Publication
Research Article
International Journal of MS Care
The Impact of Gait Disability on the Calibration of Accelerometer Output in Adults with Multiple Sclerosis
Author(s):
Accelerometer activity counts have been correlated with energy expenditure during treadmill walking among ambulatory adults with multiple sclerosis (MS). This study examined the effects of gait disability on 1) the association between rates of energy expenditure and accelerometer output in overground walking and 2) the calibration of accelerometer output for quantifying time spent in moderate-to-vigorous physical activity (MVPA) in people with MS. The sample consisted of 24 individuals with MS, of whom 10 reported gait disability based on Patient-Determined Disease Steps (PDDS) scores. The participants undertook three 6-minute periods of overground walking while wearing an accelerometer and a portable metabolic unit (K4b2, Cosmed, Rome, Italy). In the first period of walking, the participants walked at a self-selected, comfortable speed. In the two subsequent walking periods, participants walked at speeds above and below (±0.5 mph) the comfortable walking speed, respectively. Strong linear relationships were observed between rates of accelerometer activity counts and energy expenditure during walking in the overall sample (R2 = 0.90) and subsamples with (R2 = 0.88) and without gait disability (R2 = 0.91). The slope of the relationship was significantly steeper in the subsample with gait disability (β= 0.0049) than in the subsample without gait disability (β= 0.0026). The difference in slopes resulted in a significantly lower cut-point for MVPA (1886 vs. 2717 counts/min) in those with gait disability. These findings provide a metabolic cut-point for quantifying time spent in MVPA in people with MS, both with and without gait disability.
Multiple sclerosis (MS) is an immune-mediated, neurodegenerative disease of the central nervous system in which the myelin sheath is irreversibly damaged and axons are transected.1 Over time, symptoms such as muscle weakness, stiffness, and limb paralysis result from neuronal loss and brain atrophy2 and typically result in gait disability. Gait disability is reported by approximately 75% of the MS population,3 and nearly 30% of people with MS progress to a level of gait impairment that requires use of an assistive device within 10 years.4 The presence of gait disability likely influences the quantification of physical activity among those with MS.5
Motion sensors such as accelerometers have been considered the ideal tool for quantifying physical activity in the MS population. This is because data from an accelerometer are not influenced by cognitive impairment or self-report bias, which can affect survey data.5 An accelerometer is a small, lightweight device typically worn on a belt around the waist that quantifies the acceleration of the body's center of mass and expresses bodily movement in activity counts per unit time. Activity counts seem to provide a valid, objective measure of physical activity5 and have been significantly correlated with self-report, interview, and objective measures of physical activity in individuals with MS.6 7 There is additional evidence of a strong linear relationship between activity counts and energy expenditure (oxygen consumption; O2) during treadmill walking in people with MS.8 This is important because it satisfies a major assumption of using accelerometers for measuring physical activity, namely, that rates of accelerometer output are strongly associated with rates of energy expenditure during dynamic activity such as walking.8 It also allows for the generation of cut-points for quantifying time spent in moderate-to-vigorous physical activity (MVPA) for people with MS based on the rate of accelerometer activity counts per minute. This in turn allows for precise and objective quantification of the amount of physical activity performed by individuals with MS in the context of epidemiological, longitudinal, and interventional studies.
The major limitation of previous research has been validating physical activity measures without consideration of the possible impact of gait disability, particularly on the association between accelerometer output and energy expenditure during dynamic activity. Some data indicate that walking impairment may affect accelerometer output and energy expenditure in populations with neurologic disorders. For example, one recent study found that walking impairment was strongly and significantly correlated with oxygen cost of walking across different speeds of treadmill and overground walking in people with MS.9 Another study indicated that accelerometer output (ie, activity counts) was strongly and significantly associated with walking impairment in individuals with MS.10 By extension, gait disability may moderate the association between energy expenditure and accelerometer output in people with MS. This would, in turn, indicate that the cut-point for quantifying time spent in MVPA from accelerometer output would vary between groups who differ in level of gait impairment.
Therefore, this study examined the effects of gait disability on 1) the association between rates of energy expenditure and accelerometer output and 2) the calibration of accelerometer output for quantifying time spent in MVPA in people with MS. We hypothesized that the associations between the rates of accelerometer counts and energy expenditure would differ between subsamples with and without gait impairment, and that this would result in a lower cut-point for MVPA in those with a gait disability.
Methods
Overview
In this laboratory-based study, two groups of individuals with MS who differed in self-reported gait disability undertook three 6-minute periods of walking at comfortable, faster, and slower walking speeds, respectively. Participants wore an accelerometer and a portable metabolic device for measuring rates of activity counts and energy expenditure, respectively, during the periods of walking. This process is described in more detail below.
Sample
Individuals with MS who walked with or without an assistive device were recruited from the local community through telephone or e-mail messages, and 50 people underwent screening for inclusion in the study. The people recruited were participants in previous research in our laboratory. The inclusion criteria consisted of a neurologist-confirmed diagnosis of MS, no relapses during the preceding 30 days, ambulatory status with minimal assistance (ie, cane or no cane), age between 18 and 65 years, and absence of contraindications for undertaking physical activity (eg, history of cardiovascular disease, diabetes, hyperlipidemia, hypertension) based on no affirmative responses on the Physical Activity Readiness Questionnaire (PAR-Q)11 and items on a pre-exercise screening instrument. Twenty-six of the 50 individuals recruited met the inclusion criteria and expressed interest in participating; the other 24 individuals were excluded for reporting one or more risk factors for contraindications for undertaking physical activity based on the PAR-Q and pre-exercise screening. Two additional participants did not complete the protocol because of difficulty with walking 6 minutes, resulting in a final sample of 24 participants.
The sample was primarily female (n = 20, 83%), white (n = 20, 83%), and well educated (college graduate n = 18, 75%). The mean age of the sample was 42.0 years (SD = 11.7). All participants had relapsing-remitting MS (RRMS), even though this was not among the inclusion/exclusion criteria, and the mean duration of MS was 11.1 years (SD = 8.5). The median Patient-Determined Disease Steps (PDDS) score was 1 (range, 0–4), which corresponds with minor, noticeable symptoms that have only a small effect on lifestyle. The overall sample of 24 individuals with MS was divided into subsamples without gait disability (n = 14; PDDS scores ≤2.0) and with gait disability (n = 10; PDDS scores ≥3.0). This difference in groups without and with gait disability was confirmed by a significant and large difference (d = 2.4) in mean ± SD 12-item Multiple Sclerosis Walking Scale (MSWS-12) scores between groups (10.5 ± 11.1 vs. 37.8 ± 11.7), based on an independent-samples t test (t 22 = −5.81, P < .0001). We did not divide the groups based on MSWS-12 scores because there are not definitive cut-points for doing this and the PDDS value of 3 corresponded with the Expanded Disability Status Scale (EDSS) benchmark of 4.0 indicating the onset of gait disability.
Measures
Gait Disability
Gait disability was measured using the PDDS scale.12 The PDDS is a self-report questionnaire that measures gait disability using an ordinal scale of 0 (normal) through 8 (bedridden); a PDDS score of 3 corresponds with gait disability. This questionnaire was developed as an inexpensive surrogate for the EDSS, and scores from the PDDS have been linearly and strongly associated with physician-administered EDSS scores (r = 0.93).12 The PDDS has also been strongly associated with several other valid measures of walking mobility, including oxygen cost of walking (r = 0.668), the MSWS-12 questionnaire (r = 0.847), and the distance traveled during a 6-Minute Walk test (r = −0.427) in ambulatory individuals with MS.10
Accelerometry
This study used ActiGraph, model 7164, accelerometers (Health One Technology, Fort Walton Beach, FL). This model of accelerometer is small (2.0 × 1.6 × 0.6 inches) and lightweight (1.5 ounces), and contains a single, vertical axis piezoelectric bender element. The bender element has a detection range of 0.05 to 3.2 G and generates an electrical signal proportionate to the force acting on it during bodily movement. The electrical signal is band-pass filtered (0.25–2.5 Hz), digitized at 10 Hz by an 8-bit analog-to-digital converter, numerically integrated over a sampling interval (eg, 1-second epoch), and stored in random-access memory as activity counts. The activity counts represent a summation of positive and negative accelerations of the body over a sampling interval. This is not the same as a pedometer that measures the rate of steps per unit time; rather, accelerometers provide a metric of the rate of bodily acceleration per unit time. The accelerometer was worn on an elastic belt around the waist and placed on the side of the body along the nondominant hip. This location is in the sagittal plane and would capture the acceleration of the body's center of mass without interfering with normal daily functions (eg, sitting or lying down). The accelerometer provided a quantitative measure of the participant's rate of bodily acceleration (ie, movement) over time. The data were retrieved for analysis using a computer reader interface unit and software designed for the ActiGraph accelerometers. The downloaded data were imported into Microsoft Excel (Microsoft, Redmond, WA) and expressed as the average activity counts per minute.
Energy Expenditure
Energy expenditure was estimated through measurement of oxygen consumption (O2) using a portable metabolic unit (K4b2, Cosmed, Rome, Italy). This system weighs 875 g; this minimal weight, by convention, does not differentially alter oxygen kinetics or walking performance. Before each testing session, the flowmeter of the K4b2 portable metabolic unit was calibrated using a 3-L syringe (Hans Rudolph, Kansas City, MO) and oxygen and carbon dioxide sensors were calibrated using verified concentrations of gases. The K4b2 metabolic unit was worn by each participant in a harness fastened around the chest, and the associated face mask was fitted appropriately over the nose and mouth. Participants were instructed to breathe normally and refrain from talking during testing. The rate of energy expenditure was computed as the rate of O2 in mL/kg/min, and steady-state O2 was calculated by averaging O2 values across the final 3 minutes (minutes 4–6) of the 6-minute walking period. The oxygen cost of walking (mL/kg/m) was calculated by dividing O2 (mL/kg/min) by walking speed (m/min); a higher oxygen cost of walking reflects a greater energetic demand for walking.
Protocol
The study procedure was approved by a university institutional review board, and all participants provided informed consent. Each participant completed a demographic questionnaire, the MSWS-12, and the PDDS scale. The participant and one research assistant then walked the course for measuring accelerometer activity counts and energy expenditure as a familiarization protocol. The course itself was located in a handicap-accessible, rectangular hallway with four 90-m corridors that were clear of obstructions and foot traffic. We then connected the K4b2 portable metabolic unit along with the associated face mask for collecting expired gases and measuring energy expenditure, and the ActiGraph accelerometer for measuring the rate of bodily acceleration. The participant then sat and rested quietly for 5 minutes. After the 5 minutes of rest, the participant undertook three 6-minute periods of walking; this period of walking is consistent with walking endurance protocols used for people with MS8 9 and is of sufficient duration to achieve steady-state O2 during the second 3 minutes of the 6-minute period of walking.
In the first period of walking, the participants walked at a self-selected comfortable walking speed. This was followed by two subsequent walking periods in which the participants walked at 0.5 mph above (ie, faster walking speed) and below (ie, slower walking speed) the comfortable walking speed, respectively. This manipulation of walking speed was necessary for examining the association between rates of accelerometer activity counts and O2 within and between participants. Using three set speeds that would elicit appropriate metabolic demand across all individuals would not be feasible given our interest in differences based on gait disability, nor would it reflect real-world walking behavior. The comparison of O2 between groups is not invalidated by the difference in walking speeds, as people typically select a comfortable walking speed that optimizes oxygen kinetics, and we would not expect major discrepancies between groups in O2. The order of the faster and slower walking periods was randomized to minimize order effects among the walking trials, and participants had between 5 and 10 minutes of seated rest between walking periods to minimize carryover effects. To accomplish the manipulation of walking speed, one research assistant followed behind the participant during the first period of self-selected comfortable walking and recorded the actual speed using a measuring wheel (Stanley MW50, New Briton, CT). The manipulation of walking speed in the second and third periods of walking was accomplished by having the participant follow the research assistant, who controlled the speed using the aforementioned measuring wheel. Participants received $20 remuneration for their involvement in the study.
Statistical Analysis
Descriptive statistics are presented in text and tables as mean ± SD. The rates of energy expenditure and accelerometer data were entered for each participant into Microsoft Excel. This allowed computation of the squared multiple correlation coefficient (R 2), intercept, slope, and cut-point for MVPA (≥3 metabolic equivalent tasks [METs]) based on estimating a linear relationship between O2 and accelerometer activity counts for each participant. The metabolic and accelerometer data recorded across the three walking speeds and the R 2, intercept, slope, and cut-point for MVPA for the two groups that differed in gait impairment were then analyzed using PASW, version 18.0 (SPSS, Chicago, IL). The differences in R 2, intercept, slope, and cut-point for MVPA were analyzed with independent-samples t tests. The differences in metabolic and accelerometer data were analyzed using 2 (group) × 3 (speed) mixed-model analysis of variance (ANOVA). Alpha was set at .05 for all analyses.
Results
The metabolic, performance, and accelerometer data from the three 6-minute periods of slow, comfortable, and fast walking are shown in Table 1 for the overall sample and subsamples with and without gait disability. The mixed-model ANOVA indicated a speed main effect on actual walking speed (F 2,44 = 467.39, P = .0001), whereby actual walking speed increased in an expected manner across the three speeds of slow, comfortable, and fast walking. The group main effect and group by speed interaction were not statistically signifi-cant (P > .05).
Descriptive statistics for walking speed, oxygen consumption ( O2), oxygen cost, and accelerometer activity counts for the different MS samples
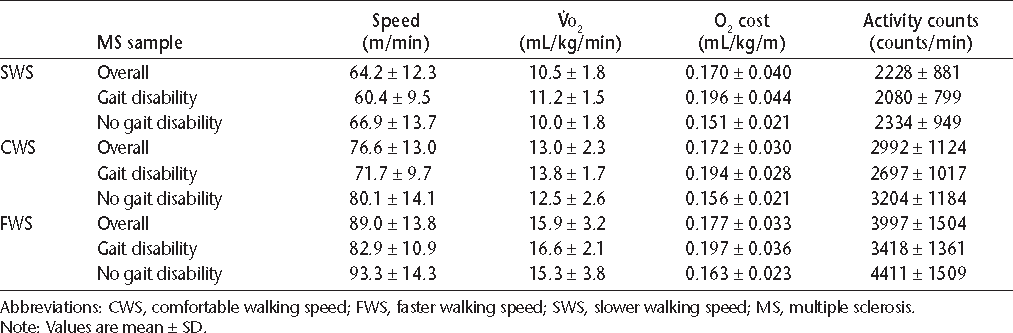
The mixed-model ANOVA identified a speed main effect on oxygen consumption (F 2,44 = 98.99, P = .0001), whereby oxygen consumption increased in an expected manner with the increase in walking speed. The group main effect and group by speed interaction were not statistically significant (P > .05). By comparison, the mixed-model ANOVA indicated a group main effect on oxygen cost of walking (F 1,22 = 15.29, P = .001), whereby the oxygen cost of walking (ie, oxygen consumption per unit traveled or mL/kg/m) was higher in people with gait disability than in those without gait disability. The speed main effect and group by speed interaction were not statistically significant (P > .05).
Lastly, the mixed-model ANOVA indicated a speed main effect on accelerometer activity counts per minute (F 2,44 = 83.87, P = .0001), whereby activity counts increased in an expected manner across the three speeds of slow, comfortable, and fast walking. The group main effect and group by speed interaction were not statistically significant (P > .05).
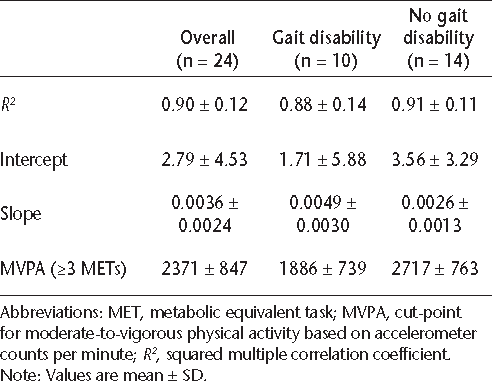
The R 2, intercept, slope, and cut-point for MVPA are shown in Table 2 for the overall sample and subsamples with and without gait disability. No statistically signifi-cant difference was found between groups in the average R2 (P > .05) or intercept (P > .05). Statistically significant differences were found in the slope of the association between accelerometer output and energy expenditure (t 22 = −2.47, P < .05) and the cut-point for MVPA (t 22 = 2.66, P < .05). The slope was significantly steeper in the sample with gait impairment, and this resulted in a significantly lower cut-point for MVPA in the sample with gait disability than in the sample without gait disability.
Squared multiple correlation coefficient (R2 ), intercept, slope, and cut-point for moderate-to-vigorous physical activity (MVPA) based on the linear relationship between oxygen consumption and accelerometer activity counts
Discussion
The main novel findings of this study were as follows: 1) The linear relationship between rates of accelerometer activity counts and energy expenditure during over-ground walking was strong overall and in each subsample, but steeper in those with gait disability than in those without gait disability. 2) The cut-point for quantifying time spent in MVPA from the accelerometer output was significantly lower in people with MS who had gait disability. These findings indicate that the association between accelerometer output and energy expenditure differs depending on the presence of gait impairment, and that, by extension, the cut-point for MVPA during overground walking is significantly lower in those with gait disability. These results have implications for using and interpreting accelerometer output for the quantification of physical activity in people with MS.
To date, no studies have been published on the impact of gait disability on the association between accelerometer output and energy expenditure in people with MS. Gait disability has been correlated with both energetic cost of walking9 and accelerometry13 in individuals with MS. Nevertheless, only one previous study examined the association between accelerometer output and energy expenditure in people with MS, and the sample was confined to those without gait impairment, limiting the generalizability of the findings to people with gait disability.8 The present study extends previous research because it accounted for gait disability and found a significant difference in the slopes of association between accelerometer output and energy expenditure, with a significantly steeper slope in the sample with gait disability than in the sample without gait disability (β = 0.0049 vs. β = 0.0026). This finding suggested that gait disability was differentially associated with the relationship between rates of energy expenditure and accelerometer output, which has implications for the interpretation of accelerometer output as a measure of sedentary or active behavior in people with MS.
Previous evidence has shown a strong relationship between accelerometry and energy expenditure in MS and a significant difference in accelerometer output between people with MS and healthy matched controls during treadmill walking.8 Researchers have suggested metabolic cut-points for moderate physical activity (MPA) (3.00–5.99 METs) and vigorous physical activity (VPA) (≥6.00 METs) in people with MS to be 591–6460 and >6460 activity counts per minute, respectively.8 This study expanded previous research by examining a more diverse sample of individuals with MS, considering the impact of gait disability, and examining overground walking. Linear regression analysis suggested that the cut-point for MVPA differed significantly depending on the presence of gait disability. The mean cut-point for MVPA (≥3.00 METs) was significantly lower in people with gait disability than in those without gait disability (1886 vs. 2717 activity counts per minute). The cut-point for those with gait disability (ie, 1886) would have fallen within the range provided for MPA in previous studies (ie, 591–6460), disregarding the increased effort and energy expenditure necessary for overground walking in people with gait disability. These findings suggest that the accelerometer output represents different levels of physical activity intensity depending on the characteristics of the sample, including the presence of a neurologic disorder and gait disability.
This study also examined the impact of gait disability on walking speed, oxygen consumption, and oxygen cost. The analysis identified a significant group (ie, gait disability, no gait disability) main effect on oxygen cost, but no group main effects on walking speed or oxygen consumption (O2). The relatively small sample size did not allow for adequate power to detect small differences in walking speed or oxygen consumption between the samples, but oxygen cost was significantly higher in those with gait disability. These findings suggest that the oxygen cost of walking is sensitive to gait impairments in the MS population, and more so than walking speed or oxygen consumption. Historically, researchers have demonstrated that the energy cost of walking is significantly higher in individuals with MS than in healthy controls,14 and that lower-limb spasticity influences the energy cost of walking in people with MS.15 Thus oxygen cost may be a useful tool for measuring energy expenditure in this population because of its sensitivity to neurologic deficits and gait impairments. Moreover, the difference in oxygen cost of walking has possible implications for therapists who prescribe walking programs. People with MS who have gait disability expend a greater amount of energy for a given speed of walking than those without gait disability; this indicates that a given walking speed is more burdensome and perhaps fatiguing for those with gait disability. This should be considered in the design and prescription of walking programs for individuals with MS, particularly those with gait disability, perhaps by prescribing a slower speed of walking.
This study has several limitations, including a relatively small sample size, the possibility that the results are limited in generalizability, and the lack of control for possible age and gender effects. Moreover, the study involved a narrowly defined sample that consisted of mostly female, well-educated, white participants, all with a diagnosis of RRMS. Yet another limitation was the use of a single brand and model of accelerometer, namely the ActiGraph model 7164. This limits support for the generalizability of our results across other brands and models of accelerometers. Finally, this study was cross-sectional, measuring accelerometer output and energy expenditure on one occasion in a controlled laboratory setting. Future research should examine the relationship between accelerometry and energy expenditure longitudinally, with specific focus on changes over time.
Conclusion
The findings of the present study provide evidence for a strong linear relationship between activity counts and energy expenditure during overground walking in people with and without gait disability. The findings also provide a cut-point for accelerometer data that allows for quantifying time spent in MVPA, and the cut-point is significantly lower in people with MS who have gait disabilities. Such results should be taken into account when using accelerometers for quantifying physical activity in future research involving individuals with MS who have gait impairments.
PracticePoints
The possible impact of gait disability has not been considered in previous research on validating physical activity measures in the MS population.
A strong linear relationship was found between accelerometer activity counts and energy expenditure during overground walking in people with MS who do and do not have gait disability.
The metabolic cut-point for quantifying time spent in moderate-to-vigorous physical activity is significantly lower in people with MS who have gait disability.
These results should be taken into account when using accelerometers for quantifying physical activity in future research involving individuals with MS who have gait impairments.
References
Vosoughi R, Freedman MS. Therapy of MS. Clin Neurol Neurosurg. 2010; 112: 365–385.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372: 1502–1517.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31–36.
Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology. 2004; 62: 51–59.
Motl RW. Physical activity and its measurement and determinants in multiple sclerosis. Minerva Med. 2008; 99: 157–165.
Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 2006; 28: 1151–1156.
Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Community Health. 2007; 30: 144–150.
Motl RW, Snook EM, Agiovlasitis S, Suh Y. Calibration of accelerometer output for ambulatory adults in multiple sclerosis. Arch Phys Med Rehabil. 2009; 90: 1778–1784.
Motl RW, Suh Y, Dlugonski D, et al. Oxygen cost of treadmill and over-ground walking in mildly disabled persons with multiple sclerosis. Neurol Sci. 2011; 32: 255–262.
Motl RW, Dlugonski D, Suh Y, Weikert M, Fernhall B, Goldman M. Accelerometry and its association with objective markers of walking limitations in ambulatory adults with multiple sclerosis. Arch Phys Med Rehabil. 2010; 91: 1942–1947.
Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci. 1992; 17: 338–345.
Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007; 127: 35–41.
Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. 2010; 290: 6–11.
Olgiati R, Jacquet J, Di Prampero PE. Energy cost of walking and exertional dyspnea in multiple sclerosis. Am Rev Respir Dis. 1986; 134:1005–1010.
Olgiati R, Burgunder JM, Mumenthaler M. Increased energy cost of walking in multiple sclerosis: effect of spasticity, ataxia, and weakness. Arch Phys Med Rehabil. 1988; 69: 846–849.
Financial Disclosures: The authors have no conflicts of interest to disclose.







