Publication
Research Article
International Journal of MS Care
Impact of Usual-Care Physiotherapy on Physical Activity and Self-Efficacy in People With Multiple Sclerosis: An Observational Longitudinal Study
Author(s):
ABSTRACT
Background:
People with multiple sclerosis (MS) experience motor and nonmotor symptoms that affect daily life. Although regular physical activity (PA) may enhance the overall well-being of people with MS, they tend to have lower activity levels than healthy individuals. This study aims to investigate the impact of usual-care physiotherapy on PA and self-efficacy in people with MS and identify prerehabilitation factors that influence positive changes in PA with physiotherapy.
Methods:
Forty-one people with MS undergoing physiotherapy with a median (IQR) age of 54.00 (17.00) years and an Expanded Disability Status Scale score of 6.00 (2.00) points were assessed on the first days (T0) and last days of their rehabilitation period (T1), and 6 weeks after it ended (T2). Instrumental assessment utilized Fitbit Versa trackers, measuring daily steps and moderate/vigorous PA engagement (MVPA). Self-efficacy, perceived fatigue, walking ability, and quality of life were measured using the Self-Efficacy in Multiple Sclerosis scale (SEMS), the Fatigue Severity Scale, the 10-Meter Walk Test, and the Short Form-12 Health Survey.
Results:
Usual-care physiotherapy did not improve (P > .05) daily steps (T0: 4139 [3333]; T1: 4438 [2505] steps per day), MVPA (T0: 6.00 [15.6]; T1: 10.52 [16.30] minutes per day), or self-efficacy (SEMS: T0: 42.0 [10.8]; T1: 40.5 [8.7] points). Low perceived fatigue, better overall PA, and good physical health perception were identified as predictors of positive changes in PA after physiotherapy.
Conclusions:
Usual-care physiotherapy focusing on mobility did not result in significant improvements in PA or self-efficacy for people with MS. Perceived fatigue and overall PA before physiotherapy impacted PA levels after rehabilitation. Future interventions may benefit from integrating motivational strategies into the rehabilitation protocol to increase PA levels.
Multiple sclerosis (MS) is one of the most common neurodegenerative diseases; it is commonly diagnosed in people between 20 and 50 years of age and impacts 2.8 million people worldwide.1 People with MS experience a wide range of symptoms affecting physical, cognitive, and physiological functions. These multifaceted symptoms can potentially influence their daily activity and active participation in life, including their daily amount of physical activity (PA).2
Recent literature emphasized the importance of PA for people with MS, suggesting that regular PA may limit the worsening of symptoms and improve disease prognosis.3 PA includes any bodily movement produced by skeletal muscle contraction resulting in increased energy expenditure.3 It broadly encompasses daily activities like work and home tasks, leisure activities, sports, and supervised rehabilitation and/or exercise.3 PA can be more precisely defined: It is light when it is not intense enough to prevent a person from singing, and it is moderate/vigorous when a person cannot sing or can barely speak while engaged in the activity.4 There is a general consensus that regular PA is crucial for people with MS to maintain and improve overall health and well-being, reduce fatigue, and enhance mobility and quality of life (QOL).5,6
Recent technological advances have made it possible to objectively measure PA in daily life through the use of wearable systems that can provide accurate and ecological measures of PA levels.7,8 To date, studies using wearable sensors show that people with MS are mostly sedentary and have lower daily PA levels compared with individuals who do not have MS.9,10 Even during periods of rehabilitation, people with MS are rarely advised on how to increase their PA to the recommended levels necessary for health benefits.11,12 A recent study showed that people with MS do not achieve the recommended daily amount of PA, even during outpatient or inpatient rehabilitation.13
Physiotherapy aims to help people with MS regain function and maintain an appropriate level of independence.14 To date, treatments for MS symptoms and impairments have included endurance, resistance, balance, and gait training, all aimed at increasing independence in daily activities.15-17 However, the effectiveness of usual-care physiotherapy in increasing daily PA in people with MS appears to be uncertain. Kuending et al found no increase after 3 weeks of inpatient rehabilitation,18 whereas Nedeljkovic et al observed some improvement post relapse.19
People with MS following usual-care physiotherapy programs are seldom given individual advice on how to increase their PA outside of the physiotherapy session, and environmental factors acting as barriers and inaccessibility affecting their PA are often not addressed. Although traditionally addressed during rehabilitation, the important issues of self-efficacy and self-management are often not addressed afterward. Motivation and self-efficacy in people with MS are key factors in maintaining a healthy lifestyle, and they are even more important when changing from a sedentary lifestyle to a more active one. Indeed, self-efficacy is linked to the belief that one can cope with challenging situations and attain goals.20 The influence of self-efficacy on physiotherapy outcomes and PA in people with MS should be considered, as it may impact an individual’s motivation to participate in beneficial PAs.21 In people who have had a stroke, self-efficacy improvement after physiotherapy appeared to be tied to better locomotor abilities, well-being, and daily living activities, suggesting that it might also be a factor in physiotherapy outcomes in people with MS.22 These issues, which are important to maintain high PA levels, are particularly relevant in people with MS with moderate to severe disability since high disability, low self-efficacy, and fatigue have been reported as factors that limit PA in people with MS.23
Given the importance of PA and the generally low levels of PA in people with MS who have higher disability levels, this study aims (1) to highlight the impact of physiotherapy on PA and self-efficacy levels in people with MS and (2) to determine the characteristics that influence changes in PA following physiotherapy.
METHODS
Study Design
This is a 1 sample, uncontrolled pre- and postintervention study including longitudinal data on PA in people with MS receiving rehabilitation. The study was conducted in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee at the Don Carlo Gnocchi Foundation in Milan (January 29, 2019; NTC04186910). All participants agreed to take part in the study, understood the study procedures, and signed informed consent releases. Participants were recruited between September 2019 and December 2022 from those who were prescribed physiotherapy in inpatient and outpatient settings.
Participants underwent assessments at 3 different time points: shortly after beginning a physiotherapy program (T0), toward the end of the program (T1), and 6 weeks after completing the program (T2). In general, the duration of a usual-care physiotherapy period was 4 weeks, and each session lasted 45 minutes. Inpatients received a high dose of physiotherapy, with a minimum of 2 sessions per day, 5 times per week. Outpatients received a low dose of physiotherapy, with only 2 to 3 sessions per week. Both inpatients and outpatients received physiotherapy from physiotherapists with experience in neurological MS rehabilitation. All treatments were tailored to the neurological and functional needs of people with MS, focusing mainly on balance/walking, strength, proprioception, and resistance training.24
Physiotherapists were not informed of the aim of this observational study, which was to document whether usual-care physiotherapy interventions to improve mobility in people with MS were effective in augmenting their daily PA and sense of self-management and efficacy. Thus, no specific interventions were performed to increase daily PA levels during the study, and all participants followed the planned physiotherapy protocol.
People 18 years or older were eligible for the study if they had a diagnosis of multiple sclerosis according to the 2010 McDonald criteria and had an Expanded Disability Status Scale (EDSS) score between 4.0 and 7.0.25 Participants were excluded if they had a Mini-Mental State Examination score of less than 20 points and/or visual or cardiac impairments that limited their ability to perform PA in daily life.
Participants provided demographic data at the baseline assessment, including age and educational level, and clinical information was obtained through their most recent neurological examination reports. Level of disability was assessed with the EDSS; in addition, information was collected about disease onset, MS type, and use of assistive devices. Clinical outcome measures assessing PA, perceived fatigue, walking ability, QOL, and self-efficacy were administered in person at T0 and T1, but at T2, questionnaires were administered over the phone.
Primary Outcomes
Instrumental and Clinical Assessment
Instrumental assessment was performed at T0 and T1 using the Fitbit Versa, a commonly used actigraph that provides information on daily steps, daily duration of light PA (LPA), moderate/vigorous PA (MVPA), and overall PA.27 Participants wore the Fitbit Versa for 1 week (≈12 hours per day) in the days after the start of their physiotherapy program and for 1 week in the days before the end of the physiotherapy program. The PA data collected included the physiotherapy sessions.
The primary instrumental outcome variables were the number of steps per day and the daily amount (minutes) of MVPA carried out during the week. The Minimal Clinically Important Difference (MCID) reflecting a significant change in walking performance has been suggested to be approximately 800 steps per day in people with MS.28
The clinical primary outcome was the participant’s score on the 15-item Self-Efficacy in Multiple Sclerosis (SEMS) scale that evaluated the individual’s ability to cope with the challenges of MS. The score for each item ranges from 0, not at all confident, to 4, very confident, with a maximum score of 60 points.29
Eligibility criteria were being between the ages of 20 and 64 years, having a diagnosis of MS confirmed by a neurologist, being employed, and not having other neurological disorders (eg, epilepsy traumatic brain injury, stroke). For the present study, only women were included.
Secondary Outcomes
Perceived Fatigue
Perception of fatigue was measured using the Fatigue Severity Scale (FSS); its 9 items are scored from 1, strongly disagree, to 7, strongly agree, and evaluate how fatigue affects daily life activities.30 The final score is the average of item scores, and the MCID is 1 point.31
Physical Activity Level
The Godin Leisure-Time Exercise Questionnaire (GLTEQ) assesses perceived PA levels, providing information on the time spent weekly doing intense, moderate, and light PA.32 People with MS with a score 24 points or higher are considered active, those between 14 and 23 points moderately active, and those with 13 points or lower sedentary.
Walking Ability
The 10-Meter Walk Test (10MWT) was used to measure gait and mobility,33 whereas the 12-item Multiple Sclerosis Walking Scale (MSWS-12) tested the impact of MS on perceived walking ability. Each item on the MSWS-12 is scored from 1, no limitation, to 5, extreme limitation, and the total score ranges from 12 to 60 points. Finally, the 2-Minute Walk Test (2MWT) assessed the participant’s ability to walk as far as possible for more than 2 minutes in a 30-meter walkway with 180° turns at both ends.34
QOL
The 12-Item Short Form Survey (SF-12) is a clinical measure providing information on perceptions of personal physical (physical component summary, PCS) and mental (mental component summary, MCS) health.35,36
Statistical Analyses
All statistical analyses were performed using R (version 4.1.0) with an α-level of 0.05. As outcome measures were normally distributed, descriptive statistics were reported as median (IQR) or percentage. Statistical differences between the groups at T0 were tested using the Pearson χ2 test or the Wilcoxon rank-sum test.
To assess the study’s first aim, we conducted a 3-way repeated measures analysis of variance (ANOVA) to determine significant changes in daily steps after physiotherapy (time effect), controlling for EDSS score (EDSS effect) and being an inpatient or outpatient (group effect). Another 3-way repeated measures ANOVA was performed to determine significant changes in MVPA after physiotherapy, controlling for EDSS and group effect. Finally, a third model was performed to determine significant changes in the SEMS scale after rehabilitation, controlling for EDSS and group effect. Each secondary variable (including T2, where data were available) was also analyzed by 3-way repeated measures ANOVA, and only statistically significant models are reported in the Results section.
For the second aim, participants were classified as responders when the daily steps change from T0 to T1 was higher than 800 steps.24 A backward stepwise logistic analysis was conducted to identify potential responder predictors of the clinical measures. At each step, predictors were chosen based on the area under the curve, and R2 was used to assess the goodness of fit of the model.
RESULTS
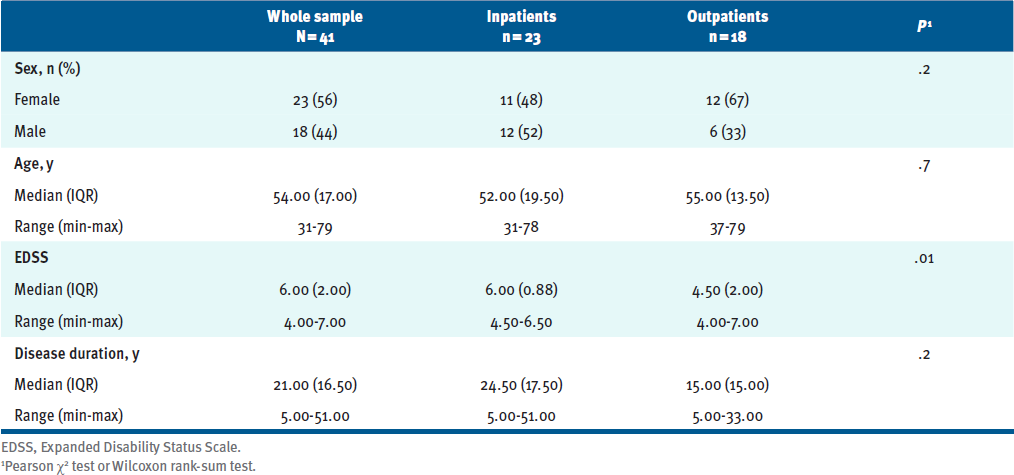
All screened participants with MS were included in the final study sample: 41 people with MS with a median (IQR) age of 54.0 (17.0) years and an EDSS score of 6.0 (2.0) points. TABLE 1 reports the complete demographic and clinical information at baseline. Inpatients (n = 23, 11 women) and outpatients (n = 18, 12 women) had comparable ages and disease durations; however, the groups differed in disability level (P = .01); inpatients had an EDSS of 6.00 (0.88) and outpatients 4.50 (2.00).
Table 1. Participant Demographics
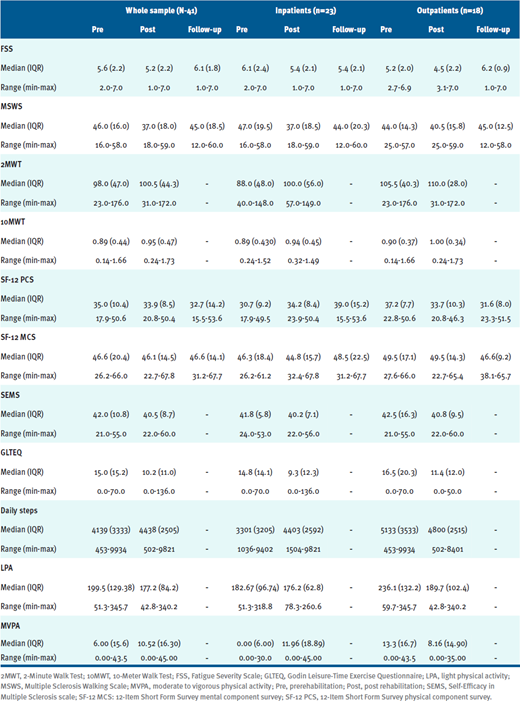
All participants attended at least 95% of their prescribed sessions; TABLE S1 summarizes the clinical information, with specific data on LPA and MVPA for the inpatient and outpatient groups, as well as the group as a whole.
Table S1. Participant Clinical Information
Aim 1: Impact of Physiotherapy on PA Levels
Instrumental Assessment
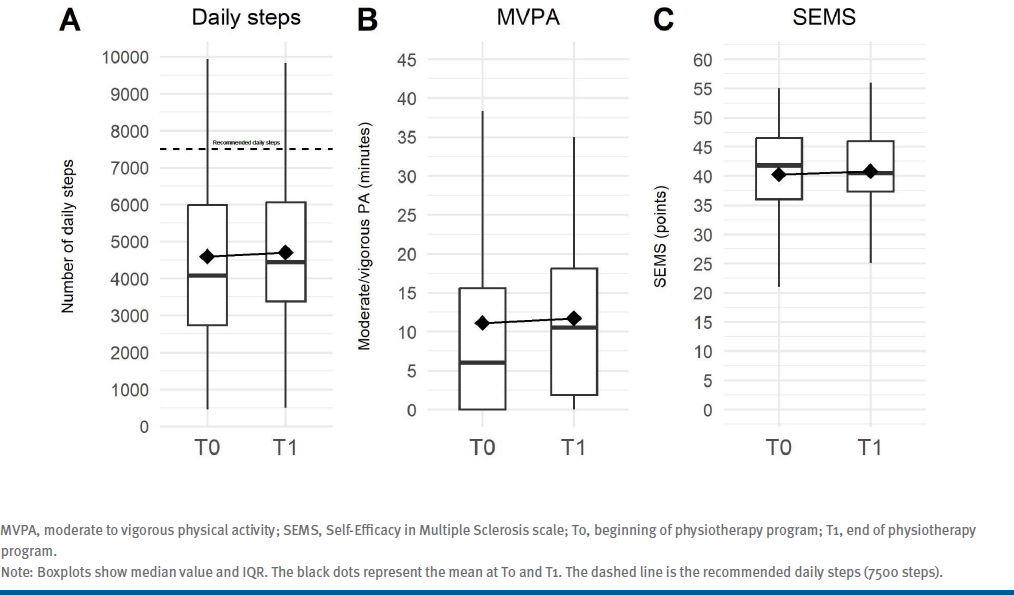
The average daily steps and minutes of MVPA are graphically reported in FIGURE A and FIGURE B, respectively. Considering the results of the 3-way repeated measures ANOVA analyses, there were no significant changes in number of daily steps (T0: 4139.0 [3333.6]; T1: 4437.9 [2505.7]; F = 0.027, P = .87) or daily minutes of MVPA (T0: 6.0 [15.6]; T1: 10.5 [16.30]; F = 0.090, P = .77) after physiotherapy (time effect).
Figure. (A) Average Daily Steps, (B) Minutes of MVPA, (C) Average SEMS Score
The 3-way repeated measures ANOVA also showed that a low EDSS score (EDSS effect) was significantly associated with both an increase in daily steps (F = 4.23, P < .001) and MVPA (F = 1.62, P = .16). Being an inpatient or outpatient (group effect) was not significantly associated with either change in daily steps (F = 2.10, P = .15) or daily minutes of MVPA (F = 0.52, P = .47).
Clinical Assessment
A graphical representation of the SEMS scale is shown in FIGURE C. Notably, there were no significant changes in the SEMS score after physiotherapy (T0: 42.0 [10.8] points; T1: 40.5 [8.7] points; time effect: F=0.008, P=.93). Nor did we observe differences (P > .05) in other clinical scales between T1 and T0 or between T2 and T0 (See Table S1.). In addition, the 3-way repeated measures ANOVA analysis showed that a low EDSS was nearly significantly associated with a better improvement in self-efficacy after physiotherapy (EDSS effect: F = 1.10, P = .37), whereas being an inpatient or outpatient was not associated with change on the SEMS after physiotherapy (group effect, F = 0.36, P = .55).
Aim 2: Characteristics That Influence PA Changes After Physiotherapy
Considering a change score threshold of 800 steps per day,24 we identified 8 of 40 participants (20%) as responders to usual-care physiotherapy. The 4 men and 4 women were 2 outpatients and 6 inpatients, with a median age of 60.0 (16.8) years and an EDSS score of 6.25 (1.13) points.
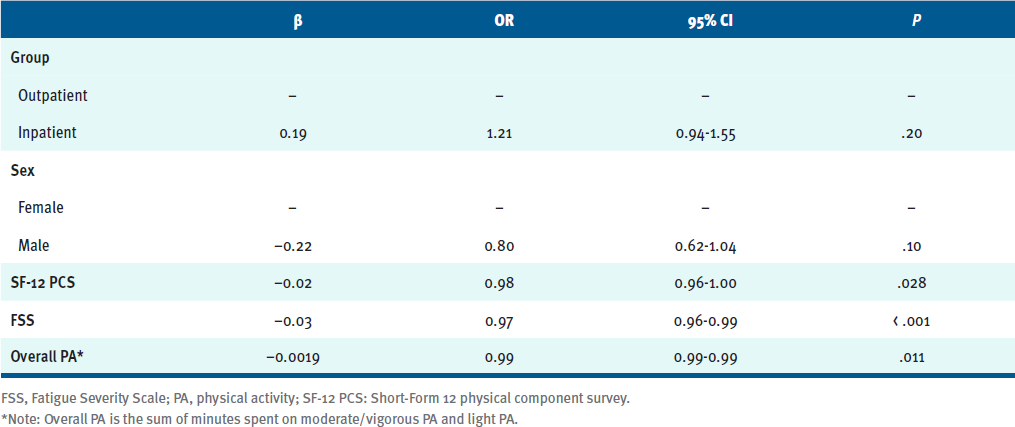
Starting from all the clinical and instrumental variables, a stepwise logistic regression model was performed. The final logistic model (TABLE S2) included 5 dependent variables; the most significant were the SF-12 PCS (OR, 0.98; 95% CI, 0.96-1.00; P = .03), the FSS (OR, 0.97; 95% CI, 0.96-0.99; P < .001), and the sum of minutes spent on light PA and moderate/vigorous PA (overall PA; OR, 0.99; 95% CI, 0.991-0.994; P = .011). The OR should be interpreted as an increase in the probability of being a responder due to an increase or decrease of 1 point on the reference clinical scale. For example, an OR of 0.97 for the FSS indicates that for every point increase in FSS at baseline (more fatigued), the participant's probability of being a responder decreases by 3%.
Table S2. Prediction Model
DISCUSSION
This longitudinal study explored the effects of usual-care motor rehabilitation on PA levels and self-efficacy in people with MS and also identified characteristics that may indicate a better outcome after physiotherapy. Overall, although small increases in both daily steps and MVPA were observed after rehabilitation, these increases were neither statistically significant nor clinically relevant; similarly, there were no differences in self-efficacy or other clinical outcomes after rehabilitation. There were indications that people with MS who have a better perception of their physical level, less perceived fatigue, and more overall activity are more likely to respond to physiotherapy with a meaningful increase in daily steps.
The sample consisted of 41 people with an average age of 54 years who were moderately to severely affected by MS with heterogeneous disease duration. As documented by other authors,9 most people with MS do not achieve the recommended number of steps per day (7500 steps per day) or sufficient levels of MVPA (150 minutes per week). Our participants took approximately 4200 steps and had 6 minutes of MVPA per day at the beginning of the rehabilitation period.36 Inpatients took fewer steps per day than outpatients, although the difference was not statistically different; however, inpatients had higher disability than outpatients and after rehabilitation they may have had fewer opportunities to engage in PA.
Aim 1: Impact of Rehabilitation on PA Levels
Daily steps and MVPA increased slightly after motor rehabilitation, but the observed increases were neither statistically nor clinically relevant. Motl and colleagues28 suggested that the minimum change in daily steps reflecting a real change in walking ability is 800 steps per day, whereas in real life or when associated with clinical anchors, the MCID ranges from 1500 to 2500 steps per day.24 Similarly, a clinically relevant increase in MVPA has been suggested to be 30 minutes or more per day for healthy people.37
To date, information on the effect of motor rehabilitation on daily steps and MVPA in people with MS is lacking. However, without specific interventions, a 2013 study found that the PA levels of people with MS decreased over a 30-month period,38 and another study demonstrated that 20 people with MS minimally increased their steps per day and time spent in MVPA over a 12-week period.39 These changes were not significant in either clinical or statistical terms, which is consistent with our results.
Physiotherapy interventions usually aim to improve balance and walking ability. In our study, the participants tended to walk faster and for longer distances and were less fatigued and more confident in their walking ability after rehabilitation. However, these improvements were not statistically significant, and they were also much lower than the MCID reported in scientific literature.40 The same was true for domains related to PA (GLTEQ) and QOL, suggesting that usual-care balance and mobility physiotherapy is insufficient to improve subjective perception of the ability to deal with MS issues. Physiotherapy programs are typically not tailored to address the specific needs related to PA or self-efficacy, and these aspects are frequently neglected by other health care professionals.41 A more comprehensive rehabilitative approach incorporating strategies to improve self-efficacy and address barriers to PA may be necessary.
As expected, people with MS with higher disability showed smaller increases in daily steps, self-efficacy, and MVPA. Increased attention to self-efficacy and PA barriers by health care professionals could increase PA and improve overall health.19,42
Aim 2: Characteristics That Predict PA Changes After Rehabilitation
The secondary analysis provides clinicians with information on which people with MS were most likely to increase PA levels following a usual-care gait and balance rehabilitation period. Perceived fatigue at baseline was the most significant predictor of a positive change. For each point less on the FSS at T0 (being less fatigued), the probability of increasing the number of daily steps by at least 800 steps after rehabilitation increased by 3%. Results of a recent large study showed that fatigued people with MS took fewer daily steps and had fewer MVPA minutes than nonfatigued people with MS, suggesting that perceived fatigue is an important aspect to consider when planning interventions.43 Similar findings were reported by other authors regarding the relationship between perceived fatigue and PA.44,45 The data indicate that fatigue in people with MS significantly affects daily activities and overall QOL and also limits their ability to engage in and sustain PA. Fatigue can be a barrier to achieving the benefits of rehabilitation programs, and in light of our findings, addressing this aspect of MS during physiotherapy appears to be essential.
In our final model, the overall PA and the SF-12 PCS results were also significant, indicating that for each minute of overall PA at baseline and for each point of the SF-12 PCS, the probability of being a responder after rehabilitation increased by 1% and 2%, respectively. This means that a better physical QOL is indicative of a better rehabilitation outcome. Intrinsically, it is possible that people with MS who are more engaged and less fatigued before the start of the rehabilitation reap more benefits from motor rehabilitation and transfer these improvements into increased PA throughout the rest of the day. Including motivational and fatigue management during the rehabilitation period could lead to further improvements in PA levels.
Finally, the model indicated that a higher dose (ie, being an inpatient) results in a higher probability to improve after rehabilitation. This finding is consistent with a meta-analysis showing that the effectiveness of balance and mobility rehabilitation is modulated by the intensity of the rehabilitation.16
General Considerations
Overall, balance and gait physiotherapy were found to be ineffective in changing PA behavior and self-efficacy in people with MS. This is a critical point, as it has been observed that people with MS often find it difficult to achieve PA levels that would lead to general health benefits, particularly regarding mobility, fatigue management, mood, cognitive functions, and QOL.46,47 Moreover, although the study focused on PA levels following physiotherapy, no significant effect was observed on mobility measures. This is consistent with Bowman et al, who examined interventions for people with MS undergoing physiotherapy and found that only about 25% of study participants achieved clinically meaningful improvements in mobility outcomes.48
Clinicians should emphasize the importance of PA for people with MS, encouraging even small steps toward a more active lifestyle; as the World Health Organization notes, “Any activity is better than none.” The Behavioral Intervention for Physical Activity in Multiple Sclerosis study (NCT03490240) demonstrated a clinically important 800 steps per day difference, as well as a clinically important increase in minutes spent in MVPA following a behavioral change intervention delivered over a 6-month period.49,50 This increase was sustained at a 6-month follow-up, indicating that people with MS can change their PA behavior and sustain it in response to a focused intervention.
In our observational study, despite the rehabilitation goal of improving gait and balance, our participants’ daily PA did not change following the rehabilitation period, and there was no impact on self-efficacy. Based on our findings and growing evidence on the effectiveness of behavioral interventions, integrating standard rehabilitation with tailored motivational strategies could be a cost-effective approach to increasing PA levels in people with MS. Physiotherapists and other health professionals may need to be trained in using motivational strategies to help people with MS achieve PA goals outside of their therapy sessions.
Limitations
This study has potential limitations. First, to detect the mean difference of 800 steps with the variability found in this study, an α-level of 0.05 and a power of 0.80, a power analysis indicated that a sample size of 89 participants would be required. Second, although Fitbit Versa’s wide availability may increase participant acceptability, it may not provide high accuracy in detecting PA parameters.51 Third, inpatients may be hospitalized for multidimensional interventions, such as speech therapy, cognitive therapy, or medication management, and not exclusively for physiotherapy. By not collecting this information, we may have missed some other beneficial consequences of being an inpatient.
CONCLUSIONS
Our study results suggest that a period of usual-care physiotherapy was not associated with significant improvement in daily PA or self-efficacy levels in people with MS. Prerehabilitation factors, including perceived fatigue, overall PA, and physical health perception, should be considered and addressed because they impact rehabilitation efficacy. For the well-being of people with MS, health care providers should endorse the benefit of being physically active and should motivate and educate people with MS to overcome barriers and increase their self-efficacy skills as part of a rehabilitation goal.
PRACTICE POINTS
- Usual-care physiotherapy alone is not effective in increasing engagement in physical activity or self-efficacy level for people with multiple sclerosis.
- Perceived fatigue predicts physical activity change after rehabilitation.
Conflicts of Interest: All authors report no conflicts of interest with any financial organization regarding the manuscript.
Funding: This research was funded by the Italian Ministry of Health, Ricerca Corrente 2023.
References
Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi:10.1177/1352458520970841
Barten LJ, Allington DR, Procacci KA, Rivey MP. New approaches in the management of multiple sclerosis. Drug Des Devel Ther. 2010;4:343-366. doi:10.2147/DDDT.S9331
Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541-556. doi:10.1097/HCO.0000000000000437
Moumdjian L, Smedal T, Arntzen EC, et al. Impact of the COVID-19 pandemic on physical activity and associated technology use in persons with multiple sclerosis: an international RIMS-SIG mobility survey study. Arch Phys Med Rehabil. 2022;103(10):2009-2015. doi:10.1016/j.apmr.2022.06.001
Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis-time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. 2019;19(11):88. doi:10.1007/s11910-019-1002-3
Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017;16(10):848-856. doi:10.1016/S1474-4422(17)30281-8
Sasaki JE, Bertochi GFA, Meneguci J, Motl RW. Pedometers and accelerometers in multiple sclerosis: current and new applications. Int J Environ Res Public Health. 2022;19(18):11839. doi:10.3390/ijerph191811839
Pau M, Caggiari S, Mura A, et al. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: comparison with patient-based measure. Mult Scler Relat Disord. 2016;10:187-191. doi:10.1016/j.msard.2016.10.007
Casey B, Coote S, Galvin R, Donnelly A. Objective physical activity levels in people with multiple sclerosis: meta-analysis. Scand J Med Sci Sports. 2018;28(9):1960-1969. doi:10.1111/sms.13214
Macdonald E, Buchan D, Cerexhe L, Renfrew L, Sculthorpe N. Accelerometer measured physical activity and sedentary time in individuals with multiple sclerosis versus age matched controls: a systematic review and meta-analysis. Mult Scler Relat Disord. 2023;69:104462. doi:10.1016/j.msard.2022.104462
Razazian N, Kazeminia M, Moayedi H, et al. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):93. doi:10.1186/s12883-020-01654-y
Kohn CG, Coleman CI, Michael White C, Sidovar MF, Sobieraj DM. Mobility, walking and physical activity in persons with multiple sclerosis. Curr Med Res Opin. 2014;30(9):1857-1862. doi:10.1185/03007995.2014.921147
Torchio A, Fusari G, Perini G, et al. Objective and subjective measures of daily physical activity in persons with multiple sclerosis beginning a rehabilitation regime: a cross-sectional study. Mult Scler Relat Disord. 2022;68:104394. doi:10.1016/j.msard.2022.104394
Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil. 2017;98(2):353-367. doi:10.1016/j.apmr.2016.04.016
Callesen J, Cattaneo D, Brincks J, Kjeldgaard Jørgensen ML, Dalgas U. How do resistance training and balance and motor control training affect gait performance and fatigue impact in people with multiple sclerosis? a randomized controlled multi-center study. Mult Scler. 2020;26(11):1420-1432. doi:10.1177/1352458519865740
Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019;1(1):CD012732. doi:10.1002/14651858.CD012732.pub2
Corrini C, Gervasoni E, Perini G, et al. Mobility and balance rehabilitation in multiple sclerosis: a systematic review and dose-response meta-analysis. Mult Scler Relat Disord. 2023;69:104424. doi:10.1016/j.msard.2022.104424
Kuendig S, Kool J, Polhemus A, Schallert W, Bansi J, Gonzenbach RR. Three weeks of rehabilitation improves walking capacity but not daily physical activity in patients with multiple sclerosis with moderate to severe walking disability. PLoS One. 2022;17(9):e0274348. doi:10.1371/journal.pone.0274348
Nedeljkovic U, Raspopovic ED, Ilic N, Vujadinovic ST, Soldatovic I, Drulovic J. Effectiveness of rehabilitation in multiple sclerosis relapse on fatigue, self-efficacy and physical activity. Acta Neurol Belg. 2016;116(3):309-315. doi:10.1007/s13760-015-0563-4
Fjeldstad C, Pardo G. Self-efficacy, physical activity and QOL in people with MS. J Neurol Neurophysiol. 2014;5(2):194. doi:10.4172/2155-9562.1000194
Motl RW, McAuley E, Doerksen S, Hu L, Morris KS. Preliminary evidence that self-efficacy predicts physical activity in multiple sclerosis. Int J Rehabil Res. 2009;32(3):260-263. doi:10.1097/mrr.0b013e328325a5ed
Szczepańska-Gieracha J, Mazurek J. The role of self-efficacy in the recovery process of stroke survivors. Psychol Res Behav Manag. 2020;13:897-906. doi:10.2147/PRBM.S273009
Young CA, Mills R, Rog D, et al. Quality of life in multiple sclerosis is dominated by fatigue, disability and self-efficacy. J Neurol Sci. 2021;426:117437. doi:10.1016/j.jns.2021.117437
Bowman T, Mestanza Mattos FG, Salvalaggio S, et al. Classification and quantification of physical therapy interventions across multiple neurological disorders: an Italian multicenter network. J Clin Med. 2023;12(20):6483. doi:10.3390/jcm12206483
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi:10.1186/1471-2377-14-58
Balto JM, Kinnett-Hopkins DL, Motl RW. Accuracy and precision of smartphone applications and commercially available motion sensors in multiple sclerosis. Mult Scler J Exp Transl Clin. 2016;2:2055217316634754. doi:10.1177/2055217316634754
Motl RW, Pilutti LA, Learmonth YC, Goldman MD, Brown T. Clinical importance of steps taken per day among persons with multiple sclerosis. PLoS One. 2013;8(9):e73247. doi:10.1371/journal.pone.0073247
Bonino S, Graziano F, Borghi M, Marengo D, Molinengo G, Calandri, E. The Self-Efficacy in Multiple Sclerosis (SEMS) scale: development and validation with Rasch analysis. Eur J Psycho Assess. 2018;34(5):352-360. doi:10.1027/1015-5759/a000350
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121-1123. doi:10.1001/archneur.1989.00520460115022
Robinson D Jr, Zhao N, Gathany T, Kim LL, Cella D, Revicki D. Health perceptions and clinical characteristics of relapsing-remitting multiple sclerosis patients: baseline data from an international clinical trial. Curr Med Res Opin. 2009;25(5):1121-1130. doi:10.1185/03007990902797675
Sikes EM, Richardson EV, Cederberg KJ, Sasaki JE, Sandroff BM, Motl RW. Use of the Godin Leisure-Time Exercise Questionnaire in multiple sclerosis research: a comprehensive narrative review. Disabil Rehabil. 2019;41(11):1243-1267. doi:10.1080/09638288.2018.1424956
Paltamaa J, West H, Sarasoja T, Wikström J, Mälkiä E. Reliability of physical functioning measures in ambulatory subjects with MS. Physiother Res Int. 2005;10(2):93-109. doi:10.1002/pri.30
Scalzitti DA, Harwood KJ, Maring JR, Leach SJ, Ruckert EA, Costello E. Validation of the 2-Minute Walk Test with the 6-Minute Walk Test and other functional measures in persons with multiple sclerosis. Int J MS Care. 2018;20(4):158-163. doi:10.7224/1537-2073.2017-046
Guicciardi M, Carta M, Pau M, Cocco E. The relationships between physical activity, self-efficacy, and quality of life in people with multiple sclerosis. Behav Sci (Basel). 2019;9(12):121. doi:10.3390/bs9120121
Huynh TLT, Silveira SL, Motl RW. Physical activity and quality of life in persons newly diagnosed with multiple sclerosis: a cross-sectional study. Arch Phys Med Rehabil. 2023;104(11):1820-1826. doi:10.1016/j.apmr.2023.04.004
Kalb R, Brown TR, Coote S, et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler. 2020;26(12):1459-1469. doi:10.1177/1352458520915629
Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013;94(12):2342-2348. doi:10.1016/j.apmr.2013.07.011
Motl RW, McAuley E, Sandroff BM. Longitudinal change in physical activity and its correlates in relapsing-remitting multiple sclerosis. Phys Ther. 2013;93(8):1037-1048. doi:10.2522/ptj.20120479
Motl RW, McAuley E, Wynn D, Vollmer T. Lifestyle physical activity and walking impairment over time in relapsing-remitting multiple sclerosis: results from a panel study. Am J Phys Med Rehabil. 2011;90(5):372-379. doi:10.1097/PHM.0b013e31820f95e1
Hobart J, Blight AR, Goodman A, Lynn F, Putzki N. Timed 25-foot walk: direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology. 2013;80(16):1509-1517. doi:10.1212/WNL.0b013e31828cf7f3
Pedullà L, Santoyo-Medina C, Novotna K, et al. Physical activity in multiple sclerosis: meeting the guidelines at the time of the COVID-19 pandemic. J Neurol Phys Ther. 2023;47(2):112-121. doi:10.1097/NPT.0000000000000430
Motl RW, Snook EM, McAuley E, Gliottoni RC. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health. 2006;29(6):597-606. doi:10.1002/nur.20161
Cederberg KLJ, Jeng B, Sasaki JE, Motl RW. Physical activity and sedentary behavior timing in fatigued and nonfatigued adults with multiple sclerosis. Arch Phys Med Rehabil. 2022;103(9):1758-1765. doi:10.1016/j.apmr.2021.12.022
Blikman LJ, van Meeteren J, Horemans HL, et al. Is physical behavior affected in fatigued persons with multiple sclerosis? Arch Phys Med Rehabil. 2015;96(1):24-29. doi:10.1016/j.apmr.2014.08.023
Kalron A, Menascu S, Frid L, Aloni R, Achiron A. Physical activity in mild multiple sclerosis: contribution of perceived fatigue, energy cost, and speed of walking. Disabil Rehabil. 2020;42(9):1240-1246. doi:10.1080/09638288.2018.1519603
Stephens S, Shams S, Lee J, et al. Benefits of physical activity for depression and fatigue in multiple sclerosis: a longitudinal analysis. J Pediatr. 2019;209:226-232.e2. doi:10.1016/j.jpeds.2019.01.040
Bowman T, Mestanza Mattos FG, Salvalaggio S, et al. Classification and quantification of physical therapy interventions across multiple neurological disorders: an Italian multicenter network. J Clin Med. 2023;12(20):6483. doi:10.3390/jcm12206483
Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008;14(1):129-135. doi:10.1177/1352458507080464
Motl RW, Sandroff BM, Wingo BC, et al. Phase-III, randomized controlled trial of the behavioral intervention for increasing physical activity in multiple sclerosis: Project BIPAMS. Contemp Clin Trials. 2018;71:154-161. doi:10.1016/j.cct.2018.06.017
Lavelle G, Norris M, Flemming J, et al. Validity and acceptability of wearable devices for monitoring step-count and activity minutes among people with multiple sclerosis. Front Rehabil Sci. 2022;2:737384. doi:10.3389/fresc.2021.737384







