Publication
Research Article
International Journal of MS Care
The TRUST (EvaluaTion of Bladder Function in Relapsing-Remitting MUltiple Sclerosis Patients Treated with Natalizumab) Observational Study
Author(s):
Background: Bladder dysfunction is a common symptom of multiple sclerosis (MS). This study was designed to evaluate effects of natalizumab on bladder function in patients with relapsing-remitting MS.
Methods: The TRUST (EvaluaTion of Bladder Function in Relapsing-Remitting MUltiple Sclerosis Patients Treated with Natalizumab) study was an open-label, single-arm, two-center study. Natalizumab-naive MS patients with disabling bladder dysfunction and initiating natalizumab were enrolled and followed for 6 months. The primary endpoint was change in the Urogenital Distress Inventory short form (UDI-6) score from baseline. Change in Incontinence Impact Questionnaire short form (IIQ-7) score from baseline was a secondary endpoint.
Results: Thirty patients were enrolled. Mean baseline characteristics were age 49.9 years, Expanded Disability Status Scale score 4.6, number of relapses in previous year 2.4, UDI-6 score 10.4, and IIQ-7 score 12.3. Mean changes in UDI-6 and IIQ-7 scores were significantly improved from baseline beginning at week 4 and up to week 24; mean improvements at 24 weeks were 4.4 (P < .0001) and 4.9 (P = .0005) points, respectively. At week 24, 85.7% and 78.6% of patients demonstrated improvements from baseline in UDI-6 and IIQ-7 scores, respectively.
Conclusions: Incontinence-related quality of life as measured by UDI-6 and IIQ-7 scores improved significantly during natalizumab treatment.
Multiple sclerosis (MS) is a progressive, degenerative disease of the central nervous system (CNS) characterized by a variety of clinical courses.1 2 The majority of MS patients present with episodic neurologic symptoms and will have bouts of disease activity (relapses) separated by periods of total or partial remission.1 2
Patients with MS may experience symptoms such as bladder dysfunction, fatigue, spasticity, pain, and depression.1 Bladder dysfunction is common in MS patients3–5 and may increase with increasing spinal cord involvement.4 6–9 The types of bladder dysfunction that typically occur in MS are failure to store, failure to empty, and a combination of both.10 11
The Urogenital Distress Inventory (UDI) and the Incontinence Impact Questionnaire (IIQ) were both developed to assess the impact of urinary incontinence on quality of life (QOL) in women.12 Shorter versions of the UDI and the IIQ, the UDI-6 and the IIQ-7, have become standard, validated measures for urologic investigations.13 14 The North American Research Committee on Multiple Sclerosis (NARCOMS) validated the bladder/bowel subscale (PSB) of its patient-reported Performance Scale (PS).15 16
In the phase 3 pivotal AFFIRM and SENTINEL studies, natalizumab (Tysabri; Biogen Idec Inc, Cambridge, MA, and Elan Pharmaceuticals, Inc, South San Francisco, CA) reduced MS relapse rates, slowed progression of MS-related disability, and improved QOL in patients with relapsing forms of MS.17–19 This observation, coupled with anecdotal reports, led us to hypothesize that natalizumab may have a demonstrable beneficial effect on bladder function in MS patients. The primary study endpoint in the TRUST (EvaluaTion of Bladder Function in Relapsing-Remitting MUltiple Sclerosis Patients Treated with Natalizumab) study was change in bladder function, as measured by the UDI-6 score from baseline through 24 weeks of natalizumab treatment. Secondary study endpoints included change from baseline over 24 weeks in the following parameters: IIQ-7 score, NARCOMS PSB score, the number of urinary incontinence episodes per patient per week, and the number of micturitions per patient per day.
Materials and Methods
Study Design
The TRUST study was an open-label, two-center (Center for Neurological Disorders and the Regional Multiple Sclerosis Center, Aurora St. Luke’s Medical Center, Milwaukee, WI, and Rocky Mountain Multiple Sclerosis Center, Salt Lake City, UT), single-arm, 24-week proof-of-concept study to evaluate potential effects of natalizumab on bladder function in patients with relapsing forms of MS. Patients were treated with natalizumab 300 mg by intravenous infusion every 4 weeks for 6 months. Enrollment began in March 2009, and the final patient visit was in April 2011.
The study was conducted in agreement with the International Conference on Harmonisation (ICH) and Good Clinical Practice (GCP) guidelines and followed Declaration of Helsinki recommendations. The Aurora Internal Review Board approved the study. All patients provided written informed consent. The trial was registered with www.clinicaltrials.gov (NCT00818038).
Patients
Natalizumab-naive patients with a relapsing form of MS and bladder dysfunction who were aged 18 years or older were enrolled into the study. Urinary incontinence in enrolled patients was defined as at least 3 incontinence episodes per week or at least 8 micturitions per day (both mean numbers). Patients were required to have a UDI-6 score of 6 or greater and an Expanded Disability Status Scale (EDSS) score of 0.0 to 6.5 at the screening visit.
Patients who were taking medications for the control of bladder symptoms or that could affect urinary output were required to maintain stable dosing of that medication for at least 1 month before the study and also during the study.
All patients were requested to maintain current hydration and caffeine intake during the study. Patients were excluded from the study if they had a history of recurrent or chronic urinary tract infection or a urinary tract infection within 30 days of initiating natalizumab treatment, used an indwelling Foley catheter or suprapubic catheter, or had a history of symptomatic benign prostatic hyperplasia or prostate cancer. In addition, patients were required to meet all prescribing criteria for natalizumab20 and be enrolled in the TOUCH® (TYSABRI® Outreach: Unified Commitment to Health) Prescribing Program (http://www.tysabri.com/tysbProject/tysb.portal/_baseurl/threeColLayout/SCSRepository/en_US/tysb/home/treatment-with-tysabri/touch-prescribing-program.xml).
Endpoints and Assessments
The primary study endpoint was change in bladder function, as measured by the UDI-6 score (range 0–18),14 from baseline through 24 weeks of natalizumab treatment. The UDI-6 is composed of six questions on irritative symptoms, obstruction/discomfort, and stress symptoms. Respondents rate each question on a 4-step ordered category scale from “not at all” (score = 0) to “greatly” (score = 3) based on how much they experienced impaired function or were bothered by a symptom. Lower scores indicate better bladder function.14
Secondary study endpoints included change from baseline over 24 weeks in the following parameters: IIQ-7 score (range 0–21),14 NARCOMS PSB score (range 0–5),15 the number of urinary incontinence episodes per patient per week, and the number of micturitions per patient per day. The IIQ-7 is composed of seven questions that measure the impact of bladder symptoms on physical activity, travel, social activities, and emotional health. Scoring is the same as for the UDI-6, and a lower score indicates higher bladder-related QOL.14 The NARCOMS PSB measures disability secondary to bowel and bladder symptoms, presumably due to MS, and is scored from 0 (no) to 5 (total disability).15 No safety analyses were conducted as part of this trial.
During the study, UDI-6, IIQ-7, NARCOMS PSB, and concomitant medication for urinary incontinence were recorded at baseline (week 0) and then every 4 weeks before each natalizumab infusion for 24 weeks. Bladder diary entries documenting incontinence episodes per week and micturitions per day were completed by the patient every 4 weeks from week 2 through week 22. The bladder diary was collected by the investigative site at each clinic visit. In the event that a natalizumab infusion was administered more than 4 weeks from the last dose, subsequent diaries were completed every 14 ± 4 days from the last patient visit.
Statistical Analysis
This exploratory study was planned to enroll approximately 30 patients from two US centers. Because no prior data are available on the effect of natalizumab on bladder function, the sample size was exploratory and not based on statistical power considerations.
The efficacy analyses were performed on all patients completing the study, and were based only on observations obtained at each assessment (ie, without imputation of missing data). Sensitivity analyses that included data from all enrolled patients were also performed. All bladder efficacy data were summarized by presenting frequency distributions and/or summary statistics (mean, standard deviation, median, and range). All analyses were conducted using a two-sided test and a significance of .05. For continuous endpoints, a paired t test was initially used to compare the change of these endpoints from baseline over 6 months of natalizumab treatment. Categorical data were analyzed using χ2, Fisher exact, and/or McNemar tests. Nonparametric analyses (eg, Wilcoxon signed rank test) were utilized as appropriate. Analyses were conducted to assess the influence of baseline characteristics (eg, age, gender, and baseline bladder functions) on the endpoints, including an analysis of variance model adjusted for covariates (ANCOVA) where appropriate.
Time effect was analyzed using a longitudinal data analysis model adjusted for baseline UDI-6 or IIQ-7 score, age, EDSS score, total number of relapses within the past 12 months and the past 3 years, disease duration, and use of medications for bladder problems. Correlations between baseline disease characteristics (age, EDSS score, total number of relapses within the past 12 months and the past 3 years, disease duration) and UDI-6 and IIQ-7 scores were assessed. Extrapolating from previous research (Corcos et al., 2002),21 the following three assumptions regarding the relationship between IIQ-7 raw score and urinary incontinence health-related QOL (UI HRQOL) were made: 1) An IIQ-7 score less than 10.5 indicates good UI HRQOL; 2) an IIQ-7 score of 10.5 to 14.7 indicates moderate UI HRQOL; and 3) an IIQ-7 score greater than 14.7 indicates poor UI HRQOL.
Results
Patients
A total of 30 patients were enrolled, and 28 patients completed the study. One patient voluntarily discontinued natalizumab prior to the first infusion (data were available for baseline and week 4 and were included in the sensitivity analysis), and another patient withdrew consent and later received three infusions (no postbaseline data were available and thus were not included in sensitivity analysis).
Baseline demographics and clinical characteristics of the 28 patients who completed the study are shown in Table 1. Seventeen patients were taking medication for bladder symptoms at study entry and continued their baseline dosage throughout the study. Bladder medications used by study participants included darifenacin, desmopressin, doxazosin, oxybutynin, solifenacin, tamsulosin, and trospium.
Baseline characteristics of patients in TRUST
There were no meaningful correlations between bladder function, as assessed by UDI-6 and IIQ-7 scores, and baseline relapse history.
Urogenital Distress Inventory Questionnaire (UDI-6)
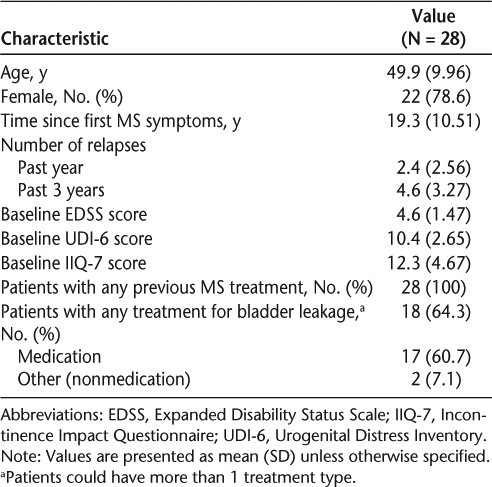
Beginning at week 4, mean UDI-6 score was significantly lower than at baseline (P < .0001), with a mean improvement at 24 weeks of 4.4 points (Figure 1A). At week 24, 85.7% of patients demonstrated an improvement from baseline in the UDI-6 score, while scores had worsened in 10.7% and were stable in 3.6% of patients (P < .0001) (Figure 2A). Patients on natalizumab with a worse (higher) UDI-6 score at baseline showed a greater initial improvement than patients with a better UDI-6 score at baseline, as indicated by a significant coefficient for baseline UDI-6 score (−0.3666, P = .0192) in the regression model. This improvement was maintained over time (data not shown).
Mean improvement from baseline to week 24 in (A) total UDI-6 score and (B) total IIQ-7 score
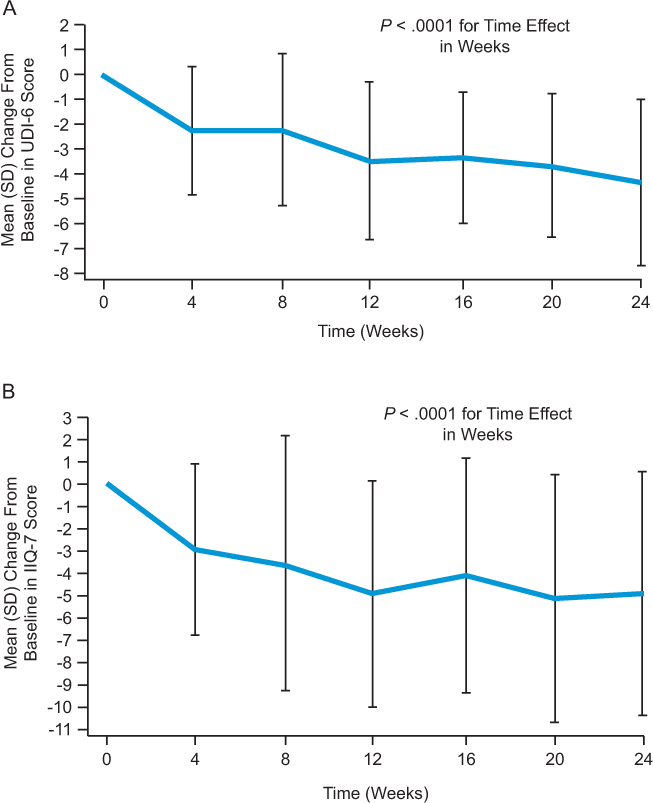
Change from baseline to week 24 in the proportion of patients with worsened, stable, or improved (A) UDI-6 score and (B) IIQ-7 score
The changes in UDI-6 score from baseline were also analyzed using a more rigorous definition as follows: improvement: score decreased by at least 3 points; stable: score unchanged or decreased by less than 3 points; worsened: score increased from baseline (>0 point). With the use of these criteria, there were significant differences in the proportions of improved, stable, and worsened patients at 12, 16, 20, and 24 weeks. At week 12, 56%, 33%, and 11% of patients showed score improvement, stability, and worsening, respectively (P < .01). These proportions remained relatively stable for the remainder of the study; at week 24, 64%, 25%, and 11% of patients showed score improvement, stability, and worsening, respectively (P < .01).
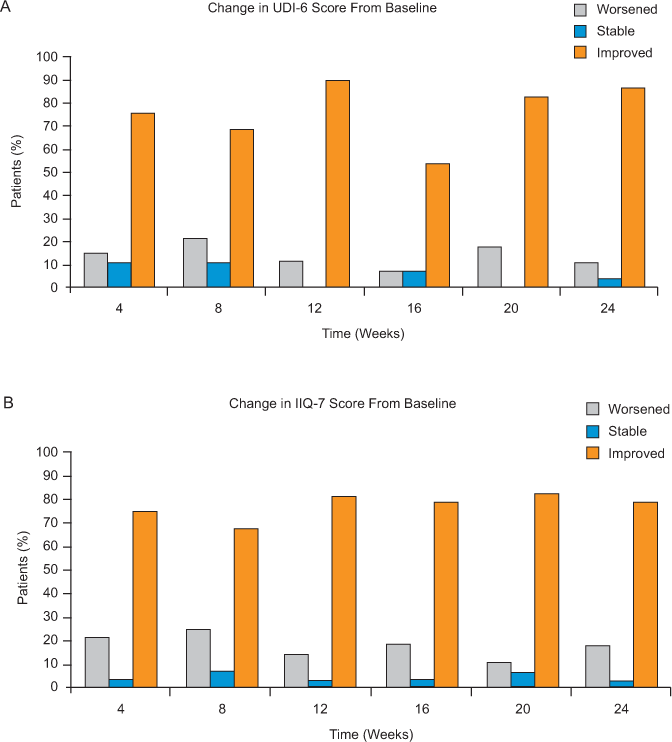
The improvement in UDI-6 score suggested an overall reduced impact of urinary symptoms. For each UDI-6 question, the combined percentage of patients who reported being bothered only “slightly” or “not at all” by symptoms increased between baseline and week 24 (Figure 3A). Additional analyses adjusting for the time since the most recent relapse (≤3 months versus >3 months) or use of medications for bladder symptoms showed no significant impact on natalizumab’s effect on UDI-6 score (data not shown).
Scores at baseline and week 24 for each (A) UDI-6 and (B) IIQ-7 question
Incontinence Impact Questionnaire (IIQ-7)
Mean IIQ-7 score was significantly lower than at baseline from week 4 onward (P = .0001), with a mean improvement at 24 weeks of 4.9 points (Figure 1B). Compared with baseline, 78.6% of patients demonstrated an improvement in the IIQ-7 score at week 24. Conversely, IIQ-7 score worsened in 17.9% and was stable in 3.6% of patients (P = .0011) (Figure 2B). With regard to each individual IIQ-7 question, the combined percentage of patients reporting that urine leakage affected them only “slightly” or “not at all” consistently increased during the study (Figure 3B).
Participants with a worse (higher) IIQ-7 score at baseline had a more pronounced initial improvement than patients with a better IIQ-7 score at baseline. This was illustrated by a significant coefficient for baseline IIQ-7 score (−0.5783, P < .0001) from the regression model that was maintained over the study period.
NARCOMS Bowel/Bladder Performance Subscale (NARCOMS PSB)
On the NARCOMS PSB, the proportion of patients who reported moderate to severe bladder/bowel disability decreased over time: 85.7%, 50.0%, 39.3%, and 35.7% at weeks 0, 8, 16, and 24, respectively. There were corresponding increases in the proportions of patients reporting normal, minimal, or mild bladder/bowel disability during the course of the study.
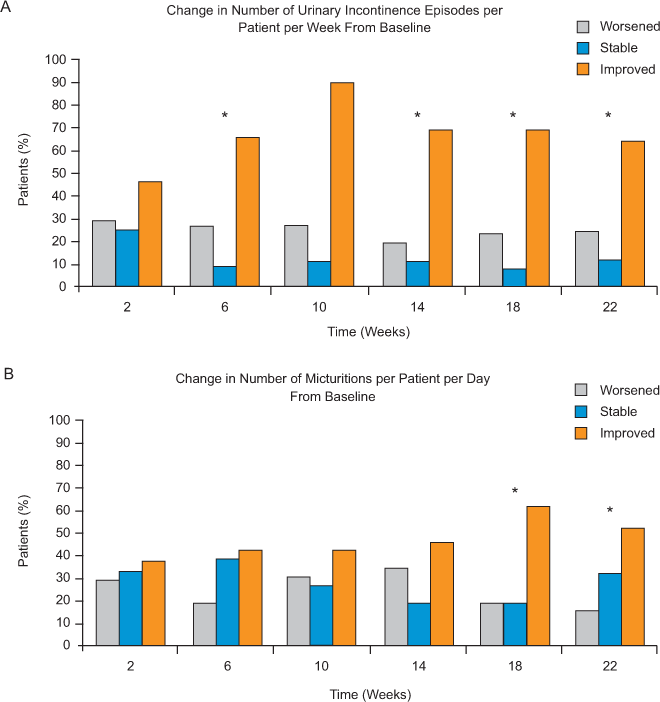
Urinary Incontinence Episodes and Micturitions (Diary Entry Data)
The majority of patients showed improvement or stability in incontinence episodes and micturitions starting at week 2 (Figure 4). At week 22, 64% of patients reported a decrease (improvement) from baseline in the number of weekly incontinence episodes reported, while incontinence episodes increased in 24% (three patients reported increased weekly incontinence episodes across all time points) and were stable in 12% of patients (P = .033) (Figure 4A). Similarly, 52% of patients reported a decrease (improvement) in the number of daily micturitions, while micturitions increased in 16% and were stable in 32% of patients at 22 weeks (P = .029) (Figure 4B). Diary data were not available for all patients at all time points. Sensitivity analyses that included data from the one patient who voluntarily discontinued natalizumab showed similar results.
Change from baseline to week 22 in the proportion of patients with worsened, stable, or improved (A) number of urinary incontinence episodes per patient per week, and (B) number of micturitions per patient per day
Discussion
Bladder dysfunction is a common and distressing symptom of MS that negatively affects QOL.22 Improvements in QOL have been shown in MS patients treated with natalizumab,19 but the effects of natalizumab on bladder function and bladder function–related QOL have not been studied to date.
In this study, we demonstrated that patients with a relapsing form of MS who were treated with natalizumab experienced significant improvement in incontinence-related QOL as measured by mean improvements in UDI-6 and IIQ-7 scores. Patients with worse (higher) UDI-6 or IIQ-7 scores as well as more weekly incontinence episodes at baseline also showed greater initial improvement in scores and maintained improvement over time while receiving natalizumab.
On the basis of other studies,12 14 16 21 23 the magnitude of the treatment effect seen in this pilot study suggests that natalizumab may be capable of decreasing the impact of urinary incontinence on QOL from moderate to mild. The QOL improvements may have been driven in part by the finding that the majority of patients had improvements in the number of incontinence episodes per week and the number of micturitions per day.
Several guidelines are available for the diagnosis and management of bladder symptoms in patients with MS.24–27 These guidelines suggest that when appropriate, pharmacologic adjunctive treatment for neurogenic bladder in patients with MS can be considered. Although somewhat effective, these approaches can increase the risk of additional side effects, add to the cost of treating MS, and do not resolve symptoms for all patients. Use of a disease-modifying therapy that has been shown to reduce bladder symptoms while having a positive impact on disease progression, relapses, magnetic resonance imaging (MRI) lesions, and other symptoms may be preferable; however, benefits should always be balanced with potential risks when considering a disease-modifying therapy.28 Adjunctive therapies and nonpharmacologic approaches could then be considered for patients with refractory bladder symptoms. However, data on the impact of approved disease-modifying therapies on bladder function are lacking. To our knowledge, our study is the first prospective clinical trial to demonstrate improvement in bladder symptoms during treatment with an approved disease-modifying therapy.
The study is limited by the small sample size and lack of a comparator arm. While it does suggest significant benefits of natalizumab on bladder function, larger, controlled studies would be required to confirm these preliminary findings. Also, the UDI-6 and IIQ-7 scales used in this study have been validated for women,14 although use of these measures with male patients has been described.29 30
In conclusion, patients treated with natalizumab experience significantly improved incontinence-related QOL as measured by UDI-6 and IIQ-7 scores. The magnitude of this effect suggests that natalizumab may decrease the impact of incontinence on QOL. Further studies evaluating the impact of natalizumab on urologic QOL are needed to support these analyses.
PracticePoints
Bladder dysfunction is a common and distressing symptom of MS.
Changes in incontinence-related quality of life (QOL) during natalizumab treatment were evaluated using Urogenital Distress Inventory short form (UDI-6) and Incontinence Impact Questionnaire short form (IIQ-7) scores.
UDI-6 and IIQ-7 scores were significantly improved in patients with MS following natalizumab treatment.
The majority of patients showed improvement or stability in number of incontinence episodes per week and in number of micturitions per day after starting natalizumab.
Natalizumab may reduce the impact of incontinence on QOL.
Acknowledgments
Medical writing assistance was provided by Ryan Woodrow, BS, and Britt Anderson, PhD, and editorial support was provided by Jackie Cannon of Infusion Communications. Their work was funded by Biogen Idec Inc and Elan Pharmaceuticals, Inc.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372: 1502–1517.
Lublin FD, Reingold SC, National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis. Neurology. 1996; 46: 907–911.
Goldstein I, Siroky MB, Sax DS, Krane RJ. Neurourologic abnormalities in multiple sclerosis. J Urol. 1982; 128: 541–545.
Hawker KS, Frohman EM. Bladder, bowel, and sexual dysfunction in multiple sclerosis. Curr Treat Options Neurol. 2001; 3: 207–214.
Litwiller SE, Frohman EM, Zimmern PE. Multiple sclerosis and the urologist. J Urol. 1999; 161: 743–757.
Betts CD, D'Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993; 56: 245–250.
Koldewijn EL, Hommes OR, Lemmens WA, Debruyne FM, van Kerrebroeck PE. Relationship between lower urinary tract abnormalities and disease-related parameters in multiple sclerosis. J Urol. 1995; 154: 169–173.
Awad SA, Gajewski JB, Sogbein SK, Murray TJ, Field CA. Relationship between neurological and urological status in patients with multiple sclerosis. J Urol. 1984; 132: 499–502.
Ukkonen M, Elovaara I, Dastidar P, Tammela TL. Urodynamic findings in primary progressive multiple sclerosis are associated with increased volumes of plaques and atrophy in the central nervous system. Acta Neurol Scand. 2004; 109: 100–105.
Andrews KL, Husmann DA. Bladder dysfunction and management in multiple sclerosis. Mayo Clin Proc. 1997; 72: 1176–1183.
Fowler CJ, van Kerrebroeck PE, Nordenbo A, Van PH; Committee of the European Study Group of SUDIMS (Sexual and Urological Disorders in Multiple Sclerosis). Treatment of lower urinary tract dysfunction in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992; 55: 986–989.
Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA; Continence Program in Women (CPW) Research Group. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual Life Res. 1994; 3: 291–306.
Lemack GE, Zimmern PE. Predictability of urodynamic findings based on the Urogenital Distress Inventory-6 questionnaire. Urology. 1999; 54: 461–466.
Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA; Continence Program for Women Research Group. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995; 14: 131–139.
Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Disparities in the management of multiple sclerosis-related bladder symptoms. Neurology. 2007; 68: 1971–1978.
Schwartz CE, Vollmer T, Lee H; North American Research Consortium on Multiple Sclerosis Outcomes Study Group. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999; 52: 63–70.
Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006; 354: 899–910.
Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006; 354: 911–923.
Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007; 62: 335–346.
Tysabri (natalizumab) [prescribing information]. Cambridge, MA: Biogen Idec, Inc; 2012.
Corcos J, Behlouli H, Beaulieu S. Identifying cut-off scores with neural networks for interpretation of the incontinence impact questionnaire. Neurourol Urodyn. 2002; 21: 198–203.
Hemmett L, Holmes J, Barnes M, Russell N. What drives quality of life in multiple sclerosis? QJM. 2004; 97: 671–676.
Ross S, Soroka D, Karahalios A, Glazener CM, Hay-Smith EJ, Drutz HP. Incontinence-specific quality of life measures used in trials of treatments for female urinary incontinence: a systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17: 272–285.
Multiple Sclerosis Council for Clinical Practice Guidelines. Urinary Dysfunction and Multiple Sclerosis: Evidence-Based Strategies for Urinary Dysfunction in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1999. http://www.pva.org/site/c.ajIRK9NJLcJ2E/b.8907639/k.298E/PDFs_Multiple_Sclerosis_MS_Publications.htm. Accessed February 4, 2014.
de Seze M, Ruffion A, Denys P, Joseph PA, Perrouin-Verbe B. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007; 13: 915–928.
Fowler CJ, Panicker JN, Drake M, et al. A UK consensus on the management of the bladder in multiple sclerosis. Postgrad Med J. 2009; 85: 552–559.
Ghezzi A, Carone R, Del Popolo G, et al. Recommendations for the management of urinary disorders in multiple sclerosis: a consensus of the Italian Multiple Sclerosis Study Group. Neurol Sci. 2011; 32: 1223–1231.
Sorensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012; 18: 143–152.
Naughton MJ, Donovan J, Badia X, et al. Symptom severity and QOL scales for urinary incontinence. Gastroenterology. 2004;126(1 suppl 1):S114–S123.
Rapp DE, Lucioni A, Katz EE, O'Connor RC, Gerber GS, Bales GT. Use of botulinum-A toxin for the treatment of refractory overactive bladder symptoms: an initial experience. Urology. 2004; 63: 1071–1075.
Financial Disclosures: Dr. Khatri has been a consultant for Bayer, Biogen Idec, Cardian, Medtronic, Pfizer, and Serono. Dr. Foley has been a consultant for Biogen Idec, Genzyme, and Teva and has received honoraria from Biogen Idec and Teva. Mr. Kramer has been a consultant for Bayer, Biogen Idec, Novartis, Pfizer, Serono, and Teva. Dr. Cha is an employee of Elan Pharmaceuticals. Drs. You and Foulds are employees of Biogen Idec. Dr. Warth was an employee of Biogen Idec at the time of the study and is currently an employee of Genzyme Corporation. Ms. Fink has no conflicts of interest to disclose.
Funding/Support: This study was funded by Biogen Idec Inc and Elan Pharmaceuticals, Inc.







