Publication
Research Article
International Journal of MS Care
Treatment Discontinuation and Disease Progression with Injectable Disease-Modifying Therapies
Injectable first-line disease-modifying therapies (DMTs) for multiple sclerosis (MS) are generally prescribed for continuous use. Accordingly, the various factors that influence patient persistence with treatment and that can lead some patients to switch medications or discontinue treatment may affect clinical outcomes. Using data from the North American Research Committee on Multiple Sclerosis (NARCOMS) database, this study evaluated participants' reasons for discontinuation of injectable DMTs as well as the relationship between staying on therapy and sustained patient-reported disease progression and annualized relapse rates. Participants selected their reason(s) for discontinuation from among 16 possible options covering the categories of efficacy, safety, tolerability, and burden, with multiple responses permitted. Both unadjusted data and data adjusted for baseline age, disease duration, disability, and sex were evaluated. Discontinuation profiles varied among DMTs. Participants on intramuscular interferon beta-1a (IM IFNβ-1a) and glatiramer acetate (GA) reported the fewest discontinuations based on safety concerns, although GA was associated with reports of higher burden and lower efficacy than other therapies. Difficulties with tolerability were more often reported as a reason for discontinuing subcutaneous (SC) IFNβ-1a than as a reason for discontinuing IM IFNβ-1a, GA, or SC IFNβ-1b. In the persistent therapy cohort, less patient-reported disability progression was reported with IM IFNβ-1a treatment than with SC IFNβ-1a, IFNβ-1b, or GA. These findings have relevance to clinical decision making and medication compliance in MS patient care.
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) involving an aberrant immune response that leads to inflammation and subsequent progressive axoglial degeneration.1 For patients with relapsing forms of MS, the injectable immunomodulatory agents interferon beta-1 (IFNβ-1) and glatiramer acetate (GA) are the most common initial treatment options. These first-line MS platform therapies, known as disease-modifying therapies (DMTs), have the potential to alter the natural history of MS by reducing the frequency and severity of relapses, reducing the number of new and enlarging brain lesions on magnetic resonance imaging (MRI), and slowing disability progression. In patients with clinically isolated syndrome, DMTs have also been shown to delay the time to diagnosis of clinically definite MS and to reduce brain lesion burden.2 Head-to-head trials have demonstrated only modest differences between injectable DMTs, and these agents are broadly perceived as having similar efficacy as first-line therapies for the treatment of relapsing-remitting MS (RRMS).3
Injectable DMTs are indicated for continuous use. Accordingly, the multiple factors that influence patient persistence with treatment, physician recommendations and patient choices on medication switching, and treatment discontinuations may affect clinical outcomes.4 5 While the precise effects of differences in patient persistence with therapy on clinical and/or MRI outcomes remain unclear, patients who do not adhere to or persist with therapy are unlikely to receive the full benefits of treatment.4 6 Conversely, patient persistence with MS therapy has been reported to be associated with lower health-care costs and health-care resource utilization,7 although this relationship has not been observed in all studies.8 A retrospective analysis of a prescription claims database found that among MS patients treated with injectable DMTs, the proportion that switched treatments did not differ significantly from one agent to another.7 However, reported discontinuation rates were highest for subcutaneous (SC) IFNβ-1b and lowest for intramuscular (IM) IFNβ-1a. Logistic regression models also showed that patients on IM IFNβ-1a had similar persistence as those on SC IFNβ-1b or SC GA, while persistence was higher with SC IFNβ-1a than with SC IFNβ-1b or SC GA.7
Understanding why patients with MS do not take their medication is an important step toward improving patient persistence. However, few studies have evaluated patient reasons for discontinuation of prescribed DMT regimens.9 10 Among patients who were not persistent with therapy, their most common reason was perceived lack of efficacy (30%), followed by injection-site reactions (12%) and flu-like symptoms (10%). Other reasons for therapy discontinuation included depression (9%), headache (8%), liver test abnormalities (7%), and fatigue (6%).4
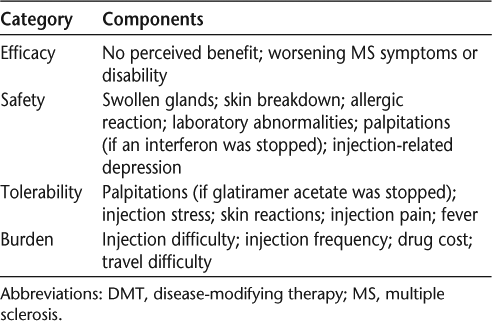
Associations between patient persistence with prescribed treatment regimens, patient disease perceptions, and disability progression may suggest avenues for improving patient persistence. One survey instrument that has been employed to characterize MS-associated disabilities and disability progression is the Patient- Determined Disease Steps (PDDS) scale. The PDDS was adapted from a physician-administered scale called Disease Steps11–13 to be used as a patient-reported measure of disability. Results generated using the PDDS assessment scale are correlated with the widely used clinician-measured Expanded Disability Status Scale (EDSS).11–13
This article presents the results of analyses of patient-reported reasons for injectable DMT discontinuations using data from the North American Research Committee on Multiple Sclerosis (NARCOMS) database. Exploratory analyses evaluated the relationship between staying on therapy and patient-reported disability progression using the PDDS and patient-reported annualized relapse rates (ARRs).
Methods
Patient Reasons for MS Treatment Discontinuation
The NARCOMS database consists of data submitted confidentially by over 36,000 people with MS who self-report detailed information about their MS disease and treatment by completing a baseline database enrollment form and subsequent semiannual follow-up surveys. NARCOMS operates as an independent MS patient registry under the auspices of the Consortium of Multiple Sclerosis Centers (Hackensack, NJ).
Participants' reasons for discontinuing injectable DMT use were investigated using a cross-sectional analysis of the spring 2005 update of the NARCOMS database. This particular survey was a single-time-point query and not a routine component of the semiannual questionnaires. The injectable DMTs included in these analyses were IM IFNβ-1a (Avonex), SC IFNβ-1b (Betaseron), SC GA (Copaxone), and SC IFNβ-1a (Rebif). Extavia, another formulation of SC IFNβ-1b, was not available for routine clinical use during the period of time covered by this particular survey, and thus no data on this drug were available.
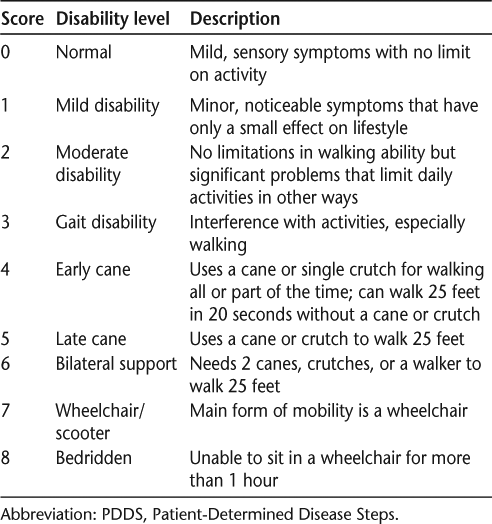
The NARCOMS discontinuation questionnaire asked those who reported stopping a DMT to select their reason(s) from among 16 possible options. Participants could select multiple responses, and all reported answers were used in the analyses. Participants were instructed to base their answers on the most recent occasion on which they had stopped using a specific MS therapy, although they were not asked to specify the date of the treatment interruption. The reasons selected by patients for DMT discontinuation were grouped into the categories of efficacy, safety, tolerability, and burden (Table 1). Analyses were performed first using unadjusted data and then after adjustment for baseline age, disease duration, disability, and sex. Patient-reported disability was assessed using the PDDS, which includes nine levels of motor dysfunction, ranging from no symptoms (scored as 0) to a bedridden condition (scored as 8) (Table 2).11 These steps relate to functional changes in ambulation that impinge on a patient's lifestyle and can be easily recognized by patients.12 The PDDS is easy to use with little interrater variability and with certain advantages over the EDSS, such as increased sensitivity for measuring progression in MS patients who use unilateral support.12 In the efficacy analysis, PDDS was used as a binary variable with two categories (≤5 and ≥6) to distinguish between those with mild or moderate disability (scores of 0–2 and 3–5, respectively) and those with more severe disability (scores of 6–8).14 For all other analyses, PDDS score was used as a continuous variable.
Reasons for stopping injectable DMTs: instrument categories and components
PDDS rating scale elements
PDDS-Defined Disease Progression and ARR
To evaluate PDDS-defined sustained disease progression and ARR, a longitudinal analysis was performed on a subgroup (n = 636) of NARCOMS participants who had completed at least 10 semiannual update surveys between 2000 and 2009 and had remained on the same DMT for at least 5 of those years. ARR estimates were from a negative binomial regression model, with ARR calculated as cumulative relapses/cumulative duration. Sustained disease progression was defined as a ≥1-point increase in PDDS score sustained for at least 6 months. In order to reduce potential bias in treatment comparisons due to unmatched patient groups, propensity score (PS) adjustments were made with respect to differences in participant baseline demographic and disease characteristics. Logistic regression models were built to predict the PS using the baseline covariates of age, sex, disease duration, number of relapses in the 6 months prior to study baseline, PDDS score, cognition, and fatigue.
Statistical Analyses
The χ2 test was used for statistical comparison of categorical data, and the t test was used for comparison of continuous data. Analysis of variance, analysis of covariance, and logistic regression analysis were employed for multiple group comparisons and analyses with covariate adjustment. Pairwise comparisons were made among all injectable DMTs included in the cross-sectional discontinuation data. For the longitudinal disease progression and ARR data, statistical comparisons utilized combined data for the SC IFNβ-1b and SC IFNβ-1a treatment groups based on their similar SC dosing frequency and group sample size limitations. PS adjustment was used to account for baseline discrepancies among different treatment groups so that the sample populations were comparable. 15 Cox proportional hazards regression, weighted for the inverse of PS, was used for analysis of disease progression. Analysis of ARR was conducted using a negative binomial regression model adjusted for PS as a covariate. The Bonferroni method was applied to adjust the P value of pairwise tests.
Results
Patient Reasons for MS Treatment Discontinuation
Among the 5625 participants who answered DMT treatment discontinuation questionnaires in the NARCOMS spring 2005 update, 3169 (56%) provided the name of the discontinued therapy. Within this subset, 1956 participants identified 1 or more of the 16 options on the discontinuation questionnaire as their main reason(s) for stopping treatment. Participants who discontinued DMT use had a mean age of 50.4 ± 9.7 years at enrollment in the database (baseline, year 2000); 92% were white, and 78% were female. At this time point, the mean MS disease duration was 21.0 ± 10.3 years and the mean PDDS score was 4.0 ± 2.3 (median = 4). When participant baseline characteristics were compared by treatment, there were no statistically significant differences among treatments for race. However, age, sex, disease duration, and PDDS score all showed significant differences (data not shown).
Unadjusted Analysis
Perceived Efficacy. Across all treatments, a lack of perceived efficacy benefit was the most frequently identified reason for discontinuation (Table 3). Differences between the four groups for discontinuations based on perceived efficacy were statistically significant (P < .0001, χ2 test). Without adjustment for baseline characteristics, SC GA had the highest discontinuation rate based on perceived lack of efficacy (46%), SC IFNβ-1a had the lowest rate (27%), and the discontinuation rates for IM IFNβ-1a (41%) and SC IFNβ-1b (40%) were similar (Table 3).
Main reason(s)a provided by patients for stopping injectable DMT use
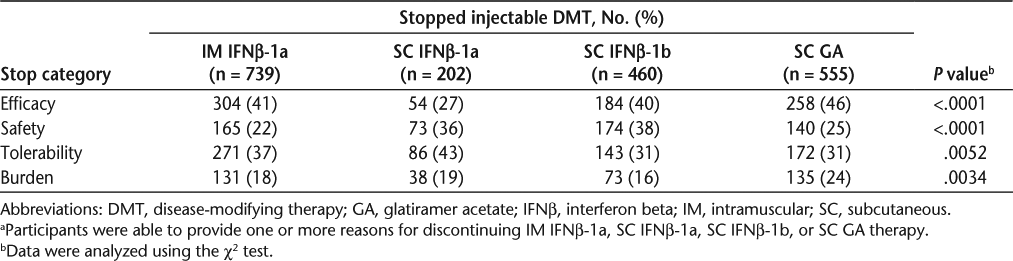
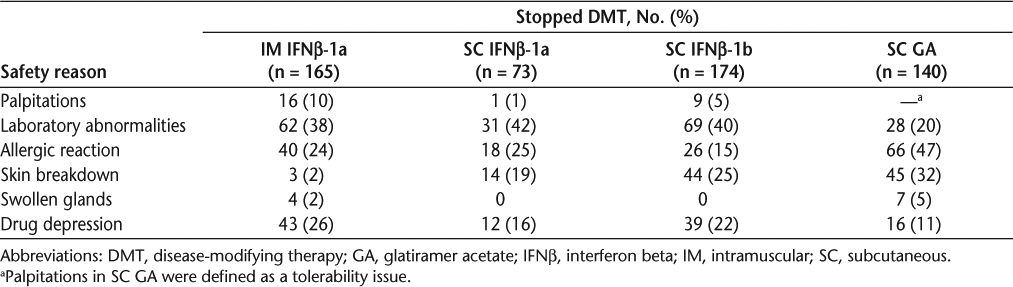
Safety. Discontinuation for safety reasons also differed significantly (P < .0001) between the four treatment groups. Safety-related discontinuations were greatest with SC IFNβ-1b (38%), followed by SC IFNβ-1a (36%), SC GA (25%), and IM IFNβ-1a (22%). Table 4 lists the reasons for discontinuation due to safety for all treatments.
Patient-reported safety reasons for treatment discontinuation
Tolerability. Discontinuations based on poor tolerability also showed a statistically significant difference (P = .0052) between groups, with SC IFNβ-1a having the highest percentage (43%), followed by IM IFNβ-1a (37%) and then both SC IFNβ-1b (31%) and SC GA (31%). Table 5 lists the reasons for discontinuation due to tolerability for all treatments.
Patient-reported tolerability reasons for treatment discontinuation
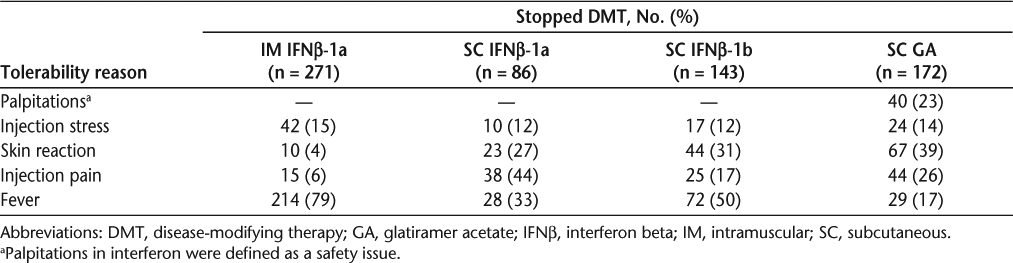
Burden. There was a significant difference (P = .0034) between treatment groups in burden-based discontinuation; the rate was highest with SC GA (24%), whereas all other therapies had similar rates (16–19%).
Adjusted Analysis
Table 6 presents the results of logistic regression analyses assessing pairwise treatment comparisons after adjustment for baseline age, sex, disease duration, and PDDS score.
Treatment group differences in patient reasons for stopping injectable DMTs
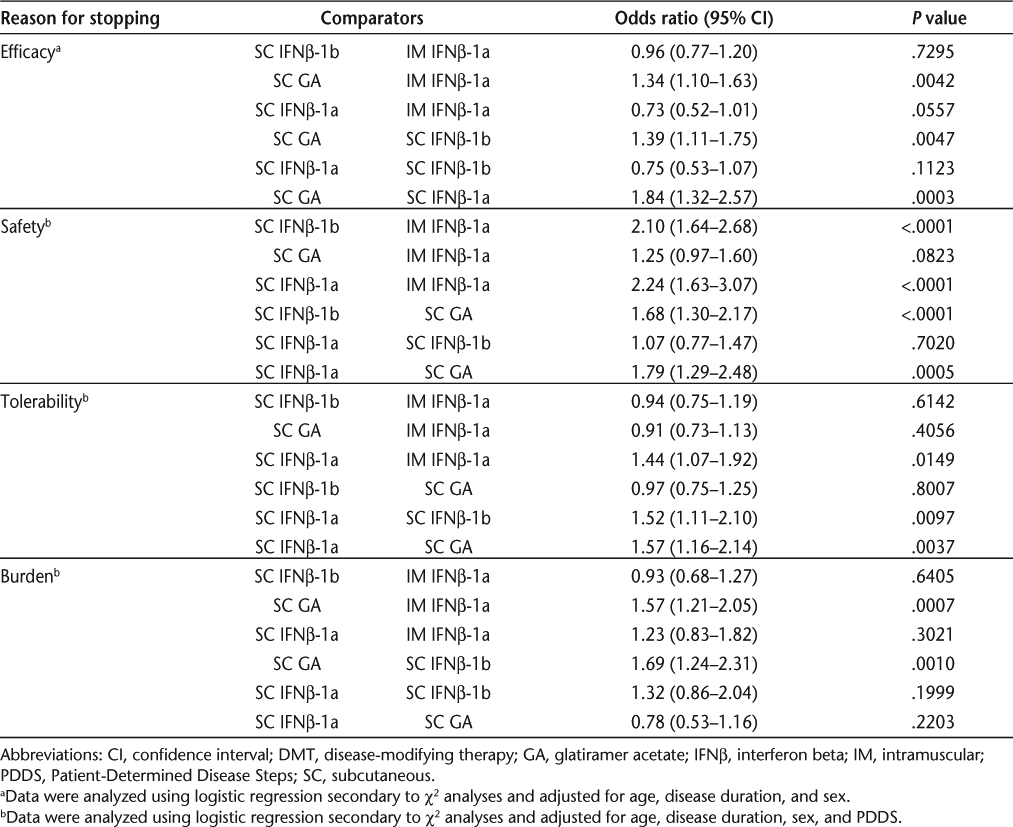
Perceived Efficacy. Patients were more likely to stop SC GA than IM IFNβ-1a (P = .0042), SC IFNβ-1b (P = .0047), or SC IFNβ-1a (P = .0003) for efficacy reasons.
Safety. Safety concerns prompted cessation of therapy more frequently with SC IFNβ-1b (P < .0001) and SC IFNβ-1a (P < .0001) than with IM IFNβ-1a. Safety-based discontinuations were also less common for SC GA than for either SC IFNβ-1b (P < .0001) or SC IFNβ-1a (P = .0005).
Tolerability. Tolerability concerns resulted in fewer discontinuations for IM IFNβ-1a than for SC IFNβ-1a (P = .0149), while tolerability-related discontinuations were more frequent with SC IFNβ-1a than with SC IFNβ-1b (P = .0097) or SC GA (P = .0037).
Burden. Patients were more likely to stop SC GA than IM IFNβ-1a (P = .0007) or SC IFNβ-1b (P = .0010) for burden reasons.
PDDS-Defined MS Progression and ARR
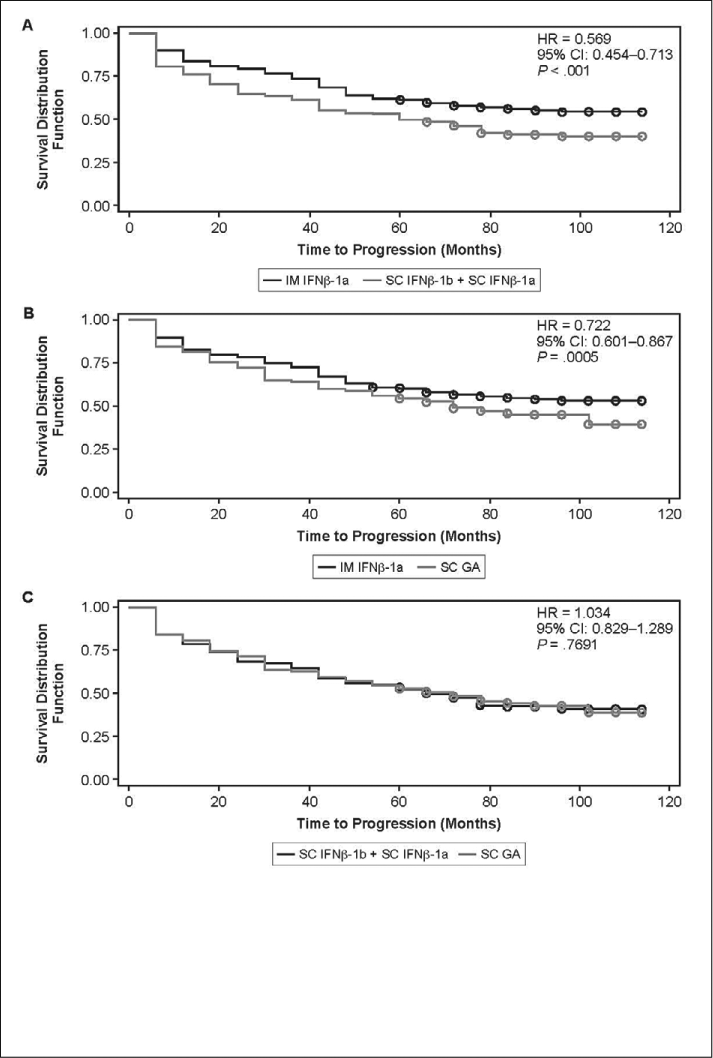
Additional subgroup analyses were conducted on longitudinal participant data from NARCOMS, with separate comparisons made between those on IFNβ-1 therapies dosed weekly (ie, IM IFNβ-1a) and those receiving more frequently dosed IFNβ-1 therapies (SC IFNβ-1b + SC IFNβ-1a, combined IFNβ data set); between participants on IM IFNβ-1a and SC GA; and between participants in the combined IFNβ data set and those on SC GA. Data were normalized using PS adjustment prior to these longitudinal analyses in order to reduce selection bias in treatment comparisons. There were no significant group differences in participant baseline data following PS adjustment (data not shown). Cognition and fatigue performance scales, which were completed concurrently with the PDDS assessment, also did not differ significantly at baseline. At follow-up, the PS-adjusted proportion of participants with sustained PDDS-defined disability progression was lower with IM IFNβ-1a than with SC IFNβ-1b + SC IFNβ-1a (44.4% vs. 59.2%, P = .007) or SC GA (45.5% vs. 54.9%, P = .042). The proportions for SC IFNβ-1b + SC IFNβ-1a and SC GA were not significantly different.
Group comparisons of time to PDDS-defined sustained disease progression are presented in Figure 1. Cox proportional hazards regression analyses, adjusted for PS, showed a lower risk of PDDS-defined disability progression with IM IFNβ-1a than with SC IFNβ-1b + SC IFNβ-1a (P < .001) or SC GA (P = .0005), but no significant difference between SC IFNβ-1b + SC IFNβ- 1a and SC GA (P = .7691). Mean ARR was lower with IM IFNβ-1a than with SC GA (0.4089 vs. 0.5382, P = .0278). No other significant differences were noted for IM IFNβ-1a versus SC IFNβ-1b + SC IFNβ-1a (0.3603 vs. 0.4680, P = .0917) or for SC IFNβ-1b + SC IFNβ- 1a versus SC GA (0.4861 vs. 0.5231, P = .6174).
Time to PDDS-defined sustained progression
Discussion
The identification of barriers to MS treatment with DMTs provides an opportunity to anticipate difficulties that patients may have with DMTs. Prescribers who are equipped with this knowledge can preemptively develop strategies to optimize persistence and, hence, the benefits of treatment. We report categorized patient-reported reasons for discontinuing injectable DMTs. These data supplement the results of previous reports on persistence and discontinuation in MS.
The primary reason for injectable DMT discontinuation in our cross-sectional analysis was perceived lack of efficacy. Our findings are consistent with several published reports indicating that patient-perceived lack of efficacy is one of the reasons most commonly cited by MS patients for discontinuation of DMTs.4 6 16 These observations are also in agreement with the results from phase 3 trials, which found that these injectable DMTs reduce ARR by only about one-third and that the impact of treatment on MS disability progression was similar across DMTs.3 Patients may have difficulty developing and maintaining realistic treatment expectations, even with the full support of a clinical disease management team. Other frequently identified reasons for treatment discontinuation included tolerability/safety issues involving treatment-related adverse events (particularly early in treatment) and treatment burden concerns associated with therapy inconvenience, frequency of injections, and needle phobia. Additional factors such as an increasing neuropsychological disease burden, including cognitive decline and depression, can further contribute to treatment discontinuations.6
In the present report, both unadjusted and adjusted statistical analyses suggest that patients who discontinued SC GA were more likely to report perceived lack of efficacy and/or the burden of therapy as their main reasons for stopping therapy than patients who discontinued IM IFNβ-1a or SC IFNβ-1b. Perceived lack of efficacy was also the most frequently reported reason for stopping treatment with IM or SC IFNβ-1a, although perceived lack of efficacy was still greater for patients treated with SC GA. Patients who discontinued SC IFNβ-1a or IFNβ-1b were more likely to report safety as a main reason for stopping therapy than those who discontinued either IM IFNβ-1a or SC GA. Patient-reported safety-related discontinuations were also more common for patients who discontinued SC IFNβ-1a or SC IFNβ-1b than for those who discontinued SC GA. Patients who stopped treatment for tolerability reasons were more likely to stop SC IFNβ-1a than any of the other injectable DMTs included in this analysis. Patients who stopped treatment for burden reasons were significantly more likely to have discontinued SC GA than either IM or SC IFNβ-1b.
For patients prescribed the same injectable DMTs considered in our analyses, discontinuation incidence at 6 months of treatment was reported to be lower with IM IFNβ-1a (11.5%) than with SC IFNβ-1b (20.0%), SC GA (13.2%), or SC IFNβ-1a (12.5%).7 In that report, the likelihood of switching among IFNβ therapies was also lower with IM IFNβ-1a (5.2%) than with SC IFNβ-1b (8.5%) or SC IFNβ-1a (7.4%).7 After 12 months of treatment, the discontinuation rate remained lower for IM IFNβ-1a (22.6%) than for SC IFNβ-1b (42.9%), SC GA (25.1%), or SC IFNβ-1a (25.0%), and the likelihood of switching among IFNβ therapies remained lower with IM IFNβ-1a (9.0%) than with SC IFNβ-1b (12.0%) or SC IFNβ-1a (12.3%).7 Earlier reports suggested that after 4 months of treatment, up to 11% of RRMS patients on a DMT discontinue treatment,17 while after 6 months, discontinuation figures ranged from 9% to 27%.4
A recent systematic review and meta-analysis of IFNβ DMTs for RRMS reported that there were no significant differences among IFNβ therapies in their impact on disability progression as assessed by the EDSS.18 However, an evaluation of possible correlations between the duration of gaps in MS treatment and the incidence of severe MS relapse found that patients with therapy gaps lasting more than 90 days were nearly twice as likely to experience a severe MS relapse as patients with shorter treatment gaps.19 In addition, nonpharmacy MS-related medical costs, including hospitalizations, emergency room visits, outpatient visits, and MRI scans, were lower in persistent patients than in those who switched or discontinued DMT treatment.7 In our analyses of baseline-adjusted NARCOMS data, among participants on the same injectable DMT for at least a cumulative 5 years, rates of sustained disease progression (as indicated by PDDS) were lower with IM IFNβ-1a than with SC IFNβ-1a or IFNβ-1b (combined data set) or with SC GA. However, MS relapses (ARR) did not differ significantly between treatment groups. Pairwise statistical comparisons among all three IFNβ DMTs were not performed for disability progression and relapse data.
The validity of the current findings from analyses of NARCOMS data is supported by the large number of participants contributing to the NARCOMS registry, but these study results also have several limitations. The data were purely observational in nature, with a possible self-selection bias for the particular subset of patients with MS who were able to participate in the registry, chose to participate, and continued treatment with the same DMT for at least 5 years. It is unknown if patient groups were segregated and different at baseline or if the first choice of therapy was biased. The study was also limited by the unavailability of data on participant total time on treatment, participant versus physician initiation of medication switching, MS treatment history, and time on previous MS therapies. Other limitations of the study included the inability to identify causal relationships among the observed outcomes using the available data or to evaluate participants who discontinued therapy because of unmet expectations. In addition, participants who were not treated with the same DMT for a cumulative 5 years were excluded from the current analyses, since those patients who discontinued DMT use after shorter intervals may have different disease characteristics, behaviors, and responses to therapy than those on persistent therapy. Finally, it cannot be known if patient-reported relapse data used in NARCOMS would be the same as those reported by a treating neurologist.
Conclusion
For a wide range of chronic medical conditions, including MS, the benefits of continuous therapy are well known and appear to include reductions in morbidity and mortality.20 Patients with MS who persist in using injectable DMTs and adhere to the prescribed timing, dosing, and frequency of medication administration have a lower risk of relapse and a better self-reported quality of life than patients who discontinue or are nonadherent.10 11 21 22 Based on our analyses, discontinuation profiles varied somewhat among DMTs for MS. IM IFNβ-1a and SC GA appeared to have the fewest safety concerns, although SC GA was associated with the highest therapy burden and with lower perceived efficacy. Difficulties with tolerability were more commonly reported with SC IFNβ-1a than with the other evaluated therapies. Tolerability was comparable among IM IFNβ-1a, SC IFNβ-1b, and SC GA. In the persistent therapy cohort, self-reported disability progression was slower for participants treated with IM IFNβ-1a than for participants treated with SC IFNβ-1a, SC IFNβ-1b, or SC GA. These findings may help healthcare providers anticipate patient difficulties with DMTs, inform clinical decision making, and influence strategies to increase medication compliance, leading to improvements in clinical outcomes and health-care resource utilization in the management of MS.
PracticePoints
Injectable first-line MS therapies are intended for continuous use. Treatment discontinuation may negatively affect patients' clinical outcomes.
The primary reasons for treatment discontinuation reported by participants in the NARCOMS database were categorized into four groups: efficacy, safety, tolerability, and burden of treatment. Specific reasons for discontinuation differed somewhat among disease-modifying therapies (DMTs).
Among the participants in this cohort who remained on the same DMT for 5 years or more, intramuscular interferon beta-1a was associated with a lower risk of patient-reported MS progression than other injectable MS therapies. Further studies are needed to assess the impact of initial selection bias on these findings.
Acknowledgments
Medical writing assistance was provided by Christopher Barnes and editorial support was provided by Joshua Safran, both of Infusion Communications. Their work was funded by Biogen Idec Inc.
References
Stys PK. Multiple sclerosis: autoimmune disease or autoimmune reaction? Can J Neurol Sci. 2010;37(suppl 2):S16–S23.
Hilas O, Patel PN, Lam S. Disease modifying agents for multiple sclerosis. Open Neurol J. 2010; 4: 15–24.
Fox RJ, Arnold DL. Seeing injectable MS therapies differently: they are more similar than different. Neurology. 2009; 72: 1972–1973.
Tremlett HL, Oger J. Interrupted therapy: stopping and switching of the beta-interferons prescribed for MS. Neurology. 2003; 61: 551–554.
Tremlett H, Van der Mei I, Pittas F, et al. Adherence to the immuno-modulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing doses. Pharmacoepidemiol Drug Saf. 2008; 17: 565–576.
Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence. 2010; 4: 1–9.
Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin. 2010; 26: 663–674.
Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011; 77: 355–363.
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009; 256: 568–576.
Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011; 18: 69–77.
Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007; 13: 1176–1182.
Hohol M, Orav E, Weiner H. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995; 45: 251–255.
Hohol M, Orav E, Weiner H. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler. 1999; 5: 349–354.
Gulick EE, Namey M, Halper J. Monitoring my multiple sclerosis. Int J MS Care. 2011; 13: 137–145.
Trojano M, Pellegrini F, Fuiani A, et al. New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol. 2007; 61: 300–330.
Rio J, Porcel J, Tellez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler. 2005; 11: 306–309.
Mohr DC, Likosky W, Boudewyn AC, et al. Side effect profile and adherence to in the treatment of multiple sclerosis with interferon beta-1a. Mult Scler. 1998; 4: 487–489.
Oliver BJ, Kohli E, Kasper LH. Interferon therapy in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis of the comparative trials. J Neurol Sci. 2011; 302: 96–105.
Al-Sabbagh A, Bennett R, Kozma C, Dickson M, Meletiche D. Medication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse: findings from a national managed care database. J Neurol. 2008; 255(suppl 2):S7.
New England Health Care Institute. Thinking outside the pillbox: a system-wide approach to improving patient medication adherence for chronic disease. http://www.nehi.net/publications/44/thinkingout-side_the_pillbox_a_systemwide _approach_to_improving_patient_medication_adherence_for_chronic_disease. Accessed June 22, 2011.
Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010; 30: 89–100.
Halpern R, Agarwal S, Dembek C, Borton L, Lopez-Bresnahan M. Comparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence. 2011; 5: 73–84.
Financial Disclosures: Dr. Fox has received consultant fees from Allozyne, Avanir, Biogen Idec, Novartis, Questcor, Teva, and Xeno-Port, and has received grant or research support from the National Institutes of Health, the National Multiple Sclerosis Society, and Novartis. Ms. Salter and Dr. Tyry have nothing to disclose. Ms. Sun and Drs. You, Laforet, and Campagnolo are employees of Biogen Idec.
Funding/Support: This study was supported by Biogen Idec Inc. The NARCOMS Registry is supported by the Consortium of Multiple Sclerosis Centers and its Foundation.







