Publication
Research Article
International Journal of MS Care
Efficacy and Safety of Alemtuzumab in Multiple Sclerosis and Impact on Nursing Role
Author(s):
Alemtuzumab, a humanized monoclonal antibody, has shown efficacy for relapsing-remitting multiple sclerosis (MS) in phase 2 and phase 3 trials. Compared with subcutaneous interferon beta-1a, alemtuzumab significantly reduced the risk for accumulation of disability and the rate of relapse, and improved mean disability level from baseline. Notable safety and tolerability concerns include infusion-associated reactions, infections of predominantly mild-to-moderate severity, and autoimmune adverse events, principally thyroid disorders and immune thrombocytopenia. As emerging therapies such as alemtuzumab are approved for the treatment of MS, nurses specializing in the care of MS patients will make increasingly significant contributions to the education of patients, caregivers, and other health-care providers about these therapies' efficacy, tolerability, safety, and administration. This article reviews the phase 2 and phase 3 efficacy and safety results for alemtuzumab, with an emphasis on the role of nurses in communication about this treatment option for those with MS.
New therapies with potential benefits for people with multiple sclerosis (MS) are being developed, tested, and approved. As new research and emerging therapies change the treatment paradigm for MS, the nursing role and its associated demands are changing as well. Nurses specializing in the care of MS patients need to stay abreast of MS research and disease-modifying agents approved by the US Food and Drug Administration (FDA), because patients are eager to make use of these new treatments. MS nurses play an important role in educating patients and their families about the treatment process, possible side effects, and the symptoms of those side effects; they are responsible for answering patients' questions, as well as providing support to patients and their caregivers. Although an emerging therapy may be promising, it may not be a suitable choice for a particular patient. Nurses must be aware of the efficacy and safety data obtained from phase 2 and 3 clinical trials if they are to explain these new therapies to patients and other health-care practitioners effectively so that appropriate treatment decisions can be made.
One category of newer therapies showing considerable promise in the treatment of MS is monoclonal antibodies. Monoclonal antibodies typically work by binding to a specific site on a target protein, which causes or blocks a specific reaction. Therapies in this drug class have unique mechanisms of action, typically involving the targeting of surface proteins on the cells of the immune system. Buttmann1 provides an excellent review of monoclonal antibodies in MS treatment. Monoclonal antibodies approved or under investigation in MS include natalizumab, daclizumab, rituximab, and alemtuzumab. Natalizumab is currently FDA-approved, and daclizumab, rituximab, and alemtuzumab are under investigation. Here we summarize the available evidence from clinical trials on the efficacy, tolerability, and safety of alemtuzumab in the treatment of MS. A literature review using the key terms “alemtuzumab” and “multiple sclerosis” identified the clinical trials discussed here and the preliminary studies on which they were based. Alemtuzumab has been in development the longest of any monoclonal antibody under investigation. The features of alemtuzumab administration and monitoring call on the nurse specialist to take on a major role in patient care in the area of risk management.
Role of Skilled Nursing Care in Multiple Sclerosis
It is currently believed that MS is an autoimmune disease occurring in genetically susceptible individuals. The disease is both inflammatory and neurodegenerative, beginning with inflammation in the brain and spinal cord that causes demyelination and leads to slower nerve conduction. Axonal injury and destruction observed in MS patients are associated with permanent neurologic dysfunction. MS is the most common cause of nontraumatic neurologic disability in individuals of working age, affecting approximately 2.1 million people worldwide.2 The age of onset typically ranges from 10 to 59 years, with most cases occurring in those aged 20 to 40 years. As with some other autoimmune disorders, MS is more common among women. It is estimated that 450,000 individuals in the United States are living with MS, with more than 200 people being diagnosed every week.2
Caring for people with MS requires a comprehensive health-care team with the MS nurse as an integral part. Smrtka and colleagues3 describe the many roles of the MS nurse as including symptom manager, patient and family advocate, collaborator, counselor, and educator. Additionally, Halper describes the MS nurse as one who assists, enlightens, refers, helps establish reasonable expectations, offers encouragement, prepares the patient, and provides explanation.4 These responsibilities are more effectively fulfilled if trust has developed between the MS nurse and the patient and open, clear communication is occurring.
Managing patients' needs, goals, and expectations can be very difficult. The patient may seek to be cured or freed of symptoms, which can be impossible given that available treatments are only partially effective and cannot repair actual axonal damage. Nonetheless, clinicians have the same set of goals for treatment: to inhibit the inflammatory and neurodegenerative components of the disease, thus reducing disease activity as measured by relapses, magnetic resonance imaging (MRI) findings, and progression of clinical disability.
When evaluating therapies for MS, three distinct characteristics are considered: efficacy, tolerability, and safety. New therapies are generally developed and tested with the aim of addressing unmet needs for greater efficacy, acceptable safety, and improved tolerability.5 Patients will often challenge the MS nurse by inquiring about ongoing research and development of new therapies, even asking about drugs that are in the very early stages of development. The MS nurse should be aware of the most current information, because these conversations offer hope to patients and their families for the availability of new therapies that could change the course of what is now an inexorably progressive illness.
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody that has been approved in the European Union and is under regulatory review in the United States and other countries for the treatment of active relapsing-remitting multiple sclerosis (RRMS). It has demonstrated efficacy in preliminary studies as well as in a phase 2 clinical trial (CAMMS223, ClinicalTrials.gov number NCT00050778) and two recently completed phase 3 trials (CAMMS323, ClinicalTrials.gov number NCT00530348; CAMMS324, ClinicalTrials.gov number NCT00548405). Alemtuzumab targets the cell-surface marker CD52, which is present at high levels on T and B lymphocytes and to a lesser extent on other immune cells. Alemtuzumab binding to lymphocytes causes lysis of these cells and their rapid depletion from circulation.6 Repopulation of the lymphocyte pool may result in changes in lymphocyte subsets, which preliminary research suggests may be beneficial in reducing the inflammatory effects of MS.7 8 The acute anti-inflammatory effect of alemtuzumab is immediately followed by the onset of a distinctive pattern of T- and B-cell repopulation that continues over time. Although the exact mechanism of action in MS is unknown, this repopulation creates a rebalanced immune system that may reduce MS disease activity. Preliminary laboratory evidence also shows that the repopulating lymphocytes produce greater amounts of neurotrophins when exposed to myelin basic protein.9 Neurotrophins are a family of proteins that help support the development, function, and survival of neurons.
Single-Arm Studies of Alemtuzumab in MS
Early investigations of alemtuzumab with secondary progressive MS (SPMS) and RRMS patients yielded promising results.10–13 Alemtuzumab significantly reduced the annualized relapse rate and the formation of new brain lesions in both SPMS and RRMS patients.10–12 Alemtuzumab-treated RRMS patients experienced a mean improvement in disability and SPMS patients experienced a reduction in the mean rate of increase in disability compared with the year before treatment. In an investigator-sponsored study of treatment-refractory RRMS patients receiving two annual courses of alemtuzumab, the results showed a significant reduction in annualized relapse rate, an increase in the proportion of patients who were relapse-free at 1 and 2 years after treatment, an increase in the time to relapse among those who did experience a relapse, and a mean improvement in disability.13
In each of the above studies, infusion-associated reactions, autoimmune adverse events (AEs) (mostly thyroid-related), and infections of predominantly mild-to-moderate severity were the primary safety concerns. One patient developed Goodpasture disease (anti–glomerular basement membrane disease), ultimately requiring a renal transplant.12 Another patient treated with alemtuzumab outside of a reported study or clinical trial was diagnosed with Goodpasture disease 24 months after an alemtuzumab course, experienced renal failure requiring a renal transplant, and is currently in remission.14
Clinical Trials
The results of the early investigations summarized above led to the development of a phase 2 and two phase 3 clinical trials. The phase 2, 3-year study (CAMMS223) compared alemtuzumab with subcutaneous interferon beta-1a (IFNβ-1a) in early, active-disease RRMS patients who were treatment-naive. CAMMS223 also had an extension phase for long-term follow-up. One phase 3 trial (CAMMS323, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis [CARE-MS I]) replicated the design of CAMMS223 with the length of study being 2 years instead of 3 years. The other phase 3, 2-year trial tested alemtuzumab in RRMS patients who had active disease while on therapy (CAMMS324, CARE-MS II). Details of the designs of these studies have been reported previously15–17 and are summarized in Table 1. An extension trial (CAMMS03409, ClinicalTrials.gov number NCT00930553) is ongoing and will evaluate long-term safety and the need for and efficacy of additional alemtuzumab courses.
Phase 2 and phase 3 study design summaries
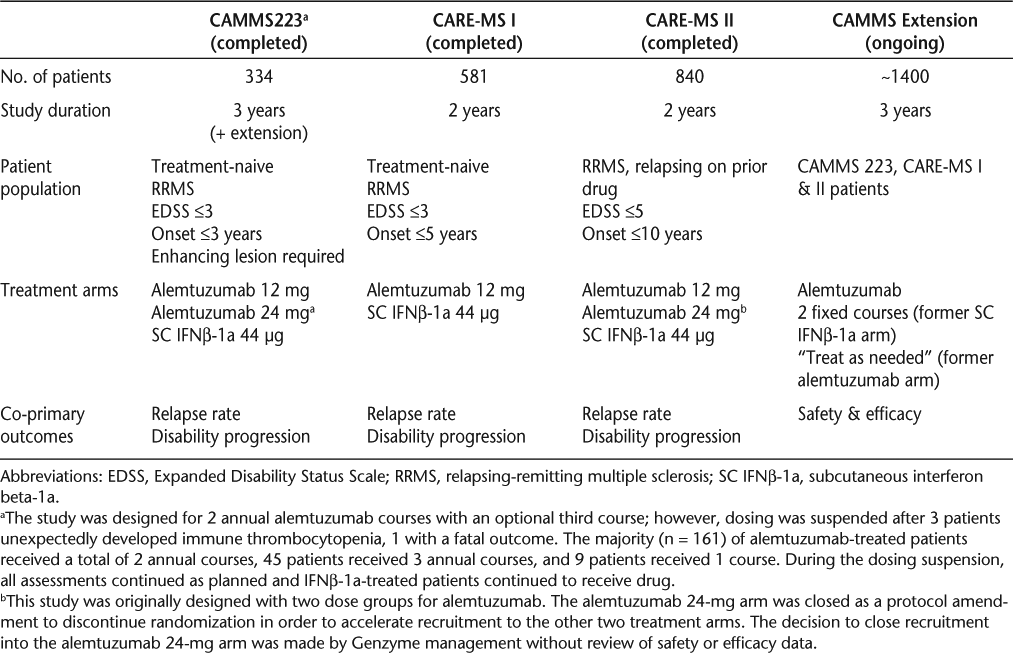
The first alemtuzumab course in each study was administered intravenously at month 0 on 5 days; subsequent annual courses were on 3 days. Interferon beta-1a was administered subcutaneously, 44 μg 3 times per week. All patients received 3 days of 1 g intravenous methylprednisolone at baseline and month 12, and in CAMMS223 also at month 24. For alemtuzumab-treated patients, this served as premedication for the infusion courses. Alemtuzumab infusions took approximately 4 to 5 hours to complete, including the administration of methylprednisolone prior to each infusion. All outcomes related to the co-primary endpoints and imaging efficacy endpoints were determined by blinded raters unaware of the patients' treatment assignments. Expanded Disability Status Scale (EDSS)18 performance was assessed quarterly, and relapses were assessed as needed and confirmed by a blinded relapse adjudication panel.
Efficacy
Co-primary efficacy endpoints in all three clinical trials were relapse rate and time to sustained accumulation of disability. In all three clinical trials, alemtuzumab was significantly superior to IFNβ-1a on relapse rate (Table 2).15–17 In CAMMS223 and CARE-MS II, alemtuzumab patients were less likely to experience a sustained accumulation of disability compared with IFNβ-1a patients (Table 2).15 17 In CARE-MS I, although the percentage of patients experiencing a sustained accumulation of disability was lower in those receiving alemtuzumab than in those receiving IFNβ-1a, the difference was not statistically significant (Table 2).16 The proportion of patients with sustained accumulation of disability in the IFNβ-1a group was lower than expected based on previous research.16
Annualized relapse rate and disability efficacy summary for clinical trial studies
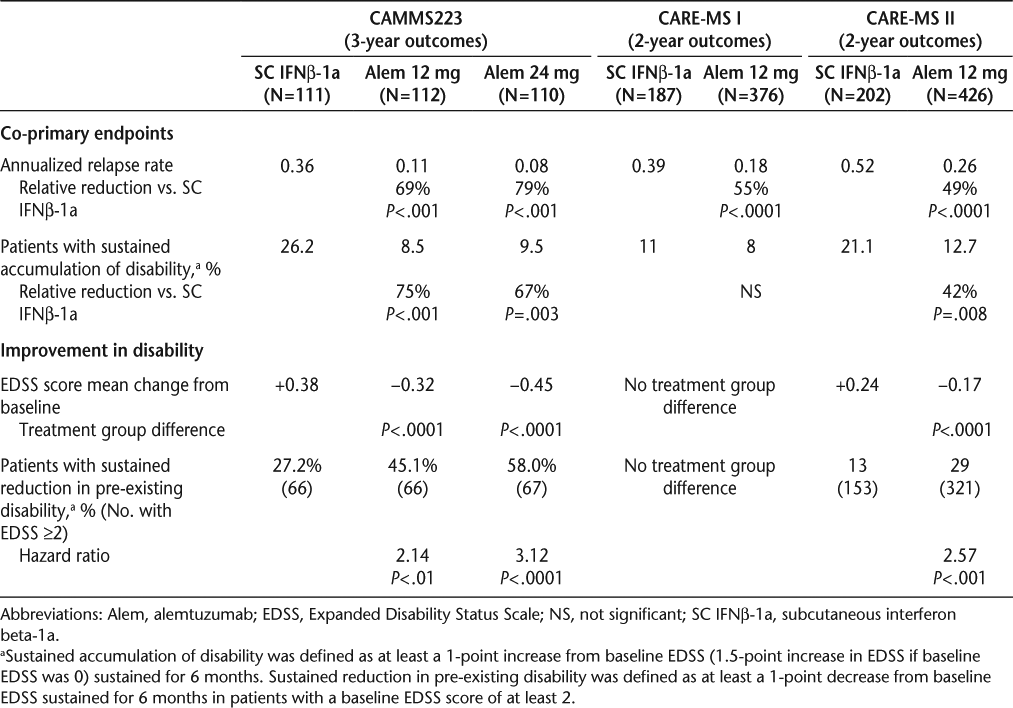
In CAMMS223 and CARE-MS II, the alemtuzumab patients showed a mean decrease in the EDSS score from baseline (improved disability), while the IFNβ-1a group had a mean increase (worsened disability); the difference between treatment groups was significant (Table 2).15 17 Among patients with baseline EDSS scores of at least 2, those receiving alemtuzumab were more than twice as likely to experience a sustained reduction in pre-existing disability compared with those receiving IFNβ-1a (Table 2).17 19 Multiple imaging outcomes were also favorable, including lesion activity and brain atrophy in the phase 3 program.16 17 Results from the extension phase of CAMM223 indicated that the efficacy of alemtuzumab through year 5 was comparable to results through year 3 of the original study period, indicating some durability of the treatment effect, particularly since most patients were last treated with alemtuzumab 4 years earlier.20
As with all studies, the clinical trials have strengths and limitations to consider when evaluating the results. Because infusion-associated reactions are a common side effect of alemtuzumab, a double-blind study design was not feasible, as patients and their treating physicians would likely know what medication they were receiving. However, to maintain the integrity of the clinical trial data, all neurologic examinations performed at the quarterly visits or to determine an MS relapse were completed by an evaluator who had no knowledge of the patient's treatment or current MS symptoms. Patients in these trials were at the earlier stages of MS, and long-term safety and efficacy require further study. To address this issue, all patients who participated in the phase 2 and 3 studies have the opportunity to be part of an extension study. This should provide evidence for the durability of the alemtuzumab treatment effect and its long-term safety. A particular strength of these studies is that alemtuzumab was compared with a treatment with demonstrated efficacy rather than a placebo, setting a higher bar for assessing alemtuzumab's efficacy. The treatment effect of alemtuzumab has been consistent across the clinical trials.
Safety and Tolerability
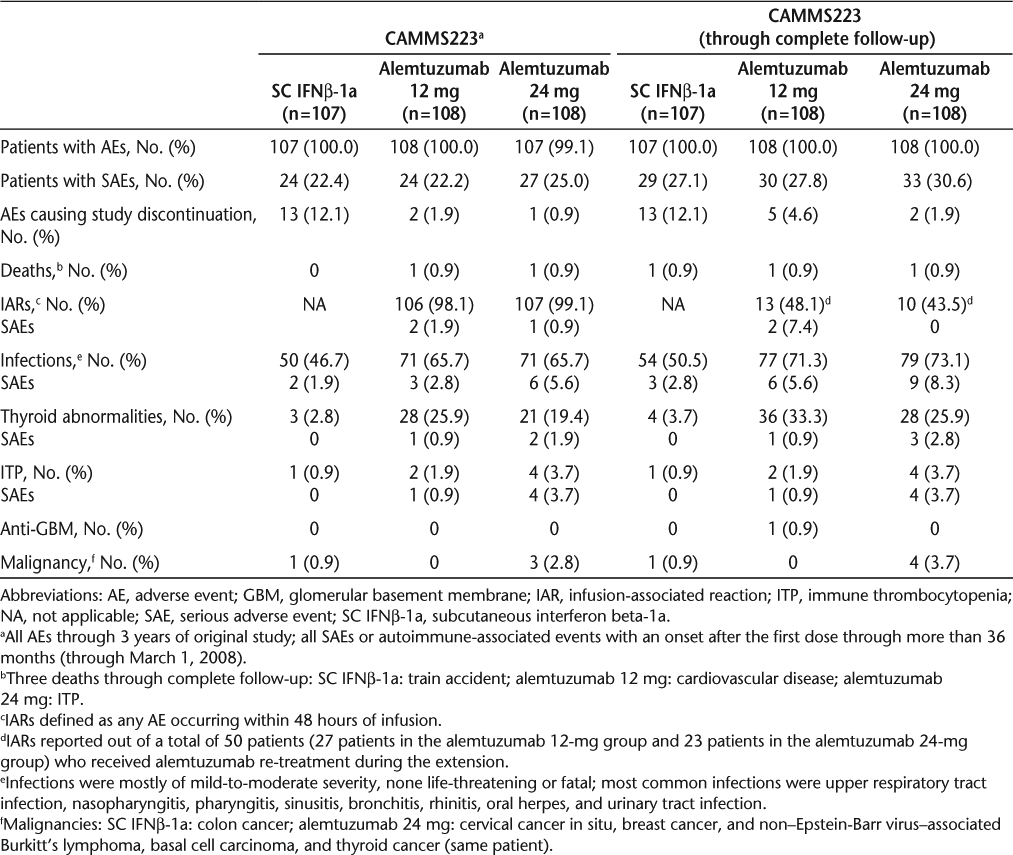
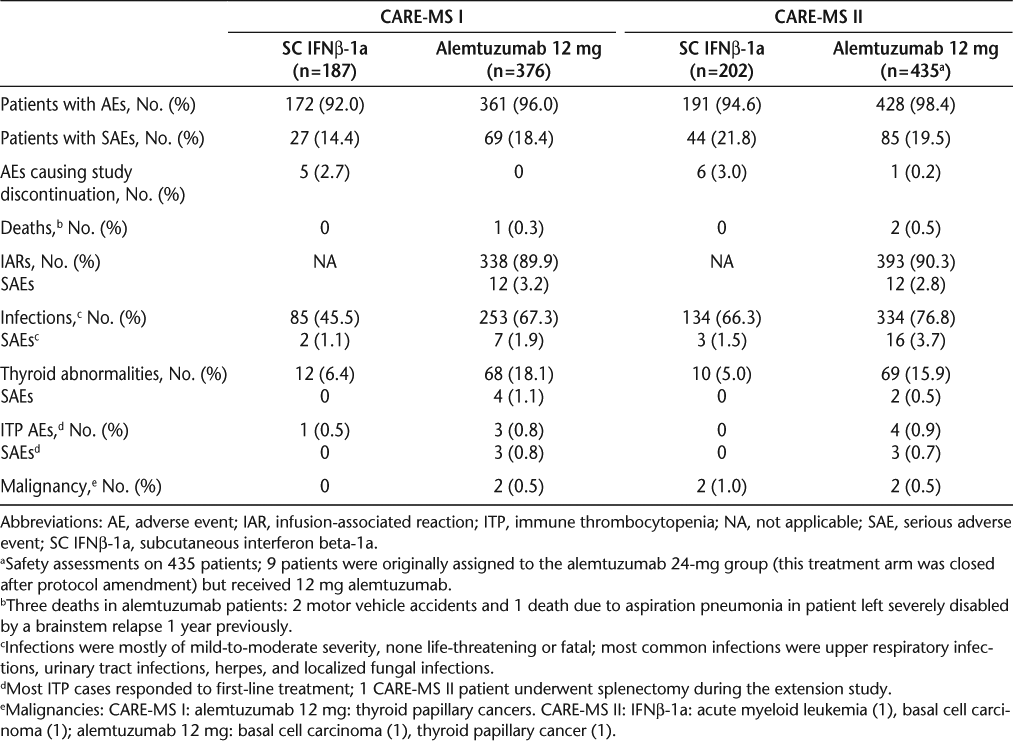
Safety and tolerability concerns were comparable in the three studies. Adverse events through 3 years and complete follow-up have been reported for CAMMS22315 20 (Table 3), and AEs through 2 years have been reported for CARE-MS I and II (Table 4).16 17 The most common AEs in the three studies were very similar (Table 5). The majority of alemtuzumab-treated patients experienced infusion-associated AEs. The specified methylprednisolone infusions on the first 3 days of alemtuzumab treatment reduced the MS-related symptoms due to cytokine release. Additional measures for managing the infusion-associated reactions have been developed independently of the study protocol but have not been formally evaluated.21
Adverse events for CAMMS223
Adverse events summary for CARE-MS I and CARE-MS II
Common adverse events (>15% of patients in at least one study)
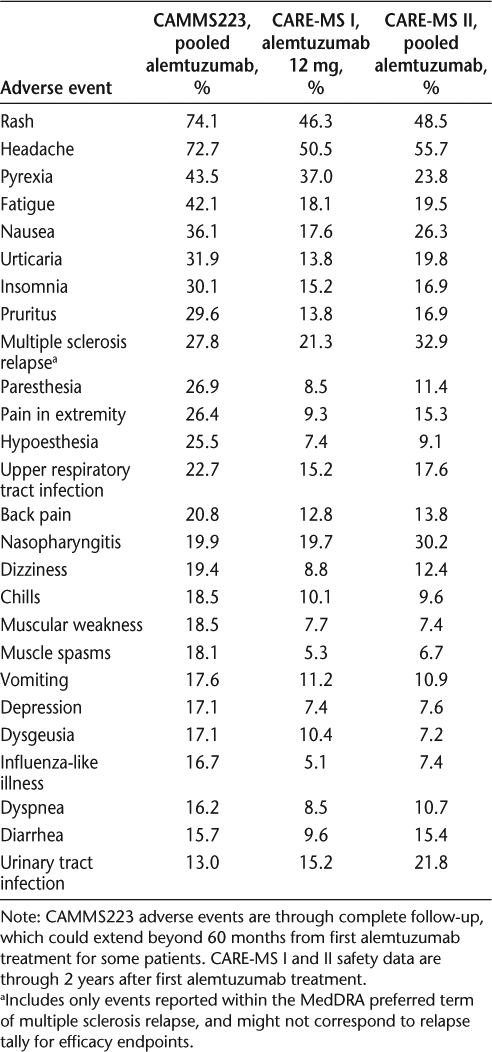
Other safety concerns with alemtuzumab were primarily infections and autoimmune AEs. The most frequently reported infections in the alemtuzumab groups were upper respiratory tract infections. Other commonly observed infections were lower respiratory tract infections, urinary tract infections, influenza, herpes simplex viral infections, vaginitis, and herpes zoster. The majority of infections were mild to moderate, with a rate of serious infections of 2% to 8% in the alemtuzumab arms (including CAMMS223 long-term follow-up) and 1% to 3% in the IFNβ-1a arms.15–17 20
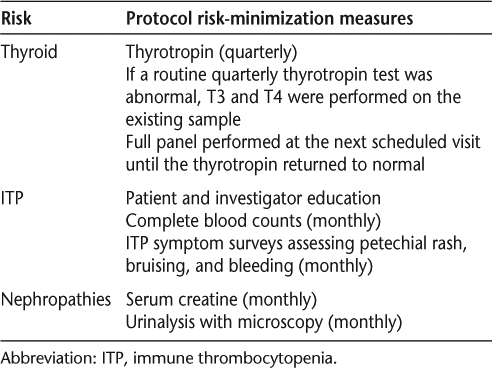
Autoimmune AEs were monitored in the extension phase of CAMMS223 and in the phase 3 studies as a result of AEs identified during CAMMS223 (Table 6). Surveillance and monitoring included thyrotropin, free T3, and free T4 for the detection of autoimmune thyroid abnormalities; and immune thrombocytopenia (ITP) through patient and investigator education, monthly complete blood counts, and monthly symptom surveys assessing petechial rash, bruising, and bleeding. Immune thrombocytopenia occurs when the immune system attacks the platelets, causing a decrease in the platelet count and increasing the risk of hemorrhage.
Surveillance and monitoring included in CAMMS223 Extension and CARE-MS I and II studies
Thyroid abnormalities were predominantly mild to moderate and largely managed with conventional therapies.15–17 20 Over 2 years in CARE-MS I and II, 18% and 16% of patients, respectively, experienced a thyroid abnormality (Table 4). Through long-term follow-up of CAMMS223 (up to 80.6 months), thyroid abnormalities, mainly hyperthyroidism, developed in a total of 30% of alemtuzumab patients and 4% of IFNβ-1a patients.20
In CAMMS223, six alemtuzumab-treated patients developed ITP. The first patient was diagnosed after the onset of a cerebral hemorrhage that proved fatal. Prodromal symptoms in this case went unrecognized. Of the five other alemtuzumab-treated patients who developed ITP, four required treatment and one did not. At 35 to 48 months after ITP diagnosis, all five surviving alemtuzumab-treated patients had normal platelet counts with no sequelae. Between 11 and 22 months after initiating alemtuzumab treatment, three patients in CARE-MS I developed ITP. In CARE-MS II, four cases of ITP occurred 3 to 24 months after receipt of alemtuzumab 12 mg and three cases developed in the exploratory alemtuzumab 24-mg arm. Most ITP cases responded to first-line treatment. One of the CARE-MS II patients underwent splenectomy during the ongoing extension study. Alemtuzumab-associated ITP appears to be limited in duration.22 Immune thrombocytopenia was not identified more than 16 months following the last alemtuzumab course in CAMMS223.22
During the extension phase of CAMMS223, one patient was identified by routine quarterly renal monitoring to have Goodpasture disease at month 51, approximately 39 months after the second alemtuzumab course.14 Goodpasture disease is a rare autoimmune disorder in which circulating antibodies are directed against an antigen present in the basement membrane of renal glomeruli and pulmonary alveoli.23 The effects may be severe and lead to rapidly progressive glomerulonephritis. Patients may develop pulmonary hemorrhage. Routine laboratory monitoring and educating the patient and health-care providers on the signs and symptoms of Goodpasture disease (gross hematuria, hemoptysis, edema, and nonspecific symptoms including malaise, fatigue, upper respiratory infection, and rash24) are essential for early detection and treatment, which has been shown to improve the outcome of the disease.25 The patient in CAMMS223 was identified early in the disease, had no pulmonary involvement, did not require a renal transplant, and was treated medically, and the disease remained in remission without clinical sequelae 17 months after diagnosis.14 Serum creatine and urinalysis were included as part of the risk monitoring in the phase 3 studies. During the 2-year study period for the phase 3 studies, no cases of Goodpasture disease were identified.16 During the ongoing extension, one alemtuzumab patient who received a third alemtuzumab treatment after the study developed glomerulonephritis and was treated medically. Six months after the original study period, 19 months after the last alemtuzumab course, one patient was successfully treated for presumed autoimmune pancytopenia. After hospital discharge, the patient failed to comply with prescribed corticosteroid therapy and developed complications resulting in death.16 Surveillance and monitoring as conducted for the CAMMS223 extension and CARE-MS studies is summarized in Table 6. Any monitoring required after FDA approval has not yet been determined.
Impact of New Therapies on MS Nursing Role
New therapies on the horizon for MS may bring hope for patients and new challenges for the MS nurse. Alemtuzumab presents an excellent example of a possible new treatment that will expand the MS nurse's role. Supporting the patient will continue to be one of the most important responsibilities of the MS nurse specialist; this task will become more complex and require the development of new skill sets, which will need to be incorporated into the complex and varied roles (educator, administrator, collaborator, consultant, researcher, advocate) currently played by the MS nurse. These new requirements include 1) understanding and interpreting the data from clinical trials, 2) expanding our knowledge of immunology so that we can understand each new drug's mechanism of action and how it affects the pathology of MS and its disease course, 3) developing a thorough understanding of how to administer these new therapies safely and effectively, 4) monitoring the patients who are receiving the medication for possible AEs, 5) counseling a patient who is considering switching treatment, and 6) developing and improving communication regarding potential side effects of a treatment. As MS nurses increase their knowledge base, the education process becomes easier and more effective. The role of educator will continue to be central to the MS nurse and of great importance to patients, their families, and health-care providers who do not specialize in the care of MS patients.
When new treatment options arise, patients and their families often turn to the MS nurse with questions. They ask whether the new therapy is an option for them. They wonder how the drug will affect their disease course. They want to know how the drug is administered, whether it is safe, and whether their insurance will cover it. As part of the treatment team, MS nurses will be called on to review and explain an increasing number of options, including efficacy and risks, administration procedures, and any needed monitoring. If a patient is advised by his or her physician that a switch in therapy is needed to help control the disease and the new therapy has the potential for greater risk, the MS nurse may have to counsel the patient on the risks versus benefits of the drug. These conversations can be challenging, as patients have differing levels of risk aversion and concern for efficacy, tolerability, and safety. Any cognitive impairment of the patient can further complicate decision-making.
MS nurse specialists are an important resource for others who treat patients with MS but do not specialize in the disease. As the treatment of MS becomes more patient-specific and complex, the education and guidance provided to these colleagues grows increasingly valuable. MS nurses will need to develop effective strategies for disseminating this information to nonspecialty nurses and providing ongoing support.
If approved by the FDA for the treatment of MS, alemtuzumab is likely to be administered as an intravenous infusion on a yearly basis via brief infusion courses that may involve the administration of other pretreatment medicines intended to improve tolerability. Alemtuzumab patients experienced high rates of infusion-associated reactions. Knowledge of the recommended practices and a versatile approach to management of the infusion process may limit the severity of these reactions and improve patient comfort and compliance. A solid understanding of both prophylactic and treatment protocols for infusion-associated events will be necessary. MS treatments that involve infusions may be administered at infusion centers that do not necessarily have personnel specifically trained in MS or MS treatments. The MS nurse will need to become an educator and a source of support for personnel at these sites in order for MS patients to receive the best possible care.
Nurses will need to be aware of other potential side effects of alemtuzumab. Infections and the development of other autoimmune diseases (thyroid abnormalities, ITP, and possibly nephropathies, including Goodpasture disease) are the main concerns. Monitoring for early detection of these potential side effects was begun during the phase 2 trial and was a part of the phase 3 studies. Such monitoring involves routine laboratory tests and patient education so that side effects can be identified early and managed before significant complications arise.
The search for biomarkers that may indicate a greater likelihood of developing autoimmune abnormalities after alemtuzumab treatment is ongoing. One study found that higher serum levels of IL-21 were associated with a greater risk of developing autoimmunity.26 Anti-thyroid peroxidase antibodies prior to treatment with alemtuzumab were also predictive of greater risk of developing autoimmune thyroid disorders, although the absence of antibodies was not protective.27 As more evidence for potential biomarkers is gathered, these data will be important for possible risk stratification in the future.
Ideally, clinicians and patients should be partners in the monitoring and identification of potential AEs following MS treatment. Thorough patient education will need to emphasize the importance of long-term compliance with risk-management procedures and teach patients to identify the early signs and symptoms that may indicate a potential AE. The infrequent administration schedule of alemtuzumab could present a challenge for MS nurses in terms of encouraging patients to maintain communication and routinely follow up.
Conclusion
Educating and counseling patients and their families, problem-solving, and providing emotional and practical support are major components of the MS nursing role. New therapies in development for the treatment of MS bring new hope and optimism to the MS community by offering potentially greater efficacy, improved tolerability, and acceptable safety. They also bring greater complexity to the decision-making process, treatment, and monitoring that is necessary. MS nursing will become more challenging as new therapies (with different mechanisms of action, side effects, and management strategies) enter the treatment arena. Many patients will be exploring these therapies on their own initiative or out of necessity owing to ongoing disease activity or an aggressive disease course. For whatever reason patients seek information about these new therapies, they will rely on their MS nurse for education and support.
In addition to offering support to patients and family members, the MS nurse will become an important resource for nurses who care for MS patients but are not specialists in the field. The knowledge and experience the MS nurse shares with nursing colleagues in the community will be critical to the safe and effective use of new therapies. Keeping abreast of the emerging therapies and developing the skills needed for their administration and patient care are essential. Should alemtuzumab receive FDA approval for the treatment of MS, nurses involved in the care of MS patients must be prepared to meet the challenges posed by this new therapeutic option.
PracticePoints
Alemtuzumab, a humanized monoclonal antibody, has shown efficacy for early, active, relapsing-remitting MS in phase 2 and 3 clinical trials; safety concerns include infusion-associated reactions, infections of predominantly mild-to-moderate severity, and autoimmune adverse events, principally thyroid disorders and immune thrombocytopenia.
As new MS treatments such as alemtuzumab emerge, the roles of the MS nurse—including educator, administrator, collaborator, consultant, researcher, and advocate—will continue to expand and become more challenging.
Patients with MS, their families, and health-care providers who do not specialize in the care of MS patients will increasingly rely on MS nurses to educate them about therapy options, including the scientific data and potential risks associated with each therapy.
Acknowledgments
We would like to thank the following Genzyme employees: Marco Rizzo for assistance with study operations and editing the manuscript; Laura Saltonstall, Jenna Hollenstein, Stephanie Jurgensen, and Colleen Miller for assistance with editing the manuscript; and Laurajae Rodriguez for assistance with manuscript submission.
References
Buttmann M. Treating multiple sclerosis with monoclonal antibodies: a 2010 update. Expert Rev Neurother. 2010; 10: 791–809.
National Multiple Sclerosis Society. About MS. http://www.nationalmssociety.org/index.aspx. Accessed February 17, 2011.
Smrtka J, Brodkey MB, Ben-Zacharia AB, et al. The Dynamic of the Multiple Sclerosis Nurse: Challenges, Expanding Role, and Future Directions. New York, NY: National Multiple Sclerosis Society; 2010.
Halper J. The evolving role of the nurse in the treatment of multiple sclerosis. J Neurosci Nurs. 2009; 41: E1–E13.
Ross AP, Thrower BW. Recent developments in the early diagnosis and management of multiple sclerosis. J Neurosci Nurs. 2010; 42: 342–353.
Xia MQ, Hale G, Lifely MR, Ferguson MAJ, Campbell D, Packman L. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993;293(pt 3):633–640.
Cox AL, Thompson SAJ, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005; 35: 3332–3342.
Thompson SAJ, Jones JL, Cox AL, Compston DAS, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab. J Clin Immunol. 2010; 30: 99–105.
Jones JL, Anderson JM, Phuah CL, et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain. 2010; 133: 2232–2247.
Moreau T, Thorpe J, Miller D, et al. Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet. 1994; 344: 298–301.
Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999; 46: 296–304.
Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006; 253: 98–108.
Fox EJ, Sullivan HC, Gazda SK, et al. A single-arm, open-label study of alemtuzumab in treatment-refractory patients with multiple sclerosis. Eur J Neurol. 2012; 19: 307–311.
Meyer D, Coles A, Oyuela P, et al. Case report of anti-glomerular basement membrane disease following alemtuzumab treatment. Mult Scler. 2010; 16:S138.
CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008; 359: 1786–1801.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012; 380: 1819–1828.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012; 380: 1829–1839.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983; 33: 1444–1452.
Coles A, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol. 2011; 10: 338–348.
Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology. 2012; 78: 1069–1078.
Mayer L. Alemtuzumab infusion in MS: nursing perspective on infusion-associated reactions. Paper presented at: 24th Annual Meeting of the Consortium of Multiple Sclerosis Centers; June 2–5, 2010; San Antonio, TX.
Fox E et al. Long-term follow-up of immune thrombocytopenia after treatment of multiple sclerosis patients with alemtuzumab in CAMMS223. Paper presented at: 62nd Annual Meeting of the American Academy of Neurology; April 10–17, 2010; Toronto, Ontario, Canada.
Levy JB, Pusey CD. Anti-GBM antibody mediated disease. In: Wilkinson R, Jamison RL, eds. Nephrology. London: Chapman & Hall; 1997:599–615.
Bolton WK. Goodpasture's syndrome. Kidney Int. 1996; 50: 1753–1766.
Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001; 134: 1033–1042.
Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. 2009; 119: 2052–2061.
Compston A et al. Autoantibody prediction of risk for thyroid adverse events after alemtuzumab treatment for relapsing multiple sclerosis. Paper presented at: 59th Annual Meeting of the American Academy of Neurology; April 28–May 5, 2007; Boston, MA.
Financial Disclosures: Ms. Caon has received honoraria for services from Teva Neuroscience, Bayer Healthcare, Novartis Pharmaceuticals, and Biogen Idec. Ms. Meyer has received honoraria for serving on speakers' bureaus for EMD Serono/Pfizer and Teva International Nurse Advisory Board, has served on a speakers' bureau for Genzyme (with no honoraria), and has received an honorarium for being a speaker at a 2011 regional meeting of the International Organization of Multiple Sclerosis Nurses (IOMSN). Ms. Mayer has received financial support from Biogen Idec, EMD Serono, Genzyme, Novartis Pharmaceuticals, Teva Neuroscience, and Acorda Therapeutics. Dr. Smith received funding from Genzyme as a contract medical writer through ReSearch Pharmaceutical Services, Inc, and contributed to the writing of this manuscript.
Funding/Support: Genzyme and Bayer Schering Pharma provided financial support for CAMMS223, CAMMS323, and CAMMS324. Genzyme provided financial support for the writing of this manuscript.







