Publication
Research Article
International Journal of MS Care
Walgreens Connected Care
Background: The Walgreens Connected Care Multiple Sclerosis (CCMS) treatment management program provides enhanced levels of monitoring, oversight, and care for patients taking MS disease-modifying agents. This study compared rates of adherence to MS medications for patients participating in the CCMS program for at least 6 months with those for patients participating for less than 6 months. For a subsample of patients, we also examined the relationship between adherence and the presence of fatigue or depression.
Methods: This was a retrospective study of patients new to the CCMS program and followed up for 1 year of participation. Adherence to MS medications was measured as the proportion of days covered, with propensity scores used to match the CCMS intervention group to the less-managed comparison group. The impact of program participation on the relationship between depression or fatigue and adherence over time was a separate analysis.
Results: Mean proportion of days covered rates improved significantly in the group managed for at least 6 months compared with those who were less managed. Positive screenings for fatigue and depression reduced adherence in the less-managed group but not in patients with longer participation in the program.
Conclusions: Overall, the CCMS program significantly increased adherence to MS medications. This improved adherence was not negatively impacted by positive screenings for fatigue and depression.
Multiple sclerosis (MS) is an autoimmune disease that affects the brain and spinal cord, leading to loss of muscle control, vision, balance, and sensation. It affects approximately 350,000 to 500,000 Americans1 2 and is often associated with additional symptomatic conditions that emerge as the disease progresses (ie, depression and anxiety, fatigue, bladder or bowel dysfunction, difficulty walking, loss of cognition, muscular weakness, spasticity, and sexual dysfunction).3 Depression has been noted to be the most frequent psychiatric diagnosis in patients with MS, and the lifetime risk of depression in patients with MS is approximately 50%.4 Fatigue is a common symptom in patients with MS, with up to 40% of patients describing it as their most distressing symptom.5 Degradation of mobility (as evidenced by falls) has also been reported in more than 50% of patients with MS surveyed.6
During the study time frame (December 1, 2010, to May 31, 2011), eight US Food and Drug Administration–approved disease-modifying agents were considered to represent primary therapy for MS: interferon beta-1a (IFNβ-1a) (Avonex, Biogen Idec Inc, Cambridge, MA; Rebif, EMD Serono Inc, Rockland, MA), IFNβ-1b (Betaseron, Bayer HealthCare Pharmaceuticals, Montville, NJ; Extavia, Novartis Pharmaceuticals Corp, East Hanover, NJ), glatiramer acetate (Copaxone, Teva Pharmaceutical Industries Ltd, Petah Tikva, Israel), mitoxantrone (various labels, various manufacturers), natalizumab (Tysabri, Biogen Idec Inc and Elan Pharmaceuticals Inc, South San Francisco, CA), and fingolimod (Gilenya, Novartis Pharmaceuticals Corp). Mitoxantrone and natalizumab are infused, fingolimod is administered orally, and the remaining five drugs are self-administered by injection (intramuscularly for Avonex and subcutaneously for the others). Achieving patient adherence to treatment regimens can be difficult because of the complexity of administering the medications through injection or infusion. In a recent study of patients with MS prescribed injectable disease-modifying agents, nonadherence rates (patients missing one or more injections) ranged from 36% to 39%.7
Specialty care management programs8 or disease therapy management programs9 have been shown to positively influence adherence to MS prescription treatment regimens. These programs suggest that pharmacists and supporting care coordinators working in specialized pharmacies can facilitate improved outcomes for patients through MS-specific programs. The Specialty Pharmacy Division of Walgreen Co launched the Walgreens Connected Care Multiple Sclerosis (CCMS) program in December 2010 to support patients receiving their MS medications through Walgreens retail locations. The program was designed to be more patient focused than other pharmacy programs available at the time and included enhanced levels of monitoring and care via periodic patient assessments and targeted education and intervention activities. The CCMS program was based on patient-focused support, with scheduled assessments and screenings conducted by registered pharmacists incorporating discussions of medication-specific adverse effects and the presence of conditions such as depression and fatigue. In addition to assisting patients in obtaining their medications, the CCMS program incorporated discussions about disease progression, information on appropriate medication dosing and administration, adherence counseling, recommendations regarding supportive care, and advice about overall health and wellness. The patients' physicians were alerted when positive responses to screening questions for depression or fatigue were reported or at any point when the patient experienced an adverse event requiring physician intervention.
The primary objective of this study was to compare patient-level adherence rates for MS disease-modifying agents between patients who participated in the CCMS program (managed patients) and those who chose not to participate in the CCMS program or who participated for less than 6 months (less-managed patients). It was predicted that mean adherence levels for MS specialty medications would be higher in CCMS-managed patients than in those who were less managed in the program.6 9 10 As noted in the literature,4 6 8 11 adherence to MS medications can be negatively affected by the presence of depression or fatigue.
A second objective of this study was to investigate the impact of the CCMS program on participants who screened positive for depression or fatigue. Information about a patient's symptoms of fatigue or depression was obtained through self-report validated screening tools. Specifically, information pertaining to fatigue and depression was collected by administering the Modified Fatigue Severity Scale12 and the Whooley Depression Screen,13 14 respectively, for those participating in the CCMS program. (Corresponding information was not available for those who chose not to participate in the program.) The subgroup analysis examined whether the association between depression or fatigue and MS medication adherence level was affected by the duration of participation in the CCMS program. We hypothesized that there would be a negative association between depression or fatigue and adherence to MS medications and that this association would be reduced or eliminated with longer participation in the CCMS program.
Methods
The Walgreens CCMS Program
Patients who participated in (ie, were managed by) the CCMS program received services and interventions beyond standard medication fulfillment, including individualized therapy management and clinical support that encompassed education about disease progression, appropriate medication dosing and administration, and managing adverse effects; adherence support and assistance; recommendations regarding supportive care; and advice about overall health and wellness. Individuals opting not to participate could still obtain partial pharmacy services or return later to the CCMS program. The CCMS program incorporated two standardized screening tools, the Modified Fatigue Severity Scale12 and the Whooley Depression Screen,13 14 to conduct semiannual or on-demand screenings for signs of possible fatigue and depression. Responses to assessment screenings for these conditions were coded dichotomously (yes/no) to reflect these constructs. Patients who screened positive at least once during the study were considered to be positive throughout the duration. This study was reviewed and approved by an independent institutional review board (Quorum Review IRB, Seattle, WA).
Study Sample
The study sample consisted of patients new to the Walgreens national retail pharmacy's CCMS program (and not in any other Walgreens MS programs) between December 1, 2010, and May 31, 2011. These patients were followed up for 1 year from the date of identification. Most study patients (97%) received a 30-day supply of medication at each dispense. Patients included in the study were prescribed any of five self-administered medications used to treat MS available during the study: Avonex (IFNβ-1a), Betaseron (IFNβ-1b), Copaxone (glatiramer acetate), Extavia (IFNβ-1b), and Rebif (IFNβ-1a). Gilenya was approved by the US Food and Drug Administration in January 2012 and, thus, was excluded from this analysis. Of note, prescriptions for Ampyra (dalfampridine; Acorda Therapeutics Inc, Ardsley, NY) were not included in this study because the drug is not a disease-modifying agent. Tysabri and mitoxantrone were also excluded because these are infused products and this study focused on self-administered medications.
Patients who had received any of the study medications during the 6 months before December 2010 were excluded because they were not considered new to therapy. Patients were also required to 1) have at least two unique prescriptions for MS medications during the study, 2) be aged 18 years or older, 3) not have switched from other MS programs to the CCMS program, and 4) not have participated in a patient management program since January 2010.
The study used a retrospective cohort design to examine medication adherence among managed and less-managed patients with MS in the CCMS program. Managed patients (n = 319) were defined as those who participated in the CCMS program for at least 6 months during the study period (Figure 1). Less-managed patients included those who were not enrolled in a retail MS program (n = 120) or who participated for less than 6 months in the CCMS program (n = 500).
Data flow diagram
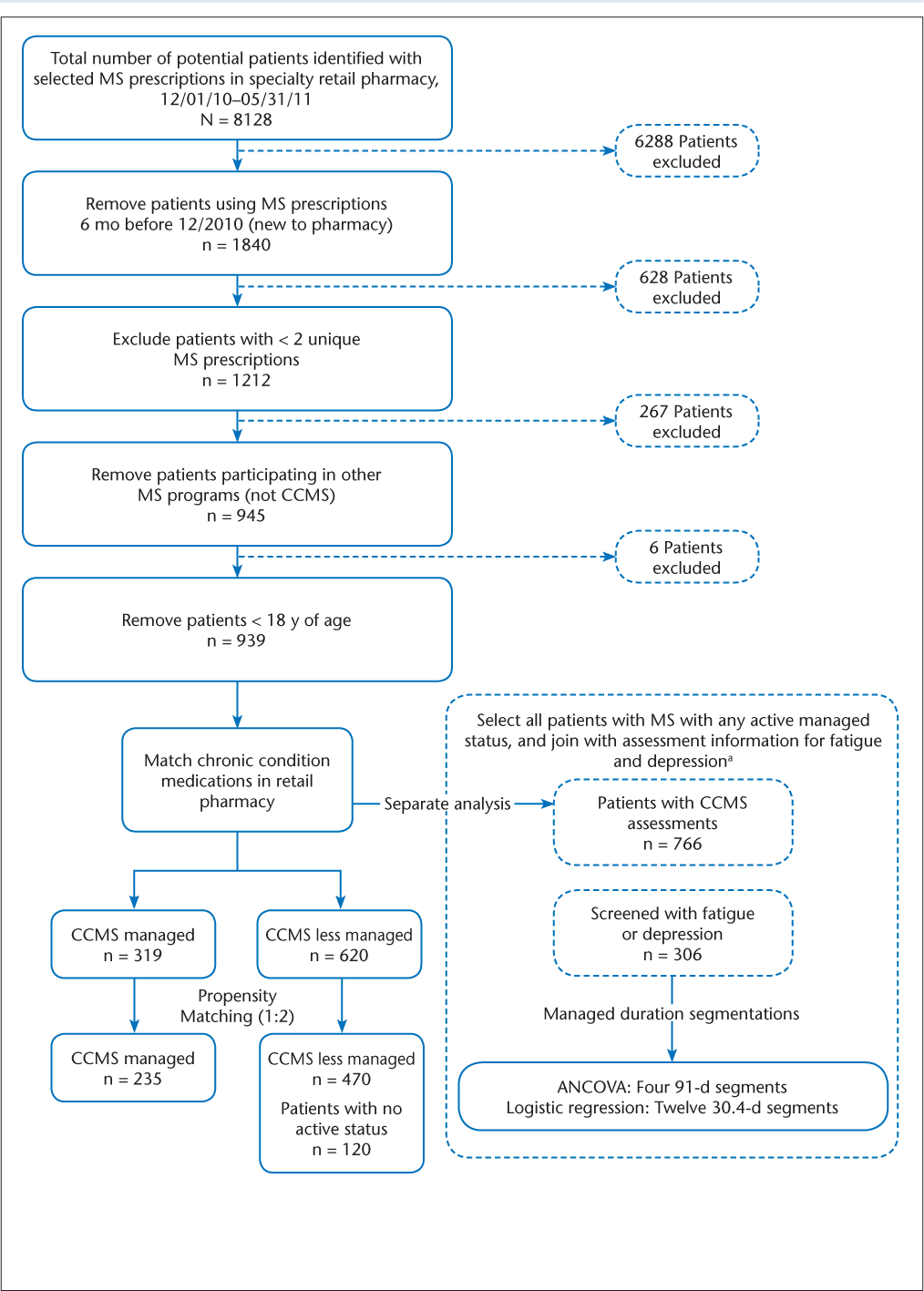
To reduce the inherent potential bias of working with observational data, one-to-many propensity score matching was also used for the primary objective. Managed patients were matched to less-managed patients based on age, sex, chronic condition, and previous adherence to long-term medication therapy, similar to adjustments in other recent MS studies.8 A one-to-many matching algorithm based on the greedy matching algorithm was implemented using SAS software version 9.3 (SAS Institute Inc, Cary, NC).15 The greedy 8-to-1 match algorithm first matches on eight digits of the propensity score, then seven digits, and so forth until the final step matches on one digit of the propensity score.15
The presence of chronic conditions in the 6 months before the first MS prescription fill was derived by mapping prescriptions to the Drug Indications Database (Medi-Span, Indianapolis, IN), which imputes a diagnosis to a patient based on the specific medications in the patient's profile. Chronic conditions were coded as the presence of none, one to two, three to five, or six or more chronic conditions other than MS. Most patients with MS were taking additional medications for more than two comorbid conditions, with the common comorbidities being depression, epilepsy, hypertension, major depression, and hypercholesterolemia. The patient's adherence to medications for a chronic condition(s) was calculated as the mean days' supply in each chronic disease classification in the previous 6 months. A patient average for all matched chronic conditions was the final covariate value used for adherence.
Patients were included in the subgroup analysis if they completed at least one screen for depression or fatigue as part of the CCMS program during the 1-year follow-up. Patients whose medication history included a medication used to treat depression or fatigue,16 thus indicating that the patient was already under a physician's care for these conditions, were excluded to minimize bias. The exclusion was condition specific for depression or fatigue and combined prescriptions for those with positive screening for both conditions. The summed duration of managed status was examined either by the total number of months a patient was managed (1–12 months) in the regression analysis or by 3-month segments in the analysis of covariance (ANCOVA) (1–3 months vs. 4–6 months vs. 7–9 months vs. 10–12 months).
Outcome Measures
The primary outcome measure for all the analyses was adherence to MS medications as determined by the proportion of days covered (PDC) metric. At the patient level, the PDC was calculated as the total medication days' supply17 18 divided by the 365-day follow-up period.10 The PDC ranged from 0% to 100%, with higher values representing greater adherence. Adherence rates for participants in the CCMS program were contrasted with those for the matched control group, and a subanalysis compared adherence rates for patients who also screened positive for depression or fatigue.
Statistical Analyses
For the primary objective, generalized estimating equation models were used to test for adherence differences between matched managed and less-managed patients with MS. Statistical significance was determined using the .05 alpha level. For the subanalysis, ANCOVA was used to examine the interaction of managed status and adverse health markers on mean adherence. To examine the impact of the same variables on a common adherence rate (PDC ≥80%) in the subgroup, a logistic regression analysis was conducted. All the statistical analyses were conducted using SAS software version 9.3 and included the same covariates of sex, age, previous comorbidity, and previous persistence with comorbid medications.
Results
Between December 1, 2010, and May 31, 2011, 8128 patients had an MS prescription filled at one of the study pharmacies (Figure 1). We excluded patients who had a prescription for one of the MS study medications during the 6 months before the study period, who did not have at least two prescriptions for an MS study medication during the study period, who participated in other MS programs, or who were younger than 18 years. The final study sample was 939 patients (Figure 1).
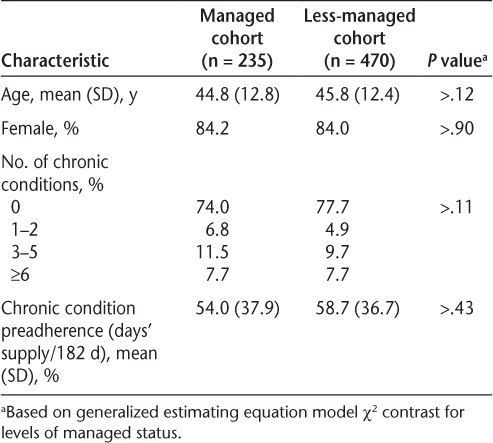
After conducting one-to-many propensity score matching for the primary analysis, 235 managed patients and 470 less-managed patients remained, with no significant differences observed between the covariates (Table 1). For the combined groups, the mean age was 45 years, 84% were women, approximately 24% had one or more chronic conditions, and adherence to medications for chronic diseases at baseline was approximately 57%. Compared with less-managed patients (ie, those participating for <6 months), managed patients had significantly more managed days (300 vs. 45), MS medication fills (9.2 vs. 5.6), mailed materials (5.6 vs. 2.2), completed telephone contacts (7.2 vs. 1.4), completed assessments (11.1 vs. 2.7), and types of completed assessments (3.1 vs. 1.4) (P < .001 for all) (Table 2). Managed patients had higher mean (SD) adherence levels for MS medications than less-managed patients (75.6% [19.3%] vs. 44.6% [27.3%], P < .001). Furthermore, nearly half of the patients in the managed group (49.5%) met or exceeded the PDC benchmark of 80% for MS medications; this was significantly higher than the proportion meeting or exceeding this benchmark in the less-managed group (17.2%, P < .001).
Patient characteristics after propensity score matching
Managed activity by cohorta

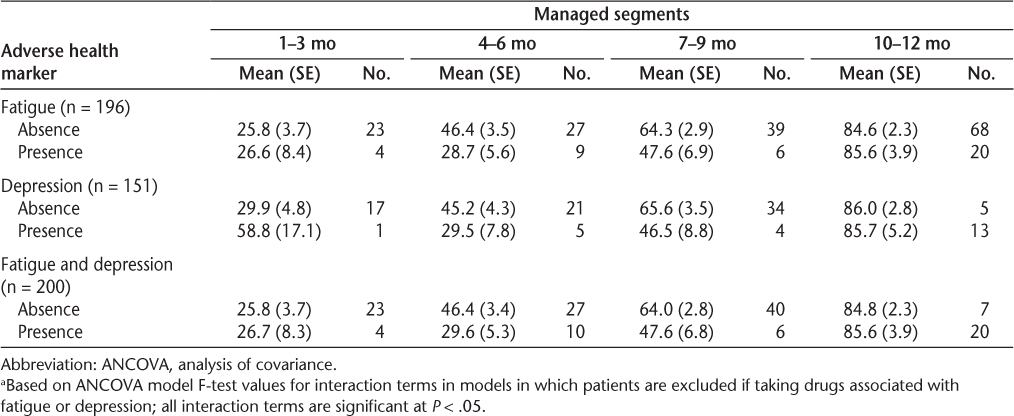
A subgroup analysis examined whether medication adherence differed according to the presence of adverse health markers. Of the reported adverse health markers, the most common was fatigue only (38.6%), followed by both fatigue and depression (19.6%) and depression only (13.4%). Some patients reporting depression or fatigue were taking antidepressant medications (43.3%) or amphetamines (12.8%) during follow-up.
The first set of results for the subanalysis implemented ANCOVA models examining the impact of managed duration on the impact of the reported adverse health marker. The ANCOVA models examined interactions between the adverse health markers and adherence (Table 3). Among patients completing assessments, the interaction of managed status and the presence of depression, fatigue, or both compared with their absence in predicting mean adherence was significant. For each subgroup (presence or absence of a specific adverse health marker), the mean PDC was higher the longer patients were managed, especially patients managed beyond 9 months. However, for patients managed for less than 7 months, the differences in mean PDC were consistently larger when the adverse health marker was present than when it was absent. For example, comparing patients without reported fatigue symptoms with those reporting fatigue symptoms, patients with managed durations of 10 to 12 months had about the same mean PDC (1% difference), whereas patients without fatigue symptoms and managed durations of 4 to 6 months had a 17.7% higher mean PDC than those with fatigue symptoms. Likewise, when patients without or with reported symptoms of depression were compared, the difference in PDC was only 0.3 percentage points at the 10 to 12 months' duration compared with 15.7% at the 4 to 6 months' duration. Finally, a 16.8% difference in mean adherence existed between patients in whom both of these adverse health markers were present versus those in whom neither was present when the managed duration was 4 to 6 months; however, for those managed for 10 to 12 months, there was a small difference in PDC (0.8%).
ANCOVA models examining a subanalysis of the effect of interaction of managed status and adverse health on adherence (proportion of days covered)a
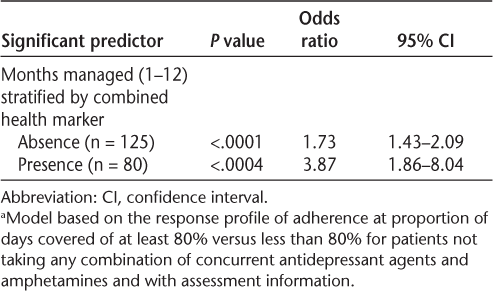
The final model included the propensity model covariates and the managed duration variable (1–12 months). The model was stratified by the combined health indication variable (depression or fatigue, neither depression nor fatigue) to better understand the interaction between managed duration and health indicators. Final predictors (Table 4) support the significant trend noted in the preceding ANCOVA table whereby the likelihood of being adherent increased by 73% for each month of management in the program (up to 1 year) for individuals who did not self-report as having depression or fatigue and by 287% each managed month for individuals with either depression or fatigue. The 287% monthly average increase among those screening positive for depression or fatigue is heavily weighted by a large increase in adherence after 10 or more months' managed status as noted in the confidence intervals and larger sample counts in this duration segment (Table 4).
Logistic regression for adherence (proportion of days covered ≥80%) in relation to managed duration by indication for depression/fatiguea
Discussion
Overall, mean adherence was 31 percentage points higher (75.6% vs. 44.6%) in individuals participating in the CCMS program for 6 months or longer compared with those who chose not to participate for that duration. This increase in adherence likely resulted from the additional managed services that CCMS patients received by participating in the managed program.
In the subgroup analysis, fatigue was a relatively common condition in patients completing at least one screening, and the literature supports this prevalence in the MS population.19 Fatigue affects patients with MS throughout the day and may last up to 6 months or longer. Depression is another common condition among patients with MS, and it significantly affects medication adherence.4 8 Fatigue and depression not only cause difficulties for patients in the day-to-day management of symptoms but also negatively affect medication adherence.6 20 21 In the subgroup analysis, a lower adherence rate was found in patients managed for less than 6 months and especially in patients who screened positive for fatigue, depression, or both. In contrast, adherence in the cohort managed for 10 to 12 months was significantly higher than that in the less-managed group, even in those with a positive screen for either or both of the aforementioned conditions. The logistic analysis highlights that the duration of managed status has a greater effect on adherence in patients reporting depression or fatigue compared with those without these indications.
Limitations
Pharmacy claims and assessment data were limited to one pharmacy chain. Absence of eligibility data resulted in an inability to determine whether a patient who ceased to be an active consumer did so because of persistence, death, termination of employment, or a change in benefit design resulting in a different pharmacy choice. Patient use of other pharmacies for prescription claims was not captured, possibly influencing adherence rates and prescription documentation of chronic conditions. Propensity scores were based on only four predictors (age, sex, chronic conditions, and preadherence); thus, other known influences on adherence (eg, socioeconomic status) were not controlled for in this study owing to data limitations. Furthermore, in the subgroup analysis, fewer than half of the patients eligible had completed a fatigue or depression assessment, potentially limiting the generalizability of the findings. Self-selection bias may be present if patients who opt in and continue to stay in specialty pharmacy managed programs are inherently different from patients who do not opt in or who fail to participate in a program once initially opted in. However, the effect of management duration on adherence suggests an incremental trend, not due solely to this possible bias.
Conclusion
This study adds evidence supporting the importance and the impact of MS disease-modifying therapy programs on increasing adherence to MS specialty medications. In addition, the study adds to the body of literature supporting clinical processes such as one-on-one patient-centric interventions and counseling in medication adherence, with robust findings that managing patients in an MS disease-modifying therapy program can overcome the negative effects of MS-related health issues on adherence. Further research is needed to determine ways to improve enrollment and continued participation in the CCMS program to optimize its benefits to patients.
PracticePoints
Quality patient-focused programs may increase adherence to MS medication therapy when implemented as part of a national retail pharmacy service for specialty medications.
The negative impact of depression and fatigue on adherence to MS medications may be minimized by long-term participation in a pharmacy-sponsored treatment management program.
Acknowledgments
We thank Jennifer Orsi, MPH, senior analyst in the Walgreens Office of Outcomes and Reporting, for editorial assistance and quantitative quality control activities.
References
Multiple sclerosis: hope through research. National Institute of Neurological Disorders and Stroke website. http://www.ninds.nih.gov/disorders/multiple_sclerosis/detail_multiple_sclerosis.htm#158915897. Accessed January 30, 2015.
What is MS? National Multiple Sclerosis Society website. http://nationalmssociety.org/about-multiple-sclerosis/index.aspx. Accessed January 30, 2015.
National Collaborating Centre for Chronic Conditions. Multiple sclerosis: national clinical guideline for diagnosis and management in primary and secondary care. National Institute for Health and Care Excellence website. http://www.ncbi.nlm.nih.gov/books/NBK48931/. Accessed January 30, 2015.
Wallin MWJ, Turner A, Williams RM, Kane R. Depression and multiple sclerosis: review of a lethal combination. J Rehabil Res Dev. 2006; 43: 45–62.
Stanton B, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler. 2006; 12: 481–486.
Costello K, Kennedy P, Scanzillo J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med. 2008; 10:225.
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009; 256: 568–576.
Tan H, Yu J, Tabby D, Devries A, Singer J. Clinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort study. Mult Scler. 2010; 16: 956–963.
Madonna MG, Keating MM. Multiple sclerosis pathways: an innovative nursing role in disease management. J Neurosci Nurs. 1999; 31: 332–335.
Stockl KM, Shin JS, Gong S, Harada AS, Solow BK, Lew HC. Improving patient self-management of multiple sclerosis through a disease therapy management program. Am J Manag Care. 2010; 16: 139–144.
Denis L, Namey M, Costello K, et al. Long-term treatment optimization in individuals with multiple sclerosis using disease-modifying therapies: a nursing approach. J Neurosci Nurs. 2004; 36: 10–22.
Krupp LB, LaRocca NG, Muir-Nash J, Steinbery AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46: 1121–1123.
Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med. 1997; 12: 439–445.
Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000; 343: 1942–1950.
Parson LS. Performing a 1:N case-control match on propensity score. In: Proceedings from the Twenty-Ninth Annual SAS® Users Group International Conference; May 9–12. 2004; Montreal, Canada. SAS Paper 165-29.
Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010; 16: 703–712.
Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharmacy Quality Alliance website. http://www.pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf. Accessed January 30, 2015.
Leslie RS. Using arrays to calculate medication utilization. In: Proceedings from the 2007 SAS Global Forum; April 16–19, 2007; Orlando, FL. Paper 043-2007.
Zifko UA. Management of fatigue in patients with multiple sclerosis. Drugs. 2004; 64: 1295–1304.
Caon C, Saunders C, Smrtka J, et al. Introduction. J Neurosci Nurs. 2010; 42(suppl):S1–S4.
Mohr DC, Goodkin DE, Likosky W, Gatto N, Baumann KA, Rudick RA. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997; 54: 531–533.
Financial Disclosures: All the authors are current employees of Walgreen Co, the sponsoring organization.
Funding/Support: Walgreen Co funded the study development, analysis, and publication costs.







