis Walking Scale (MSWS-12); nonresponders also reported an improvement in the MSWS-12, although a treatment effect was not established. Objective measures of daily walking and physical activity recorded in a community setting have not been evaluated with D-ER in a randomized trial.
Accelerometers provide continuous measurement of ambulation and have been shown to correlate with walking mobility in community-dwelling people with MS.7 We chose several accelerometer- and community-based outcome measures for this therapeutic trial of D-ER. Ordinary community walking is characterized by multiple brief bursts of walking (90% of most walking bouts last ≤1 minute).8 Therefore, a measure of peak cadence (steps per minute) may be a relevant measure of how well a person is walking in the community. We used the peak activity index (PAI) as the primary outcome measure. The PAI is the average rate (strides per minute) of the most intensive 30 individual minutes during the day and is a measure of maximum walking intensity.9 In a 6-month observational MS study, we found that the PAI had significant associations with several clinical tests, including the Expanded Disability Status Scale (R 2 = 0.44), the T25FW test (R 2 = 0.41), and the MSWS-12 (R 2 = 0.47).10 11 The hypothesis for this study was that D-ER therapy would increase the PAI relative to any change with placebo use.
Methods
Protocol and Participant Assessment
This was a double-blind, randomized, placebo-controlled, crossover trial with two parallel treatment sequence groups (D-ER/placebo and placebo/D-ER, 1:1 randomization). Each treatment period was 4 weeks, separated by a 2-week off-treatment washout period (Supplementary Figure 1, which is published in the online version of this article at ijmsc.org).
Participant eligibility required a confirmed diagnosis of MS based on the McDonald criteria, age 18 to 75 years, a screening 6-Minute Walk Test (6MWT) distance of 50 to 500 m, and an Expanded Disability Status Scale score of 0 to 6.5. Patients with contraindication to D-ER, use of any aminopyridine product or mitoxantrone in the past 6 months, or conditions that would not allow a 6MWT were excluded. The study was approved by the research committee of EvergreenHealth Medical Center (Kirkland, WA) and by Western Institutional Review Board (Puyallup, WA) (WIRB protocol 20101785).
After screening, eligible participants returned 2 weeks later (week 0) for randomization (Figure 1). They then entered a 4-week, double-blind, placebo-controlled period. Active and placebo drugs were provided by Acorda Therapeutics (New York, NY) and appeared identical. Treatment with D-ER was 10 mg twice a day, and participants were instructed to take one blinded tablet (supplied in appropriate quantities at each clinic visit) approximately every 12 hours during the treatment phase. At week 6, participants entered a second 4-week double-blind period during which they were allocated to receive the opposite treatment from that during the initial 4-week period. The final visit was at week 10, and a telephone follow-up visit was made 2 weeks later to check on adverse events (AEs).
CONSORT diagram
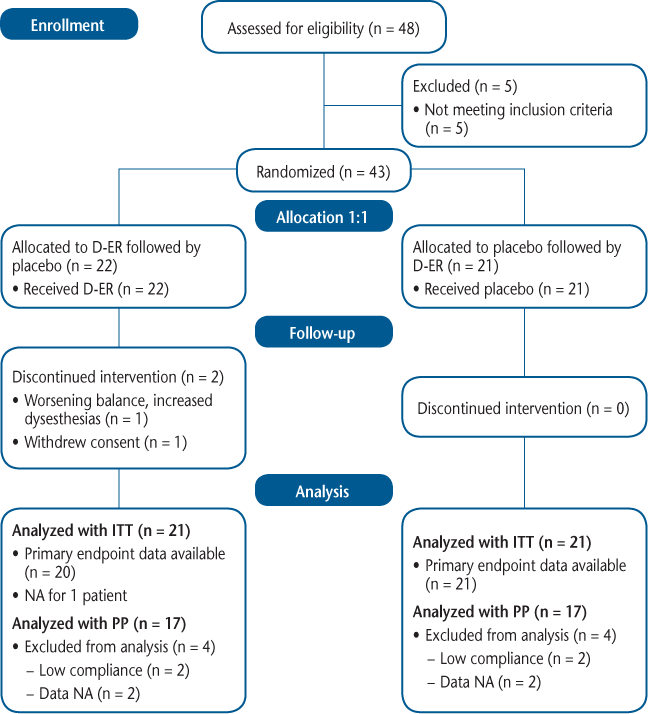
Accelerometry measurement was performed by wearing the instrument for 1 week at baseline and during week 3 of both the active and placebo periods of the study. We used an ankle-worn accelerometer (StepWatch3, OrthoCare, Washington, DC) that measured cadence (right steps per minute) on a minute-by-minute basis for periods of 7 continuous days (Supplementary Figure 2). Daily results were averaged over 1 week of wear. Accelerometer activity logs were reviewed by a blinded reviewer (TRB), and days with less than 10 hours of wear were rejected. Minimum compliance was 5 days of wear.
The primary efficacy measure was the change in PAI recorded by the accelerometer. Secondary efficacy measures included accelerometer measures of step counts, percentage of the day spent inactive, and the maximum activity rate (strides per minute) for 5, 20, and 60 continuous minutes. For step counts, the daily number of strides (right leg only) was doubled and averaged over 7 days. Additional secondary outcomes were clinic-based measures of 6MWT distance and Timed Up and Go (TUG) test and patient-reported outcomes of Physical Activity and Disability Survey–Revised (PADS-R),12 MSWS-12,13 and Subject Global Impression of change (SGI). The SGI asked patients to rate their impression of the effects of the study medication during the preceding week on their physical well-being. Additional exploratory outcomes are not included in this report. Except for the SGI, all nonaccelerometry testing was performed at baseline and at the end of both study periods. A separate evaluator, blinded to the participant's overall clinical and safety assessments and global scores, performed all the functional outcome measurements, and assessments were performed by the same individual at each visit, whenever possible. Safety was assessed by AE and vital sign monitoring and by clinical laboratory tests, electrocardiography, and urine pregnancy testing, as indicated.
Statistical Analysis
The primary endpoint for the study was the change from baseline in the PAI. The primary efficacy analysis compared the difference in the intraparticipant changes from baseline from the period in which the participant was taking D-ER to the period in which the participant was taking placebo. The statistical results were based on a mixed-effects repeated-measures model predicated on maximum likelihood, using the change from baseline as the response variable. The model included sequence (AB or BA), period (1 or 2), and treatment (D-ER or placebo) as fixed effects. The model included participant nested within sequence as a random effect. Incorporated into the preestablished statistical analysis plan was a responder analysis using a responder definition of a participant who experienced at least a 20% increase in PAI from baseline to period (this 20% cutoff point was established a priori, but no minimal clinically important difference for the PAI has been determined). A P < .05 was judged as having provided evidence of efficacy. Safety was assessed based on the incidence of AEs in the safety population, defined as participants who received at least one dose of any study medication. The study was powered for an 80% probability to detect treatment effect on the PAI with 43 participants and assuming a 10% dropout rate.
Results
A total of 43 participants were enrolled and randomized, and 41 completed the study and had data available for efficacy analyses. Two participants withdrew from the study, one due to moderate AEs (dizziness, weakness, fatigue) during the D-ER phase and one during the placebo phase; both withdrawals were in the sequence A (D-ER/placebo) group. Reasons for exclusion from per-protocol analysis included inadequate compliance with study medication accountability (n = 4), inadequate accelerometer wear (n = 1), and other missing data (n = 3). See Figure 1 for more details.
The two groups were well matched for demographic variables except for a difference in sex composition, for which there was a significantly higher proportion of females in sequence A relative to sequence B (86% vs. 52%; P = .022) (Table 1). The use of disease-modifying therapies was similar in the two groups (all but two participants in each group were taking disease-modifying therapies). There were no differences in outcome measures at baseline between the two randomization groups for the following outcomes: accelerometer: peak 30 minutes, peak 5 minutes, peak 20 minutes, peak 60 minutes, steps per day, and percentage of the day inactive; nonaccelerometer tests: 6MWT, TUG, and PADS-R (Total score and Exercise and Leisure subscore) (Table 1).
Demographic and baseline clinical characteristics of the randomized study population
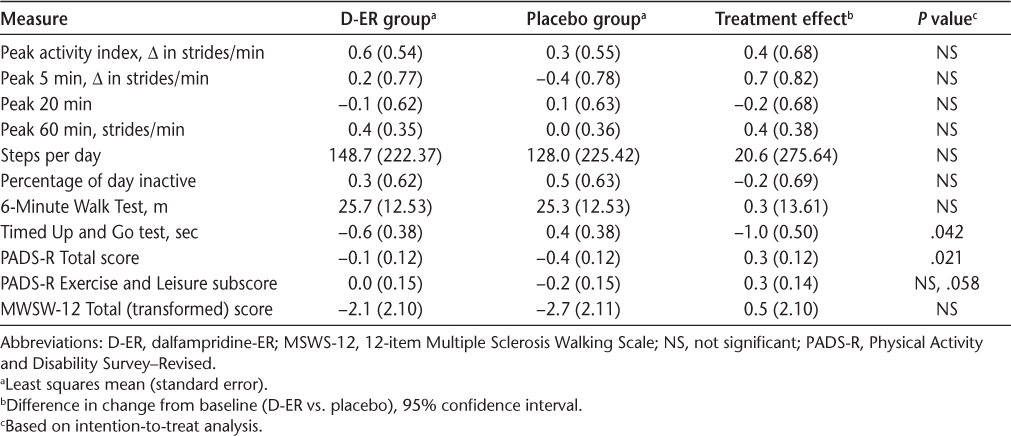
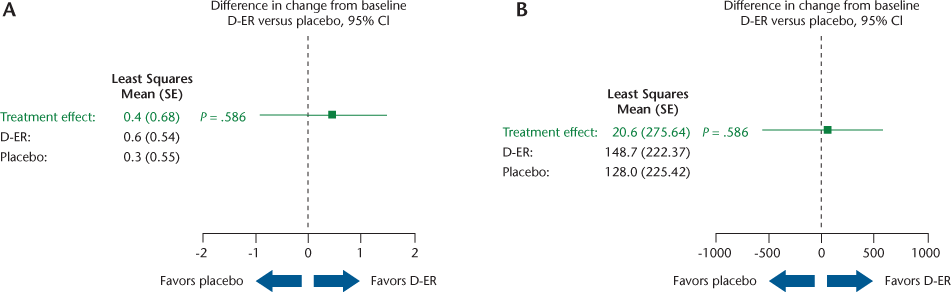
Results for the key outcomes (Table 2) are illustrated in Figures 2, 3, and 4 as forest plots that present the point estimate and 95% confidence interval for the difference in change from baseline for D-ER relative to placebo; a confidence interval that crosses zero indicates no significant difference between treatments.
Change from baseline for key outcome measures
Primary and key secondary accelerometry measures
Clinic-based measures
Patient-reported outcomes
Accelerometry Results
No statistically significant differences were found between D-ER and placebo for the primary efficacy outcome of PAI (Figure 2A). Similarly, the number of steps per day did not show any difference between the two treatments (Figure 2B), and neither did the other four accelerometry outcomes (percentage of the day inactive, peak 5 minutes, peak 20 minutes, and peak 60 minutes) (data not shown). Changes from baseline in accelerometry activity were small for all of the accelerometer-based measures. We performed responder analysis using a prespecified responder definition as having a 20% change in PAI relative to baseline. We found five participant responders to D-ER, five responders to placebo, and two responders to both D-ER and placebo. A similar post hoc responder analysis using steps per day showed no treatment effect. We found no evidence of significant sequence or period effects. Additional analysis of PAI and steps per day outcomes for period 1 alone (in which there could be no sequence or period effect) showed no significant differences between D-ER and placebo on any accelerometer measure.
Clinical and Self-report Results
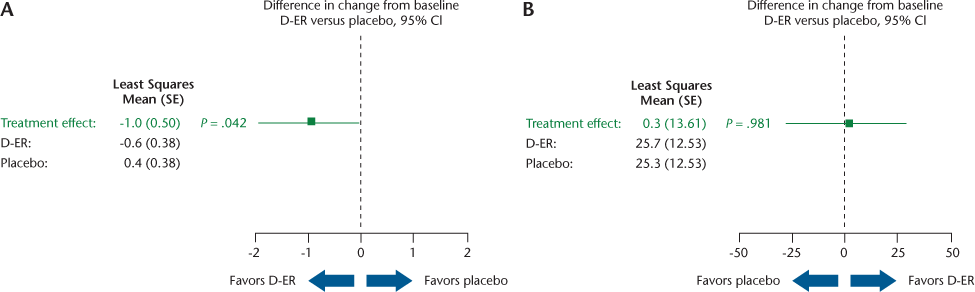
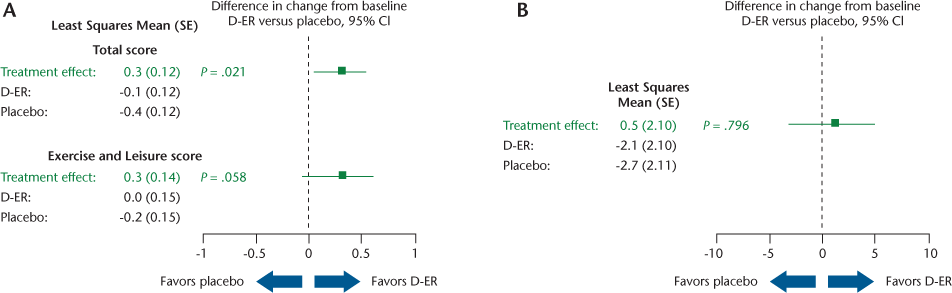
On the clinic-based measures, a significant difference favoring D-ER was observed on the TUG test (P = .042) (Figure 3A), but not for the 6MWT (Figure 3B). On patient-reported outcomes, D-ER resulted in a significantly greater improvement on the PADS-R Total score relative to placebo (P = .021), with a trend favoring D-ER (P = .058) on the Exercise and Leisure subscale (Figure 4A). No statistically significant differences between treatments were observed on the PADS-R subscales of General Activity, Therapy, Employment, and Wheelchair Use (data not shown). No differences between treatments were observed for the MSWS-12 (Figure 4B) or the SGI (data not shown).
Results are provided for the intention-to-treat analysis. With per-protocol analysis, the only differences were that the change in TUG test time became nonsignificant, the PADS-R Total score significance became borderline (P = .055), and the PADS-R Exercise and Leisure subscore became significant (P = .027).
All randomized participants who took at least one dose of study medication (N = 43) experienced one or more AEs. The AEs were generally mild and similar between treatments. The numbers of mild or moderate AEs with a possible relationship to the drug were 22 with D-ER and 22 with placebo. There was one participant with AEs leading to treatment discontinuation with D-ER (weakness, fatigue, nausea) and none with placebo. Insomnia was the only AE that occurred disproportionately: two events with placebo (4.7%) and seven with D-ER (16.3%). The other most common AEs (in ≥5% of participants) with placebo versus D-ER were falls in 7 (16.3%) versus 6 (14.0%); headache, 4 (9.3%) versus 5 (11.6%); dizziness, 2 (4.7%) versus 4 (9.3%); nausea, 3 (7.0%) versus 3 (7.0%); cold, 2 (4.7%) versus 3 (7.0%); back pain, 3 (7.0%) versus 3 (7.0%); diarrhea, 1 (2.3%) versus 2 (4.7%); dry mouth, 2 (4.7%) versus 2 (4.7%); and loss of balance, 3 (7.0%) versus 2 (4.7%). There were no serious AEs, no seizures, and no relapses.
Discussion
One of the important, but challenging, aspects of research is the translation of a clinically determined research finding into everyday experience. Is the effect of D-ER on the clinic-based T25FW test accompanied by changes in the behavior and activity of people with MS-related walking difficulty? We addressed this question by measuring ambulatory activity using accelerometers worn during periods while taking D-ER and placebo using a crossover design.
Accelerometers provide continuous measurement of ambulation. They capture the daily variation in walking habits, including brief bursts of fast walking that reflect peak activity, such as crossing the street or walking to answer the telephone. Continuous recording reveals how much of the day a person spends on his or her feet using measures such as total ambulatory time and steps per day. Accelerometer output is strongly associated with rate of oxygen consumption and energy expenditure in people with MS.14 The accelerometer that we used (StepWatch3) is more expensive and bulky than are commercially available consumer accelerometers; it has the advantage of detailed recording of walking speed and has been shown to record ambulatory activity with good to excellent accuracy at comfortable and fast walking speeds and even in the presence of severe gait impairment in MS, Parkinson disease, and neuromuscular disease.15–19 We chose the PAI, an accelerometer-based outcome measure that reflected peak walking intensity (strides per minute), as the primary outcome. The secondary outcomes included steps per day and total percentage of the day spent in ambulation. Thus, there were quantitative as well as qualitative measures of ambulatory activity.
We found no evidence that D-ER had an effect on accelerometer measures. There were no significant differences relative to placebo or relative to baseline values for the PAI; fastest 5, 20, and 60 minutes of the day; steps per day; and total ambulatory time.
A 12-week double-blind crossover study that also used accelerometers found no significant effect of D-ER (fampridine in Europe) on the total cohort, as with the present study.20 They did responder analysis, defining responders as those who had improvement in T25FW test values, as was done in the pivotal trial. They found that responders, who composed 36% of the cohort, had a significant increase in daily activity. We did not perform the T25FW test but based the responder analyses on accelerometer results (a 20% change in the PAI or a 20% change in steps per day). With either criterion, responder rates did not differ between D-ER and placebo.
As measured by accelerometers, this MS cohort had no statistically significant changes in ambulatory activity during the trial. On the self-reported PADS-R, there was a statistically significant difference favoring D-ER over placebo. The PADS-R was developed specifically for use in people experiencing disability and has been validated in MS.21 A particular strength of the PADS-R is its ability to discriminate between different levels of activity. It is possible that the PADS-R identified a subjective change in physical activity that is not measurable with accelerometers, such as a perceived ability to be more physically active that did not translate into an actual change in behavior. However, the MSWS-12, a self-report measure of qualitative walking ability, did not indicate a therapeutic effect.
The clinical measures included the 6MWT and the TUG test. The TUG test, which measures time to walk a short distance with change in direction and station, showed a statistically significant difference favoring D-ER. As a brief test of maximum walking speed, this measure shares qualities with the T25FW test, which was the primary outcome in the D-ER pivotal trials. The 6MWT, which is more a test of walking endurance, showed no statistically significant differences. The PAI is a measure of peak walking speed over thirty 1-minute segments of the day, and it showed no difference. The T25FW and TUG tests are clinic-based measures with prompting to perform a task as fast as possible, and the PAI records continuously in the community. These findings suggest that brief tests of speed conducted in a clinical setting may be more sensitive to the effects of D-ER than long-duration tests or passive recordings. However, note that the primary outcome was not the TUG or 6MWT, and neither was the study powered for these outcomes.
The two groups in this study were generally well balanced, except for a difference in sex composition. The crossover design should have mitigated the impact of this difference. However, this study has several limitations. The patient numbers were small. The crossover design may have introduced period and sequence effects, although the tests for such effects were negative. Key accelerometer measurements were recorded during week 3 of each treatment period. It is possible that this timing was not the optimal therapeutic response time, which has been described as 2 to 6 weeks. We used a 2-week washout period between treatment periods, which may have been too short to remove the effects of the first period. However, no significant changes were seen during period 1 relative to baseline, so such a carryover effect may have been negligible. The key outcomes involved routine daily activities over 7 days. This may have reduced the detection of changes in peak walking performance, although measurement of peak 5- and 20-minute activity rates (both not statistically significant) were included. Potentially, therapeutic effects may have been found using a different protocol that required participants to attempt an increase in physical activity. We could find no relevant published clinical trials. The study did not show a change in the MSWS-12 score related to treatment, whereas larger, longer-duration randomized studies showed that responders improved on the MSWS-12 and that nonresponders also reported an improvement on the MSWS-12, although a treatment effect was not established. This suggests fundamental differences between the present single-center population and that of earlier larger studies of responsiveness to D-ER.
In conclusion, D-ER did not show an effect on accelerometer measures of ambulatory activity in this double-blind crossover study of people with MS-related walking difficulty. Further work may investigate other measures or other study designs for possible effects of D-ER on community ambulation in MS.
Acknowledgments
We thank Carey Gonzales and Sherry Alboucq for help during the study and in manuscript preparation.
PracticePoints
Dalfampridine extended release (D-ER) is available to improve walking in people with MS as demonstrated by improvement in walking speed.
Accelerometers can be used to quantify physical activity and to qualify the intensity of walking in daily living in people with MS.
This was the first study to use accelerometer outcomes in a randomized, double-blind, placebo-controlled crossover trial of D-ER therapy in people with MS with walking difficulty.
Use of D-ER did not show an effect on accelerometer-measured ambulatory activity in this study.
References
Kraft GH, Freal JE, Coryell JK. Disability, disease duration, and rehabilitation service needs in multiple sclerosis: patient perspectives. Arch Phys Med Rehabil. 1986;67:164–168.
Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study, I: clinical course and disability. Brain. 1989;112:133–146.
Schapiro RT. Multiple Sclerosis: A Rehabilitation Approach to Management. New York, NY: Demos; 1991.
La Rocca N. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. The Patient. 2011;4:189–201.
Goodman AD, Brown TR, Krupp L, et al. Sustained release of oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738.
Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68:494–502.
Weikert M, Motl R, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. 2010;290:6–11.
Orendurff MS, Schoen JA, Bernatz GC, et al. How humans walk: bout duration, steps per bout and rest duration. J Rehabil Res Dev. 2008;45:1077–1090.
Sample grant text for StepWatch-related research. Orthocare Innovations website. http://www.orthocareinnovations.com. Published in 2008. Accessed July 30, 2009.
Wundes A, Brown TR, Severson BJ, Bowen JD. Clinical predictors of falls and ambulatory activity in MS: 6-month prospective study [abstract]. Int J MS Care. 2007;9:81.
Wundes A, McAleenan J, Severson B, Bamer A, Brown TR. Predictors of community-based ambulatory activity: a prospective six month study of multiple sclerosis patients [abstract]. Mult Scler. 2009;15(suppl):S47–S48.
Kayes NM, Schluter PJ, McPherson KM, et al. The Physical Activity and Disability Survey–Revised (PADS-R): an evaluation of a measure of physical activity in people with chronic neurological conditions. Clin Rehabil. 2009;23:534–543.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-item MS Walking Scale (MSWS-12). Neurology. 2003;60:31–36.
Sandroff BM, Motl RW, Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev. 2012;49:467–475.
Sandroff BM, Motl RW, Pilutti LA, et al. Accuracy of StepWatch™ and ActiGraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS One. 2014;9:e93511.
McDonald CM. Physical activity, health impairments, and disability in neuromuscular disease. Am J Phys Med Rehabil. 2002;81(suppl):S108–S120.
Pearson OR, Busse ME, van Deursen R, Wiles CM. Quantification of walking mobility in multiple sclerosis (MS) using an ambulatory activity monitor. J Neurol Neurosurg Psychiatry. 2003;74:1450.
Busse ME, Pearson OR, van Deursen RWM, Wiles CM. Activity indices for measuring mobility in neurologically impaired patients. J Neurol Neurosurg Psychiatry 2004;75:884–888.
Schmidt AL, Pennypacker ML, Thrush AH, et al. Validity of the StepWatch activity monitor: preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J Geriatr Phys Ther. 2011;34:41–45.
Weller D, Sutter T, Zörner B, et al. Prolonged-release fampridine enhances physical activity during everyday life in patients with multiple sclerosis (FAMPKIN) [ACTRIMS/ECTRIMS abstract P906]. Mult Scler J. 2014;20(suppl 1):463.
Kayes NM, McPherson KM, Taylor D, et al. The Physical Activity and Disability Survey (PADS): reliability, validity and acceptability in people with multiple sclerosis. Clin Rehabil. 2007;21:628–639.
Financial Disclosures: Dr. Brown has done consulting with and has received speaking honoraria from Acorda Therapeutics. Dr. Simnad has no conflicts of interest to disclose.
Funding/Support: This study was funded by an independent medical grant from Acorda Therapeutics.







