Publication
Research Article
International Journal of MS Care
Five-Year Follow-up of a Longitudinal Cohort Study of the Effectiveness of Functional Electrical Stimulation for People with Multiple Sclerosis
Abstract
Background:
Few studies have examined the long-term consequences of using peroneal nerve functional electrical stimulation (FES) for people with multiple sclerosis (MS). This study examines orthotic effects on a longitudinal cohort and explores additional benefits of FES on self-reported measures such as joint pain.
Methods:
One hundred forty-five people with foot drop and MS were included (mean age, 52 [range, 28–74] years). Orthotic effects, unassisted walking speed, and clinically important differences (ie, ≥0.05 and ≥0.10 m/s) were derived from walking speed over 10 m. Visual analogue scales examined joint pain, walking effort, trips, confidence, and quality of life. Measures were taken on day 1, after 6 months, and at 2, 3, 4, and 5 years.
Results:
A significant difference was found overall for walking with FES compared with walking without FES for the 5-year period (P < .001). Despite a significant decline in overall unassisted walking speed at baseline (0.58 m/s) compared with 5 years later (0.46 m/s) (P < .001), participants achieved an orthotic effect with (0.52 m/s) versus without (0.46 m/s) FES after 5 years (P < .001). A significant decrease in joint pain was found after 6 months compared with day 1 (P = .004), which was maintained after 5 years (P < .001).
Conclusions:
Despite progression of MS, long-term users of FES still benefit from an orthotic effect after using FES for 5 years. The study highlights the need for further work to assess the perceived benefits of FES regarding the experience of joint pain.
Lower-limb function is perceived by people with multiple sclerosis (MS) to be of primary importance compared with other types of body function measured on the United Kingdom Disability Scale.1 Lower-limb paralysis or weakness, consisting of a reduced ability to dorsiflex, is commonly referred to as foot drop. Foot drop is associated with an increased likelihood to trip and fall along with a reduction in walking speed. Functional electrical stimulation (FES) of the peroneal nerve seeks to restore more natural functional movement during gait by enabling dorsiflexion.2
The term orthotic effect is used to describe the immediate difference in walking speed between unassisted walking and FES walking. The existence of an orthotic effect through using FES over a short period is well established.3–6 In an initial case series that included 23 people with MS, researchers found a 16% increase in walking speed after 4.5 months of using FES.3 The findings from the case series were further supported by a two-group randomized controlled trial (N = 44) in which a significant improvement in orthotic effect was found after 12 weeks (6%). The observed improvement in orthotic effect continued to be significant after 18 weeks (9%).4 A significant orthotic effect has also been found in a long-term observational study that included a progressive group of 32 people with MS and familial spastic paraplegia.5 Researchers found a significant improvement in orthotic effect after 3 months of using FES (7%), which was still significant after 11 months (4%). The authors note that many participants did not continue to the end of the study due to the distances involved in traveling to the clinic, which may have influenced the results.5 In contrast to previous studies, a more recent observational study found a higher orthotic benefit from FES, with a 27% increase in walking speed.6 The inconsistency in the size of the orthotic effect could potentially be influenced by the level of disability of the participants, the size of the cohort, the different lengths of follow-up, and the way that walking speed was measured. Encouragingly, although there is an overall reduction in walking speed, which is most likely associated with a gradual deterioration due to MS, these studies suggest that there is a significant orthotic effect after the use of FES.
There has been little examination of the use of FES for people with MS over a long-term period of more than a year. One observational study that included 39 people with MS found the average time of FES use was 5 years, with 11 participants continuing for the full 11.1 years.7 A large and substantially clinically meaningful orthotic effect was found, with participants walking 29% faster using FES compared with unassisted walking. It was also found that 40% of participants had improved their functional walking category in terms of an orthotic effect. The results of this study are encouraging, particularly because participants were followed up for an extended period, indicating the gains that can be made by some people with MS. However, walking speed measures were averaged across the entire period rather than at set time points. Therefore, it was not representative of the gains that are made for most participants included in the study around 5 years.
Although walking speed is commonly accepted as the best overall measure of gait improvement, participants often report wider benefits of using FES. Confirmation of patient-reported benefits has been found in a variety of studies of FES use for people with MS. These benefits include reduced walking effort,3,8,9 improved quality of life,8 improved confidence, decreased tripping,9 and fewer falls.10 Studies in these areas are potentially limited by examining short-term use of FES. Examining the wider reported benefits of FES over the average amount of time that FES is used by people with MS would be more representative of any additional gains achieved.
Compensatory gait patterns after foot drop are common and may lead to joint pain during and after walking. An area that has been neglected in the FES literature is self-reported changes in the experience of pain during walking by FES users. People with foot drop may engage in compensatory gait patterns, such as hip-hitching and circumduction of the leg, to prevent the foot from scuffing on the ground, increasing the likelihood of a trip and fall. This behavior may lead to increased pain in joints and referred pain to other areas of the body. One of the presumed benefits of FES is the establishment of a more “natural,” functional gait pattern, which should lead to a reduction in pain.
The aim of the present study was to examine whether there were significant and clinically meaningful orthotic effects in people with MS after the use of FES. It was expected that significant and clinically meaningful orthotic effects would be found at regular intervals over a 5-year period. The study also sought to explore the potential impact of FES on joint pain and to examine other wider benefits reported by patients with MS over a 5-year period.
Methods
Participants
One hundred forty-five people with MS-related foot drop (mean age, 52 [range, 28–74] years; 19 bilateral, 60 left limb, 63 right limb, three not documented) participated in this study. Fifty-four participants had an ankle-foot orthosis (AFO) before the start of treatment, and seven had received botulinum toxin treatment for spasticity (this was not in the 3 months before the start of the study). Seventeen participants did not use a walking aid, 113 used one stick, ten used a frame, one used a wheelchair, and four were not documented. Forty-one people were working before treatment. Patients meeting the inclusion criteria were referred by general practitioners or by consultants in the National Health Service in the United Kingdom to a specialist outpatient FES clinic. Exclusion criteria consisted of the inability to walk 10 m with the assistance of a walking aid, poorly controlled epilepsy, and fixed skeletal deformities. Patients were excluded if they received “no functional benefit” from FES at the initial assessment. This may have included patients who did not achieve any ankle dorsiflexion from electrical stimulation and patients with mild occasional problems with their gait that did not yet require an assistive device. It would also include patients who did not achieve improvement in their walking in terms of walking speed, heel strike, and foot clearance during the swing phase from using FES. This may have led to patients being referred to another service (eg, referral for an AFO).
Patients were also excluded if the clinician judged that their AFO equipment was adequate for providing the support required for safe walking and patient choice. For patients who were using an AFO, the clinician made a comparison between AFO and FES by examining walking speed, heel strike, and foot clearance during the swing phase. If these were found to be similar between AFO and FES, the decision as to which device the patient used was made through patient choice. Patients were further excluded if they experienced sensitivity to the sensation of stimulation or had a recent injury, fracture, or surgery; major skin conditions; or cancerous tissue in proximity to the site of stimulation. Ethical approval was not required as the data were collected as part of a routine clinical audit. This practice is consistent with guidelines that have been developed to enable the differentiation of research, clinical audit, and service evaluation in the United Kingdom.11 The study was conducted following the guidelines of the Declaration of Helsinki. Patients included in the study provided consent for the storage and use of their data for the purposes of audit and publication.
Clinical Procedure
Participants were set up over 1 day using the ODFS Pace FES device (Odstock Medical Ltd, Salisbury, UK). All the devices were fitted by the same physiotherapist (C.S.). Participants were mostly funded through the local health authority, with some privately funded participants. The full clinical procedure is described in a 2015 article by Street et al.6 Participants who were followed up after 6 months were provided with the opportunity for further appointments before and after 12 months of treatment if required. Data were collected on day 1, at 6 months, and at 2, 3, 4, and 5 years.
Ten-meter walking speed was measured using a random order of FES and unassisted 10-m walks. The average was taken of three walks with FES and three walks without FES without a rest period. A minimal clinically important difference in walking speed was defined as 0.05 m/s or greater and a substantial difference as 0.10 m/s or greater.12 Functional walking categories were also derived from the walking speed data to define the baseline and subsequent level of impairment of participants (<0.4 m/s = household, 0.4–0.58 m/s = most limited community, 0.59–0.79 m/s = least limited community, and ≥0.8 m/s = community walkers).13
Self-reported visual analogue scales (VASs) were used to record joint pain, tripping, confidence, walking effort, quality of life, and spasticity. Individual VAS scores were used as appropriate for each patient. Baseline VAS scores were recorded without FES. Subsequent recordings were with FES. The VAS scores were presented from 0 to 10 as whole numbers on a vertical line with no intervals. People chose whole numbers, including “0.” High scores for confidence and quality of life represent improvement, and the inverse is true for joint pain, spasticity, trips, and walking effort. The scoring was presented as such to ensure that people thought about their answers and did not score a particular number across all categories. The VAS questions are presented in Appendix S1, which is published in the online version of this article at ijmsc.org.
Skin irritation was recorded, and advice was given for skin care. Hypoallergenic electrodes were started and used thereafter as soon as the skin was healed, or adjacent to the irritated area, so as to allow participants to continue with device use.
Statistical Analysis
Statistical analysis was completed using NCSS 10 statistical software (NCSS LLC, Kaysville, UT). The data were explored using histograms and box plots and were found to be from a normal distribution. A repeated-measures analysis of variance was used to examine the data over 5 years and determine whether there was an overall significant treatment effect between walking with FES and walking unassisted. Planned comparisons were conducted using t tests. A significance level of P < .05 was used. The planned comparisons included an initial orthotic effect, measured as the difference between walking unassisted and with FES at baseline, and a continuing orthotic effect, measured as the difference between unassisted walking and walking with FES after 5 years. For the VASs, Friedman tests were used to determine whether there was an overall significant difference for each VAS. Wilcoxon signed rank tests were used to compare day 1 with 6 months later and day 1 with 5 years later.
Results
Seventy-five people were excluded before starting the study (Figure 1), 58 of whom were unable to complete the 10-m walking speed protocol (eg, unable to walk without FES). These patients were still able to benefit from FES and went on to start FES treatment outside the study (eg, electrical exercise stimulation to strengthen muscles for assistance in transfers). Of the 145 people with MS who started the study, 21 (14%) had issues with skin irritation over the 5-year period but were able to continue with FES after receiving appropriate care. Eighty-three participants were discharged from treatment over 5 years (Figure 1). Table 1 describes the reasons for participants to be discharged at each follow-up period over the 5-year period. Most participants who discontinued treatment were discharged within the first 2 years. The overall predominant reason for discharge was due to MS-related deterioration in function (n = 45). The data for 24 participants were not available for the final analysis at 5 years although they were still using FES; the reasons for the data being unavailable are provided in Figure 1. For the final analysis, 38 participants were included (Figure 1).
Flow diagram of participant progress through study stages
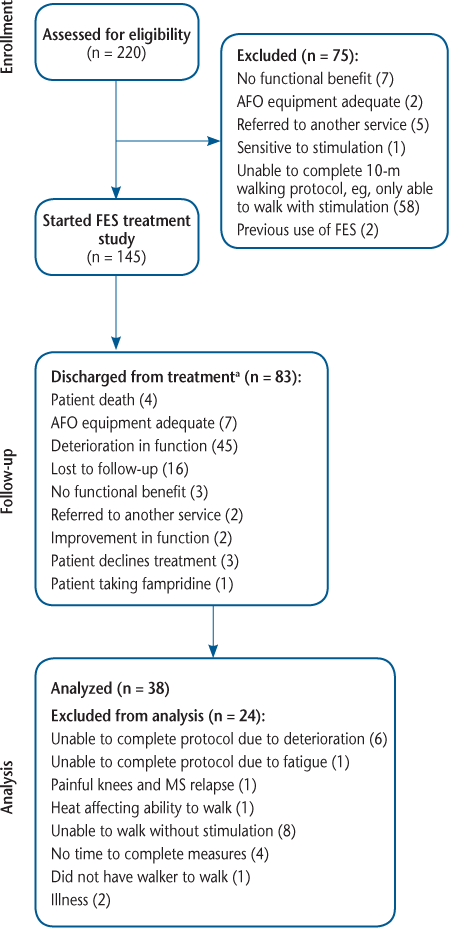
Reasons for patients to be discharged at each follow-up period (n = 83)
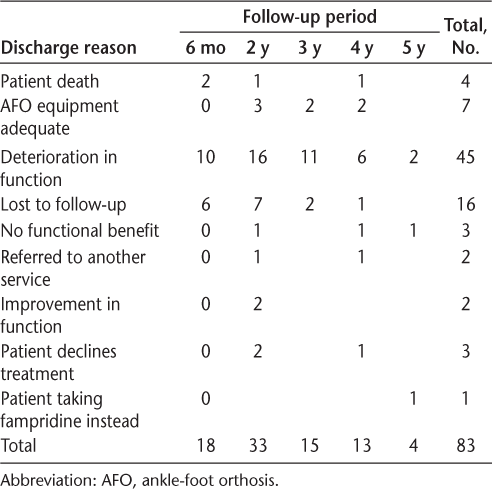
The data were normally distributed, and 14% of the data collected over the 5-year period were missing. Missing data imputation was judged suitable to further examine the data using a multivariate normal procedure. A significant overall main effect was found for an increase in walking speed with FES compared with unassisted walking (F 5,37 = 48.19, P < .001) and a significant main effect for time (F 5,37 = 48.19, P < .001). A significant difference was found for initial orthotic effect at baseline (t 37 = −4.62, P < .001), and a significant difference for continuing orthotic effect was found with FES (0.52 m/s) and without FES (0.46 m/s) after 5 years (t 37 = −5.70, P < .001). A significant decline in overall unassisted walking speed at baseline (0.58 m/s) compared with 5 years later (0.48 m/s) was found (t 37 = 4.15, P < .001).
During the study, participants who were unable to complete the walking speed protocol without FES completed it with FES. After 6 months there were four participants who walked with FES at a mean (SD) speed of 0.4 (0.2) m/s (n = 4); 2 years, 0.18 (0.08) m/s (n = 3); 3 years, 0.32 (0.19) m/s (n = 4); 4 years, 0.2 (0.07) m/s (n = 4); and 5 years, 0.45 (0.16) m/s (n = 8).
Table 2 shows the means of the differences for the planned comparisons. A minimal clinically important difference (≥0.05 m/s) derived from walking speed was found between walking unassisted and walking with FES at baseline, after 6 months, and at 3, 4, and 5 years. Table 2 shows that mean walking speed with FES at 4 years (0.60 m/s) was similar to mean walking speed at baseline without FES (0.58 m/s).
Mean walking speed for walking with and without FES over 5 years and means of differences (n = 38)
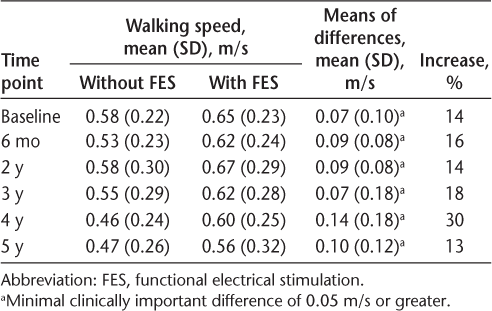
Table 3 shows the number of participants in each functional walking category before stimulation and details the number of participants who improved their functional walking by one category or more while wearing FES at baseline, after 6 months, and at 2, 3, 4, and 5 years. The category with the highest proportion of participants was the most limited walkers. On day 1, 14 of 38 (37%) improved on their functional walking by one category or more. After 6 months, 16 of 38 (42%) improved on their functional walking by one category or more. After 5 years, 11 of 38 (29%) improved on their functional walking by one category or more. Community walkers were not able to show an improvement in functional walking category unless they had a deterioration in their unassisted walking speed.
Number of patients who increased functional ambulation by one category or more while using stimulation from day 1 to 5 years (n = 38)
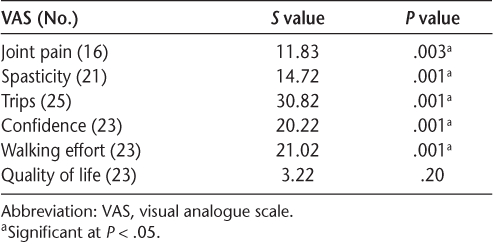
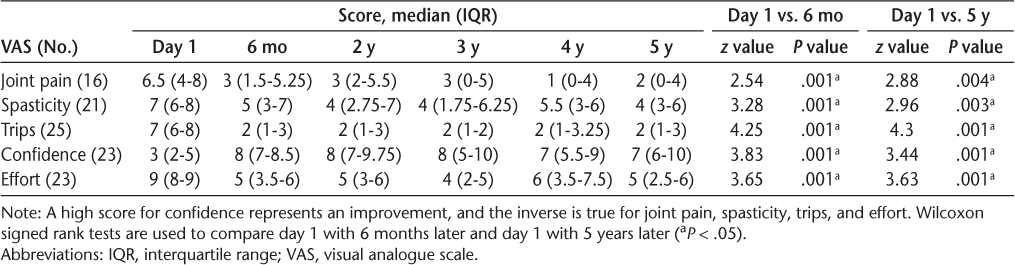
An overall significant difference between repeated measurements was found for all the VASs except quality of life (Table 4). Table 5 displays the median (interquartile range) VAS scores (possible range, 0–10) for joint pain, spasticity, trips, confidence, and walking effort over 5 years. Table 5 also displays a comparison between day 1 and 6 months later and between day 1 and 5 years later (P < .05). The results revealed that joint pain, spasticity, trips, confidence, and walking effort were all significantly improved after 6 months and after 5 years with FES compared with day 1 without FES.
Nonparametric overall Friedman test for VASs (adjusted for ties)
VAS Scores (0–10) from day 1 to 5 years
Discussion
To our knowledge, the present study provides the largest long-term follow-up of people with MS using FES. The findings suggest that FES provides a clinically meaningful immediate benefit or initial orthotic effect on day 1. People with MS also gain a continuing orthotic effect after 6 months and a long-term continuing orthotic effect after 5 years of using FES. Despite a significant reduction in the speed of unassisted walking, participants were still able to gain a significant orthotic effect after 5 years of using the device. After 6 months, 42% of people improved on their functional walking by one category or more while using FES. After 5 years, 29% of participants were still able to improve on their functional walking by one category or more. The findings are consistent with previous research that has found a continuing orthotic effect for people with MS through the use of FES.3–7
Participants also self-reported a variety of improvements to walking in other areas; most interestingly, this included a reduction in pain experienced in joints that has not been reported previously. A reduction in joint pain could be used to suggest that FES helps to decrease harmful compensatory gait patterns that may lead to joint pain. Another potential explanation for a reduction in joint pain is research supporting the use of electrical stimulation in producing an analgesic effect by activating opioid receptors.14 The technique is most commonly referred to as transcutaneous electrical nerve stimulation. It would be beneficial to conduct further research examining the specific body areas where people experience pain along with the intensity. It would also be useful to identify whether there is a correlation between identified qualitative changes in gait and self-reported pain outcomes.
The other self-reported qualitative measures, which examined spasticity, trips, confidence, walking effort, and quality of life, found significant improvements over a 6-month period of using FES, which is consistent with other studies that have been conducted on the short-term use of FES. These improvements include reduced walking effort,3,11,12 better quality of life,8 improved confidence, decreased tripping,9 and fewer falls.10 Self-reported quality of life was not found to be significantly improved after 5 years compared with baseline, which is likely to be expected due to the progressive nature of MS. In addition, the quality-of-life measure was the only VAS scale that did not specifically relate to walking. Interestingly, the present study suggests that, despite the progressive nature of MS, FES continued to have a long-term impact in terms of reduced walking effort, improved confidence, decreased tripping, and fewer falls. In particular, a reduction in trips and falls has consequences for safety and limitation of future injury. Through reducing hospital visits and the associated reduction in habitual physical activity that may cause further complications to health, the long-term health and economic consequences are far reaching.
This study has some limitations. The study started with 145 participants; after 5 years, the data from only 38 of these participants were available for analysis. Therefore, there is a potential for attrition bias in the study. Unavoidably in any study examining a progressive disorder, dropout numbers are high, which makes research in this area problematic. As expected, the predominant reason for discharge from treatment was deterioration in function. Note that some participants were on a waiting list for treatment for extended periods and, therefore, the dropout rate from treatment is not necessarily representative of the amount of time that participants could benefit from FES treatment.
During the study, several people who were still using FES were excluded from the analysis because they were unable to complete the walking speed protocol. Some of these individuals were unable to walk without FES. Further research would benefit from using other measures, such as the 2-Minute Walk Test. Using this test would enable researchers to avoid the floor effects encountered through use of the 10-m timed walking test. Alternatively, participants who cannot complete the 10-m walk trial could have their walking speed measured over the distance they could walk. Distance could be measured afterward, allowing the reporting of walking speed and distance for these participants. This method of analysis would circumvent the need to include a 2-Minute Walk Test.
The use of self-reported VAS scores to measure quality of life, trips, walking effort, confidence, joint pain, and spasticity may be limited by a lack of detail compared with other outcome measures. Nevertheless, the simplicity of the VAS scores provides an efficient means of exploring new areas and confirming established areas in a busy, time-restrained clinical setting with participants who are likely to fatigue quickly.
The implications for clinical practice from the present study support the use of FES to encourage self-management with minimal input from a clinician. The structure of care with annual reviews allows for the clinician to adjust stimulation parameters accordingly to any changes that may have occurred in the condition. It is also important for the clinician to use a holistic approach and take into consideration comorbidities, allowing for the opportunity for signposting and planning for further changes. The structure of care using annual reviews is an effective use of resources. With minimal intervention, one FES clinician can effectively manage a large number of participants, making treatment more patient led.
In conclusion, despite progression of MS, long-term users of FES still benefit from an orthotic effect after using FES for 5 years. The study highlights the need for further work to assess the perceived benefits of FES in terms of the experience of joint pain. The findings also highlight the need to explore the potential benefit to people who are at a more progressed stage of their mobility, particularly those unable to walk without FES.
PRACTICE POINTS
After 5 years, some people with MS continued to exhibit improved walking speed when using a functional electrical stimulation (FES) device for foot drop, compared with walking without FES (orthotic effect), despite progression of walking impairment over time.
It is helpful to assess patients who use FES for foot drop annually to make necessary adjustments, troubleshoot any issues, and plan and signpost for any changes using a holistic patient-led care approach.
Measuring walking speed and distance with and without FES is important to monitor functional outcomes. Self-reported outcomes, such as pain, trips, perceived spasticity, and walking effort, can also provide useful information.
Financial Disclosures
The authors declare no conflicts of interest.
References
Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold S. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991.
Carnstam B, Larsson L, Prevec T. Improvement of gait following functional electrical stimulation, I: investigations on changes in voluntary strength and proprioceptive reflexes. Scand J Rehabil Med. 1977;9: 7–13.
Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–1583.
Barrett C, Mann G, Taylor P, Strike P. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009;15:493–504.
Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–167.
Street T, Taylor P, Swain I. Effectiveness of functional electrical stimulation on walking speed, functional walking category, and clinically meaningful changes for people with multiple sclerosis. Arch Phys Med Rehabil. 2015;96:667–672.
Taylor P, Humphreys L, Swain I. The long-term cost-effectiveness of the use of functional electrical stimulation for the correction of dropped foot due to upper motor neuron lesion. J Rehabil Med. 2013;45:154–160.
Barrett C, Taylor P. The effects of the Odstock drop foot stimulator on perceived quality of life for people with stroke and multiple sclerosis: effects of the Odstock drop foot stimulator. Neuromodulation Technol Neural Interface. 2010;13:58–64.
Bulley C, Mercer TH, Hooper JE, Cowan P, Scott S, van der Linden ML. Experiences of functional electrical stimulation (FES) and ankle foot orthoses (AFOs) for foot-drop in people with multiple sclerosis. Disabil Rehabil Assist Technol. 2015;10:458–467.
Esnouf J, Taylor P, Mann G, Barrett C. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Mult Scler. 2010;16:1141–1147.
Brain J, Schofield J, Gerrish K, et al. A Guide for Clinical Audit, Research and Service Review. London, UK: Healthcare Quality Improvement Partnership; 2011. http://www.ns2.pollenuk.co.uk/documents/HQIP_A-Guide-for-Clinical-Audit-Research-and-Service-Review.pdf. Accessed June 26, 2017.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults: meaningful change and performance. J Am Geriatr Soc. 2006;54:743–749.
Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989.
Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4:197–209.







