Publication
Research Article
International Journal of MS Care
Nurses’ Perspective on Approaches to Limit Flu-Like Symptoms During Interferon Therapy for Multiple Sclerosis
Author(s):
Background: Several interferon beta (IFNβ) formulations are approved for first-line use as disease-modifying therapies to treat patients with multiple sclerosis (MS). Systemic post-injection reactions, often termed flu-like symptoms (FLS), occur in approximately half of all patients treated with IFNβs and can affect adherence to therapy. These symptoms, which include pyrexia, chills, malaise, myalgia, and headaches, usually resolve within 24 hours or persist intermittently following each injection. Because FLS, which usually occur early in the treatment course and diminish over time, are a primary cause of nonadherence to IFNβ therapy, it is important to employ strategies that can attenuate these side effects.
Methods: To identify interventions effective in limiting FLS, a panel of United States–based nurses with expertise in MS patient care was convened and a literature review completed.
Results: Panel consensus was reached on specific interventions that can attenuate FLS. These prevention and mitigation strategies include dose titration, analgesia, and optimal injection timing, as well as other techniques that panel members have found useful in their clinical practice experience.
Conclusions: These measures, in addition to effective patient education, will help to reduce the incidence of FLS secondary to IFNβ therapy, improve patient medication adherence, and positively affect long-term clinical outcomes.
Multiple sclerosis (MS) is a chronic, progressive, neurodegenerative disease characterized by demyelination and the subsequent loss of both gray and white matter in the central nervous system.1 It is currently estimated that 2.5 million people worldwide, including 400,000 people in the United States, have MS. In the United States alone, approximately 200 people receive a diagnosis of MS each week.2 Treatment for relapsing-remitting MS is indicated to slow MS disease progression, minimize axonal damage, reduce the frequency and severity of relapses, delay the accumulation of irreversible neurologic damage, and lessen the disability associated with the disease. Moreover, in long-term studies of individuals diagnosed with a clinically isolated syndrome, which often precedes relapsing MS, the benefits of early intervention were sustained, whereas delayed treatment was not associated with comparable therapeutic benefits.3 4 In general, early therapeutic intervention is associated with better long-term clinical outcomes in MS.5
Several therapeutic options are currently available to treat MS. Interferon beta is used in a number of different formulations as first-line MS therapy, based on its efficacy in slowing disease progression and in reducing the number and frequency of MS relapses.6 7 Interferons are members of a large class of glycoproteins, known as cytokines, that broadly activate immune cells and are involved in the upregulation of antigen presentation to T lymphocytes.8 9 However, these medications may have downstream effects that include aching muscles, fever, and a host of other systemic post-injection reactions, often referred to as flu-like symptoms (FLS).10 11
Adherence to therapy is increasingly recognized as critical to improving long-term outcomes in MS patients, yet long-term adherence rates for MS treatment rarely exceed 75%.12 While convenience-related factors, such as dosing frequency, can affect adherence,13 tolerability issues also represent an important potential barrier to adherence. Thus, effective management of side effects is essential to maintain treatment and prevent disease progression.14 15
Patients frequently report FLS as a concern, and untreated FLS negatively affect adherence to IFNβ therapy. However, such symptoms can often be effectively managed with minor lifestyle and medication modifications. Flu-like symptoms usually resolve within 24 hours after injection or persist intermittently following each injection; they typically diminish during the first few months of treatment and may cease completely over time.16 17 Clinicians working with the MS population need to be proactive in assessing the tolerability of treatment and providing education and strategies for managing treatment-related symptoms while setting realistic expectations. The goal of the panel was to generate a consensus statement about FLS mitigation, primarily aimed at nursing staff working with MS patients.
Methods
A panel of nine United States–based expert MS nurses was convened and a literature review completed with the goal of generating a set of practice guidelines for nurses and other caregivers of MS patients on managing FLS secondary to IFNβ therapy. Five FLS—pyrexia, chills, malaise, myalgia, and headache—were examined. A number of possible interventions derived from clinical trials (Class 1 evidence), clinical reports (Class 2 evidence), or the experts’ own 100-plus cumulative years of clinical experience with MS patients (anecdotal evidence) were discussed. Consensus regarding each of the proposed interventions was obtained, and treatment recommendations were made to generate a best practice model for addressing the most common FLS reported by patients treated with IFNβ.
Results
The panel recommended combining early patient counseling regarding the possibility of developing FLS with specific interventions effective in limiting these injection-related reactions. Seven interventions were unanimously recommended by the panel, as described below and summarized in Table 1.
Interventions for limiting the incidence of FLS
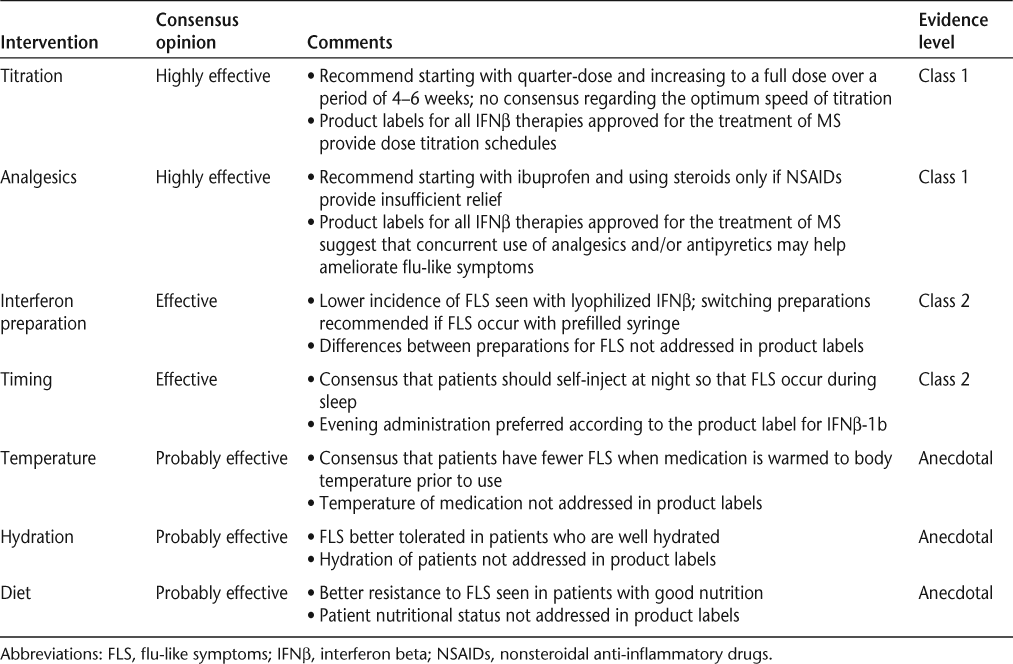
Titration
The panel agreed that the most effective and best-supported strategy to reduce FLS is dose titration during the initiation of IFNβ therapy.10 18 19 Dose-titration schedules are included in the prescribing information for IFNβ therapies currently approved for the treatment of MS20–23 and are supported by the results of several studies.16 19 24 A study of 98 patients randomized to either placebo or two different titration schedules of IFNβ-1b found that slower titration (reaching full dose on day 31) was associated with FLS in 32.9% of patients, while rapid titration (reaching full dose on day 15) was associated with FLS in 41.9% of patients.24 In an open-label pilot study of 47 patients, the combination of analgesics and quarter- or half-dose titration of intramuscular (IM) IFNβ-1a significantly reduced FLS during the first 2 weeks of therapy compared with analgesics alone.16 These findings were confirmed and extended by a larger, randomized, dose-blinded study assessing the frequency and severity of FLS in healthy volunteers using self-injecting IM IFNβ-1a with no titration or with one of two titration schedules.19 One group increased its dosage by a quarter-dose each week for 3 weeks, reaching full dose at week 4; the other group underwent a longer 6-week titration, starting at a quarter-dose with quarter-dose increases every 2 weeks, reaching full dose after week 6. Compared with no titration, the incidence of FLS at the 4- to 6-hour time point and at the 12- to 15-hour time point was significantly reduced with both 3-week titration (P < .001 at 4–6 hours; P = .006 at 12–15 hours) and 6-week titration (P = .023 at 4–6 hours; P = .027 at 12–15 hours). The severity of FLS was reduced by 76% at 4–6 hours (P < .001) and by 37% at 12–15 hours (P < .001) with 3-week titration, while symptom severity was reduced by 50% at 4–6 hours (P < .001) and by 32% at 12–15 hours (P = .002) with 6-week titration, compared with no titration.19 Thus, there is compelling evidence in both healthy volunteer and MS patient populations that the incidence and severity of FLS can be reduced by carefully titrating IFNβ dosage as patients start therapy.
The panel agreed that dose titration is critical to minimize FLS incidence when initiating any IFNβ therapy. Many participants on the panel customized the titration rate based on the patient’s tolerance and any reports of FLS during initiation of therapy.
Analgesic Management of FLS
For patients who experience ongoing FLS, there is good evidence that analgesics are effective in limiting symptom occurrence and severity.16 25 26 Concurrent use of analgesics and/or antipyretics to help ameliorate IFNβ-associated FLS is mentioned in the product labels for all IFNβ therapies currently approved for the treatment of MS.20–23 In clinical practice, clinicians recommend a variety of treatment protocols to prevent and mitigate FLS, including ibuprofen, naproxen, and oral steroids, as well as acetaminophen.
The panel reviewed three studies25 27 28 that attempted to determine the most effective analgesic for FLS management. When the efficacies of acetaminophen, ibuprofen, and the steroid prednisone were compared, no significant difference was found between the treatment options in the first month of therapy with IM IFNβ-1a. However, ibuprofen appeared to provide better control of symptoms immediately after IM IFNβ-1a injection than either acetaminophen or steroids.27 Similarly, Reess et al.25 compared acetaminophen and ibuprofen and found them equally effective at FLS management in patients initiating IM IFNβ-1a. Leuschen et al.28 compared the efficacy of naproxen, ibuprofen, and acetaminophen and found that the first two were more effective than the last at minimizing many of the physical symptoms associated with IM IFNβ-1a. However, none of these therapies was as effective as originally hypothesized for minimizing fatigue or for effectively managing joint and muscle pain.28
Based on these studies, the panel recommended that patients take ibuprofen or naproxen 1 hour prior to IM IFNβ-1a injection, as both compounds were shown to be better than acetaminophen at providing overall prophylaxis of FLS. In one clinical trial a course of steroids with acetaminophen significantly reduced FLS compared to acetaminophen alone; however, patients taking steroids should be carefully monitored, and steroids should generally not be used for long-term symptom management.27 Using low-dose oral steroids to help manage FLS-related pain was recommended only if no improvement was noticed with nonsteroidal anti-inflammatory drugs (NSAIDs).
Timing of Injections
Patient education regarding the potential impact of injection timing on the development of FLS is a key component of successful symptom management, especially during the first 6 months of therapy, when the risk of nonadherence is highest.29 Based on clinical reports,29–31 the panel recommended that IFNβ injections, in general, be administered in the evening to mitigate FLS. Patients are encouraged to determine when their symptoms peak and administer the injection at the appropriate interval prior to bedtime, thus allowing the worst of the side effects to occur during sleep and to fully resolve prior to waking. However, the panel underscored the importance of evaluating individual patients for alternate dosing times, especially in light of evidence that many patients suffered fewer FLS with morning injections.31 These data demonstrate the need for clinicians to counsel each patient to develop an individual routine that best allows the patient to manage any FLS, based on his or her own responses to IFNβ injection.
IFNβ Formulation
IFNβ therapies for MS are available in a number of different formulations, including prefilled syringes and lyophilized preparations (reconstituted immediately prior to injection).20–23 Flu-like symptoms have been reported in 59% to 88% of patients during the initial dosing period using prefilled syringe preparation22 32 and in 49% to 57% of patients receiving reconstituted formulations.20–23 30 32 In their clinical practice, members of the panel noted that a substantial number of their patients who were switched from lyophilized preparations to prefilled syringes experienced more FLS with the latter preparation. Therefore, the panel agreed that clinicians should consider evaluating the IFNβ formulation for those patients who continue to experience FLS despite following the dose-titration, analgesic, and administration timing recommendations.
Solution Temperature
A review of the literature did not identify any formal reports on the effect of solution temperature on FLS, and this issue is not discussed in the prescribing information for the IFNβ products used to treat patients with MS.20–23 However, in the panel’s experience, the severity and duration of FLS are reduced when the prepared medication is near body temperature at the time of administration. Thus, to optimize solution temperatures, IFNβ therapies should be removed from the refrigerator several hours prior to dosing.21 Patients need to be cautioned that these medications should never be artificially warmed in hot water, in a microwave, or with another intense heat source, as they will lose potency. However, gently warming the solution by holding the vial or syringe in hands, axilla, or a pocket can safely bring the medication closer to a more comfortable temperature. While this evidence is anecdotal, the panel concluded that gently warming the solution prior to injection is associated with a reduced incidence of FLS in a substantial number of their patients and is, therefore, recommended, especially as no risks to the patients are incurred.
Hydration
Low-grade fever is often a significant component of FLS. Because fever can cause dehydration, it is important to counsel patients to hydrate themselves adequately, particularly during the period they experience FLS. Hydration is also important for many aspects of normal cognitive and physical functioning.30 Patients should be counseled on the importance of maintaining adequate fluid intake, as well as on the signs of dehydration, such as dry skin, headache, fatigue, irritability, confusion, and reduced urine output. As patients with MS experience urinary bladder symptoms including incontinence, urine leakage, and hesitancy, they may restrict their fluid intake to limit these bladder-related issues.33 These symptoms may exacerbate FLS in patients with fever resulting from IFNβ treatment. Maintenance of adequate hydration is particularly important in patients who inject during the evening, as restricting fluid intake in the hours before sleep is often recommended to manage nocturia.
The panel recommended that MS therapy guidelines for injectable IFNβs emphasize the importance of patients’ maintaining adequate hydration, particularly prior to IFNβ injection, in order to minimize any effects of fever-induced dehydration. The guidelines should recognize that for patients who suffer from FLS and nocturia, fluid loading prior to evening injections may not be appropriate and alternative dosing times should be considered. This recommendation is not based on formal clinical trial data, but on the panel’s clinical experience in their efforts to mitigate discomfort during low-grade fever.
Diet
Finally, proper nutrition is critical for maintaining robust immune responses.34 While there is no evidence that a specific food can moderate FLS, it is axiomatic that maintenance of a healthy diet makes a large contribution to the body’s ability to withstand FLS. Proper nutrition that includes a reasonable combination of proteins, carbohydrates, and fats from fresh fruits and vegetables, grains, meats, fish, and dairy products has been associated with better health outcomes across all patient populations. Therefore, patients with MS can benefit by avoiding or limiting their intake of foods that are high in fat and sugar in the interest of their overall health. While no formal trials have been conducted on the effect of specific foods or diets on FLS, the panel felt that patients should be counseled on nutrition issues for overall health.
Discussion
As the maximal therapeutic benefit requires good adherence to therapy, it is essential to address factors that lead to patient nonadherence. Adverse events, including FLS, are a major cause of discontinuation of IFNβ-1a therapy in MS patients.33 35 36 The majority of currently approved first-line therapies are IFNβ formulations, which may cause such FLS as fatigue/malaise, chills, fevers, headaches, and myalgia that many patients find difficult to tolerate over long periods. Moreover, the relatively low rate of clinically relevant relapses that most MS patients experience makes long-term adherence more challenging, because the clinical benefit of treatment may not be readily apparent while FLS may continue to occur regularly.
Clinical trial data clearly show that effective management of FLS can increase patient adherence to IFNβ-based disease-modifying therapies (DMTs).37 38 Because FLS most often occur during therapy initiation, it is critical that patients be counseled on both the probability of symptoms and their mitigation. Because FLS can occur in long-term users of IFNβ-based DMTs as well, it is important to routinely monitor patient adherence so that clinical staff can intervene as necessary. Nurses should be prepared to discuss the probability of FLS with patients initiating IFNβ therapy and offer practical methods to limit these side effects. This counseling has been shown to be an important aspect of patient care, as adherence rates are higher in patients with realistic expectations of treatment-related side effects.38 In summary, nurses play a crucial role in ensuring the best long-term clinical outcomes in MS patients by working with patients to develop strategies that increase their overall compliance and adherence to therapy. The guidelines described will help health-care providers educate their patients on effective strategies to limit FLS and thus increase the probability of long-term adherence.
PracticePoints
Flu-like symptoms (FLS) associated with interferon beta (IFNβ) injections negatively affect patient adherence to therapy for MS, and strategies that limit FLS can improve adherence and, hence, overall clinical outcome.
FLS can be mitigated by dose titration, use of analgesics, evening dosing, drug formulation, solution temperature, adequate hydration, and a nutritious diet.
Overall adherence to IFNβ therapy can be significantly improved by working with patients to effectively incorporate all of these interventions into their daily routines and injection practices.
Acknowledgments
Anne Williamson, PhD, and Joshua Safran from Infusion Communications copyedited and styled the manuscript according to journal requirements.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002; 359: 1221–1231.
National Institute of Neurological Disorders and Stroke. Multiple sclerosis: hope through research: treatments to help reduce disease activity and progression. http://www.ninds.nih.gov/disorders/multiple_sclerosis/detail_multiple_sclerosis.htm#193353215. Accessed December 17, 2012.
Kinkel RP, Kollman C, O'Connor P, et al. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006; 66: 678–684.
Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009; 8: 987–989.
Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology. 2011;76(suppl 1):S26–S34.
Jacobs LD, Cookfair DL, Rudick RA, et al.; The Multiple Sclerosis Collaborative Research Group (MSCRG). Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996; 39: 285–294.
Zivadinov R, Locatelli L, Cookfair D, et al. Interferon beta-1a slows progression of brain atrophy in relapsing-remitting multiple sclerosis predominantly by reducing gray matter atrophy. Mult Scler. 2007; 13: 490–501.
Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011; 25: 491–502.
Wiendl H, Kieseier BC. Disease-modifying therapies in multiple sclerosis: an update on recent and ongoing trials and future strategies. Expert Opin Investig Drugs. 2003; 12: 689–712.
Galetta SL, Markowitz C. US FDA-approved disease-modifying treatments for multiple sclerosis: review of adverse effect profiles. CNS Drugs. 2005; 19: 239–252.
Ruggieri RM, Settipani N, Viviano L, et al. Long-term interferon-beta treatment for multiple sclerosis. Neurol Sci. 2003; 24: 361–364.
Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010; 30: 89–100.
Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011; 28: 51–61.
Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011; 18: 69–77.
Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012; 18: 932–946.
Brandes DW, Bigley K, Hornstein W, Cohen H, Au W, Shubin R. Alleviating flu-like symptoms with dose titration and analgesics in MS patients on intramuscular interferon beta-1a therapy: a pilot study. Curr Med Res Opin. 2007; 23: 1667–1672.
Moses H Jr, Brandes DW. Managing adverse effects of disease-modifying agents used for treatment of multiple sclerosis. Curr Med Res Opin. 2008; 24: 2679–2690.
Galetta SL. The controlled high risk Avonex multiple sclerosis trial (CHAMPS Study). J Neuroophthalmol. 2001; 21: 292–295.
Matson MA, Zimmerman TR Jr, Tuccillo D, Tang Y, Deykin A. Dose titration of intramuscular interferon beta-1a reduces the severity and incidence of flu-like symptoms during treatment initiation. Curr Med Res Opin. 2011; 27: 2271–2278.
Betaseron [package insert]. Montville, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2010.
Avonex [package insert]. Cambridge, MA: Biogen Idec Inc; 2011.
Rebif [package insert]. Rockland, MA: EMD Serono, Inc; 2009.
Extavia [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2011.
Wroe SJ. Effects of dose titration on tolerability and efficacy of interferon beta-1b in people with multiple sclerosis. J Int Med Res. 2005; 33: 309–318.
Reess J, Haas J, Gabriel K, Fuhlrott A, Fiola M. Both paracetamol and ibuprofen are equally effective in managing flu-like symptoms in relapsing-remitting multiple sclerosis patients during interferon beta-1a (AVONEX) therapy. Mult Scler. 2002; 8: 15–18.
Rice GP, Oger J, Duquette P, et al. Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Can J Neurol Sci. 1999; 26: 276–282.
Río J, Nos C, Bonaventura I, et al. Corticosteroids, ibuprofen, and acetaminophen for IFNbeta-1a flu symptoms in MS: a randomized trial. Neurology. 2004; 63: 525–528.
Leuschen MP, Filipi M, Healey K. A randomized open-label study of pain medications (naproxen, acetaminophen and ibuprofen) for controlling side effects during initiation of IFN beta-1a therapy and during its ongoing use for relapsing-remitting multiple sclerosis. Mult Scler. 2004; 10: 636–642.
Kleinman NL, Beren IA, Rajagopalan K, Brook RA. Medication adherence with disease modifying treatments for multiple sclerosis among US employees. J Med Econ. 2010; 13: 633–640.
Singer B, Lucas S, Kresa-Rehal K, Ross AP, Blake P. Optimizing adherence to multiple sclerosis therapies. Int J MS Care. 2008; 10: 113–126.
Nadjar Y, Coutelas E, Prouteau P, et al. Injection of interferon-beta in the morning decreases flu-like syndrome in many patients with multiple sclerosis. Clin Neurol Neurosurg. 2011; 113: 316–322.
Phillips JT, Rice G, Frohman E, et al. A multicenter, open-label, phase II study of the immunogenicity and safety of a new prefilled syringe (liquid) formulation of Avonex in patients with multiple sclerosis. Clin Ther. 2004; 26: 511–521.
Cohen BA. Identification, causation, alleviation, and prevention of complications (ICAP): an approach to symptom and disability management in multiple sclerosis. Neurology. 2008;71(24 suppl 3):S14–S20.
Issazadeh-Navikas S, Teimer R, Bockermann R. Influence of dietary components on regulatory T cells. Mol Med. 2012; 18: 95–110.
Bayas A, Rieckmann P. Managing the adverse effects of interferon-beta therapy in multiple sclerosis. Drug Saf. 2000; 22: 149–159.
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009; 256: 568–576.
Denis L, Namey M, Costello K, et al. Long-term treatment optimization in individuals with multiple sclerosis using disease-modifying therapies: a nursing approach. J Neurosci Nurs. 2004; 36: 10–22.
Mohr DC, Goodkin DE, Likosky W, et al. Therapeutic expectations of patients with multiple sclerosis upon initiating interferon beta-1b: relationship to adherence to treatment. Mult Scler. 1996; 2: 222–226.
Financial Disclosures: Dr. Filipi has served on speakers’ bureaus for Biogen Idec, Acorda, Teva, and the International Organization of Multiple Sclerosis Nurses (IOMSN), and on the clinical advisory board for the National Multiple Sclerosis Society. Ms. Beavin is an employee of Biogen Idec; a substantial portion of her contributions to this article were made while she was employed at Forget-Me-Not Home Memory Care, Raleigh, NC. She has also served on speakers’ bureaus for Biogen Idec, Novartis, the NMSS, the IOMSN, and the Consortium of Multiple Sclerosis Centers (CMSC) and has been a consultant for Biogen Idec, Genzyme, Janssen, and Pfizer. Ms. Brillante is an employee of Biogen Idec; a substantial portion of her contributions to this article were made while she was employed at Rush Multiple Sclerosis Center, Chicago, IL. Ms. Costello has been a scientific advisory participant and consultant for and received honoraria from Teva, Biogen Idec, EMD Serono, Genzyme, Acorda, Questcor, and Novartis; she has also received a research grant from Novartis. Gail C. Hartley has served on speakers’ bureaus and been a consultant for Acorda, Biogen Idec, EMD Serono, Genzyme, Novartis, Pfizer, Questcor, and Teva. Ms. Namey has served on speakers’ bureaus and been a consultant for Biogen Idec, Teva, Pfizer, EMD Serono, Acorda, Novartis, Genzyme, and Questcor; she has also been a consultant for Novartis and Allergan. Ms. O’Leary has served on speakers’ bureaus for Biogen Idec, Genzyme, and the IOMSN and has been a consultant for Novartis and Teva; a substantial portion of her contributions to this article were made while she was employed at Texas Neurology, Dallas, TX. Ms. Remington has served on speakers’ bureaus for Biogen Idec, Teva, the NMSS, the CMSC, and the IOMSN and been a consultant for Biogen Idec, Teva, Genzyme, and Acorda. Kay Hartley has no conflicts of interest to disclose.
Funding/Support: Biogen Idec provided funding for editorial support in the development of this article, provided support for the Flu-Like Symptom Expert Panel, and reviewed the manuscript and provided feedback on it to the authors. The authors had full editorial control of the manuscript and provided final approval of all content.







