Publication
Research Article
International Journal of MS Care
Evaluating Walking in Patients with Multiple Sclerosis
Walking limitations are among the most visible manifestations of multiple sclerosis (MS). Regular walking assessments should be a component of patient management and require instruments that are appropriate from the clinician's and the patient's perspectives. This article reviews frequently used instruments to assess walking in patients with MS, with emphasis on their validity, reliability, and practicality in the clinical setting. Relevant articles were identified based on PubMed searches using the following terms: “multiple sclerosis AND (walking OR gait OR mobility OR physical activity) AND (disability evaluation)”; references of relevant articles were also searched. Although many clinician- and patient-driven instruments are available, not all have been validated in MS, and some are not sensitive enough to detect small but clinically important changes. Choosing among these depends on what needs to be measured, psychometric properties, the clinical relevance of results, and practicality with respect to space, time, and patient burden. Of the instruments available, the clinician-observed Timed 25-Foot Walk and patient self-report 12-Item Multiple Sclerosis Walking Scale have properties that make them suitable for routine evaluation of walking performance. The Dynamic Gait Index and the Timed Up and Go test involve other aspects of mobility, including balance. Tests of endurance, such as the 2- or 6-Minute Walk, may provide information on motor fatigue not captured by other tests. Quantitative measurement of gait kinetics and kinematics, and recordings of mobility in the patient's environment via accelerometry or Global Positioning System odometry, are currently not routinely used in the clinical setting.
Approximately 400,000 people in the United States have multiple sclerosis (MS).1 Although this represents only 0.13% of the total US population, MS is the third most common neurologic diagnosis cited as the cause of disability.2
The International Classification of Functioning, Disability, and Health defines walking as “moving along a surface on foot, step by step, so that one foot is always on the ground, such as when strolling, sauntering, walking forwards, backwards, or sideways.”3 Walking limitations are a key component of disability in patients with MS. Approximately 75% of patients with MS experience clinically significant walking disturbance,4 5 which may be present even in early stages of the disease and in patients with mild disability. A study by Martin and associates6 reported significant abnormalities in temporospatial parameters of gait, such as walking speed and stride length, in patients with recent-onset MS compared with matched controls without the disorder, even in the absence of pyramidal dysfunction. Similarly, Johansson and colleagues7 reported that whereas 89% of patients with moderate Expanded Disability Status Scale (EDSS) scores (4.0–5.5) had walking disability, 22% of those with mild scores (1.0–3.5) also had clinically significant gait deviations.
The importance of walking is increasingly being recognized from the MS patient's perspective.8 9 In a study conducted in 1980, gait and motor disturbances were the primary complaints in 85% of patients with MS.10 In a more recent study in which patients prioritized the importance of 13 bodily functions, lower-limb function was ranked the highest regardless of actual level of disability and disease duration.11 Similarly, among factors affecting quality of life, mobility was given the highest priority by 65% of patients with MS.12 Gait deviations were also shown to be a significant predictor of patient independence, with slower speed, shorter stride length, and decreased distance walked identified as contributing factors to patients' perceptions of their ability to perform activities of daily living.13
In 1994, the lifetime cost of the disease for a patient with MS was estimated to be approximately $2.2 million.14 Nonmedical costs have been identified as a major component of the economic burden of MS.14–17 In particular, productivity losses have been reported to be the single highest contributor to the societal burden associated with MS.16 The contribution of walking limitations to lost productivity was demonstrated by Edgley and coworkers18 in a Canadian study of determinants of unemployment among patients with MS. In this study, participants who were unemployed had significantly more walking limitations than did those who were employed, with these limitations being most frequently cited as the reason for unemployment. These observations were consistent with those of an earlier study that used a statistical technique termed “path analysis” to construct a causal model to explain employment status in patients with MS.19 The model showed that loss of mobility was the major determinant predicting unemployment.
The increased recognition of the importance of walking limitations in the lives of patients with MS leads to a need for regular assessment of walking in order to monitor clinical disease activity and assess the efficacy of symptomatic and rehabilitation therapies. The corollary is that the instruments used for such assessments must satisfy a number of requirements.
Challenges in the Assessment of Walking Performance in MS
General Requirements
In order to allow meaningful interpretation of results, the measurement tools must have demonstrated psychometric properties, such as validity, reliability, and responsiveness to change (Table 1). Additionally, in the clinical setting, particular attention should be paid to feasibility, including costs (eg, license to use the instrument, equipment needed, personnel to complete the assessment and enter data); space requirements (eg, room for a gait course); and time requirements, including responder burden for questionnaires.
Requirements of instruments for assessing walking performance in patients with multiple sclerosis

Generic versus Disease-Specific Instruments
Generic instruments are designed to be used across a broad range of disease states or populations, but their constructs may not measure domains specifically associated with MS. Consequently, generic instruments may be less relevant and responsive to change than disease-specific instruments. Therefore, it is preferable to use disease-specific instruments, or generic instruments that have been used and validated in patients with MS.
Objective Assessment versus Self-Report
Walking is an observable function, and the clinician's preference is often objective assessments based on observation of gait. However, there is no clinician-administered scale or test that captures all aspects of walking. In particular, direct observation is most often performed in the clinic, over a short period of time, and may not reflect the patient's performance over the course of an entire day or week in his or her own environment, as might be detected by a self-report questionnaire.
Challenges Specific to MS
Although there is no aspect of gait disturbance that is specific to MS, unique features of the disease affect the evaluation of walking performance. Because of the variability of lesion distribution in the brain and spinal cord, clinical presentations are heterogeneous, and walking limitations are often the result of multiple impairments. Performance is likely to fluctuate from day to day, or from one time of the day to another, even in the absence of new disease activity, and limitations also occur as a consequence of fatigue.20–22 Furthermore, for many walking tests or scales, the clinical meaningfulness of scores or change in scores has not yet been defined. An additional challenge for determining clinical meaningfulness is the dichotomy between the patient's and the clinican's perceptions of change. For example, a study by Paltamaa et al.23 identified several measures of activity, including walking distance and change in heart rate during the 6-Minute Walk (6MW) test, 10-m gait test velocities, and stride length and cadence, as being among those most responsive as measures of MS deterioration. However, when the participant's perception of change and the clinician's perception of change (EDSS scores) were used as external criteria for deterioration, 51% of the participants showed deterioration as measured by their own perceptions, compared with 26% as rated by the clinician.
Goals and Methods
In clinical practice, there is a growing emphasis on measuring outcomes. In addition, an increasing number of treatments and interventions are available to address walking limitations in patients with MS, leading to a need to identify those patients who might benefit, and the results. Therefore, the purpose of this review is to examine measures of walking that may help in quantifying abnormalities and in monitoring change related to disease progression, or to medical and rehabilitation management. A review of instruments that quantify walking ability in neurologic disorders, focusing on the psychometric properties of these instruments, has been previously published.24 That review recommended that monitoring of total ambulatory activity, defined by total distance or steps covered over a representative period in the patient's usual environment, be adopted as a gold standard for measuring walking activity. Although this type of standard is a desirable goal, it cannot be routinely applied in clinical practice.
Identification of articles pertaining to the assessment of walking in patients with MS was initially made via a PubMed search using the terms “multiple sclerosis AND (walking OR gait OR mobility OR physical activity) AND (disability evaluation)” with a date of publication subsequent to January 2004. Abstracts of the 93 retrieved citations were searched to focus on relevant articles (N = 43) describing or using measurement instruments. The text of relevant articles that were not validation studies was subsequently searched to determine the source of the original instrument description and its validation in MS, and the references of all relevant articles were also searched to identify additional articles and instruments that may have been missed because of limitations in the search terms or publication in periodicals not indexed in PubMed.
Instruments Used to Evaluate Walking in MS
The instruments were classified as clinician-assessed rating scales (usually ordinal measures) and performance tests (usually continuous measures), and patient self-report questionnaires (usually ordinal measures). This list of measurement tools is not exhaustive, but instead focuses on those instruments that are the most relevant to the stated purpose of this article.
Rating Scales
Kurtzke EDSS
The Kurtzke EDSS25 is a disease-specific instrument that has become the gold standard for characterizing disability levels and determining disability progression in patients with MS. The EDSS evaluates impairments at lower scores and disability at higher scores.26 Although a numerical score from 0 to 10 is generated, each step on the ordinal scale does not reflect an equal change in disability.
Walking is assessed in the middle range of the scale (4.5–7.5). Whereas assessment of walking is based on maximum distance walked up to 500 m and use of an assistive device, the latter factor has been given more weight than the former for assigning scores. Maximum distance walked demonstrated an overall correlation with EDSS scores within the EDSS range of 4.5 to 7.0.27 However, wide variability was observed in maximum distance walked within each EDSS level, with patients at a higher level of disability often walking the same maximum distance as those at a lower level of disability, by virtue of their use of a walking aid.
Additional patient variables and environmental factors can affect the maximum distance walked, potentially leading to mischaracterization of disability level or disease progression. Albrecht and colleagues20 reported that there is daily variability in maximum walking distance within a single patient, which can result in an EDSS score that may vary across a range of 1.5 points. The authors also suggested that mean walking speed is a more stable variable than maximum walking distance. Although the reason for the variability in maximum walking distance was not determined, the authors noted that fatigue was a possible cause.
The EDSS was developed prior to the codification of methods for the psychometric validation of assessment instruments. Nevertheless, two studies subsequently evaluated the psychometric properties of the EDSS.28 29 These studies suggested that the psychometric properties of the EDSS may be less than optimal, generally demonstrating low sensitivity, poor reliability, and, most importantly, suboptimal responsiveness to change. In addition, the EDSS is somewhat cumbersome to administer, as it relies on a detailed neurologic examination performed by a trained clinician, in addition to the observation of walking performance over a long distance (up to 500 m). For this reason, in clinical practice, the EDSS is rarely used outside of a neurologic setting and is most often used as an outcome in clinical trials of MS-modifying therapies.
In summary, although the EDSS continues to be a useful instrument for assessing disease severity in MS, it is, at best, only marginally useful as an assessment of walking performance for routine patient management.
Hauser Ambulation Index
The Hauser Ambulation Index (AI)30 is similar to the EDSS in that it is a single-item, ordinal scale that was developed for use in patients with MS. Consequently, many of the limitations of the EDSS also apply to the AI, which is scored using a 10-point scale (0–9), with 0 representing no impairment (fully active) and 9 representing confinement to a wheelchair with the inability to transfer oneself independently. In contrast to the EDSS, scoring of the AI is dependent on the need for an assistive mobility device, such as a cane, walker, or wheelchair, and on the ability and time needed to walk 25 feet (approximately 8 m).
The AI was initially developed to assess ambulation-related disability in patients with MS undergoing intensive immunosuppressive therapy.30 It exhibits high reliability, but its responsiveness to clinical change is not optimal.28 Although better correlations were observed between walking speed and AI scores than between maximum distance walked and EDSS scores, walking speed nevertheless varies within each level of the AI.27
The AI is a good tool for classifying patients based on current walking performance. It is easier to use than the EDSS, as the AI does not require a full neurologic examination, it evaluates walking ability on only a 25-foot distance, and it has high reliability. However, its low responsiveness to change makes the AI less suitable for measuring the results of interventions.
Other Scales
The Dynamic Gait Index (DGI) is an eight-item assessment tool that relies on the rater's observation of degree of limitation during a patient's performance of specific tasks.31 Although this instrument was originally designed to determine the likelihood of falling among older adults and, as such, may also be construed as a measure of balance, it nevertheless provides an adequate overall assessment of ambulation based on its incorporation of walking, stair climbing, and balance. In patients with MS, the DGI demonstrated good interrater and intrarater reliability, and inversely correlated with the timed 6.1-m (20-ft) walk (r = –0.801, P < .01), suggesting concurrent validity and relevance to the clinical assessment of mobility as related to walking in this patient population.32 Because of the need for a detailed assessment by a trained evaluator, the DGI is used mostly in rehabilitation settings.
Qualitative gait analysis can be performed using the Rivermead Visual Gait Assessment (RVGA), a validated objective measure of gait performance developed for clinical use in patients with neurologic deficits.33 The RVGA comprises 20 items that score the severity of deviations from normal postures and joint movements during the stance and swing phases of gait using a 4-point ordinal scale (0 = normal; 3 = severe), with one item (elbow flexion) scored between 0 and 2. The total score therefore ranges from 0 to 59, with higher scores indicating more severe deviations from normal. The RVGA has demonstrated good interrater and intra-rater reliability, as well as sensitivity to treatment effects, with a correlation between change in RVGA score and change in walking time.33 The RVGA provides a quantified assessment of gait pattern, which is not provided by other tools. However, scoring takes a relatively long time and must be performed by a clinician experienced in gait assessment. Therefore, use of the RVGA is essentially reserved for a rehabilitation setting.
Timed Walking Tests
Timed walking tests are objective assessment instruments with the inherent advantage of readily providing a quantitative measure of walking performance. Although these measures are not disease-specific, they have demonstrated good reliability and reproducibility in patients with MS.34
Timed 25-Foot Walk
Although not developed as a disease-specific instrument, the Timed 25-Foot Walk (T25FW) has been validated in MS as one of the three components of the Multiple Sclerosis Functional Composite (MSFC); the instructions on administering the T25FW are presented in the MSFC manual. The MSFC itself was designed specifically as a change-sensitive outcome measure for use in clinical trials to evaluate three key domains: walking (T25FW), upper-extremity function (Nine-Hole Peg Test), and cognitive function (Paced Auditory Serial Addition Test).35 The MSFC correlates moderately with the EDSS—a correlation that is driven primarily by a strong association between the T25FW and the EDSS.36 The T25FW correlates independently with the EDSS across disability severity and MS type, with the strongest associations observed among patients with severe disability and those with primary progressive MS.37 However, a limitation of the T25FW is a floor effect, rendering it less sensitive for detecting differences among patients with very mild disability. Nevertheless, the T25FW is useful as part of the MSFC and also as a stand-alone quantitative measure of walking, because it has been extensively validated. In fact, the T25FW is frequently used as a standard for evaluating correlation with other walking and mobility instruments. A minimum change of 20% on the T25FW was found to be clinically meaningful in comparison to clinically observable changes and patients' perception of change.38–41 The T25FW is also of high practical value in the clinical setting, requiring a minimum of time and space, and providing an objective evaluation of walking ability. Assessment of walking speed on a short distance is increasingly recommended as an outcome measure in individuals with neurologic conditions and in the elderly.42 The T25FW is only one of many proposed tests, with various distances (in particular, the Timed 10-Meter Walk [T10MW] and the Timed 30-Meter Walk [T30MW] tests), starting instructions (static vs. dynamic start), and paces (comfortable vs. “maximum but safe” pace).43 Differences in test administration instructions have been shown to affect the results of the assessments; therefore, a universal protocol would be helpful in comparing results from outcomes studies.44 Our preference is for maximum speed (as long as it is deemed safe) with static start, but further research is needed to determine which methodology is the most effective.
The 6-Minute Walk (6MW) Test
The 6MW records the maximum distance a patient walks in 6 minutes. For validation in patients with MS, the original instructions were modified to emphasize speed and eliminate instructions for permitted rest.45 The 6MW correlated strongly with overall measures of disability, including the EDSS (r = 0.73, P < .0001) and the MSFC (r = 0.72, P < .0001), and with a patient-reported measure of walking, the 12-Item Multiple Sclerosis Walking Scale (MSWS-12; r = 0.81, P < .001).45 Although the 6MW also showed a very strong correlation with the T25FW, it is most likely a better measure of walking endurance than the T25FW. Distinct patterns of walking speed throughout the 6-minute period were observed among groups of subjects, with patients having moderate and severe disability demonstrating a slowing of their gait speed over the duration of the test.
One practical limitation of the 6MW test in the clinical setting is the need for a walkway of sufficient length to enable comfortable walking while minimizing turns. Also, in MS patients with severe fatigue and moderate or severe disability levels, walking for 6 minutes can be exhausting and may prevent them from performing any significant physical activity or testing until they have rested. An alternative is the 2-Minute Walk (2MW) test. This shorter test, with greater feasibility and reduced patient burden, has been validated in patients with cardiovascular and respiratory disease46 and in those having undergone lower-limb amputation,47 and has been used in neurorehabilitation trials in patients with stroke48 or MS.49 The 2MW appears more feasible and less burdensome for patients than the 6MW and was shown to have similar psychometric properties in patients with respiratory problems50; however, this has not been confirmed in patients with MS.
The Timed Up and Go Test
The Timed Up and Go (TUG) test51 is a quantitative assessment of the time required for a person to rise from a chair, walk 3 m, turn around, walk back to the chair, and sit down. This test was evaluated concomitantly with the T10MW and T30MW tests in patients with MS.34 Overall, there was a strong correlation among all three tests (r = 0.85), but correlations were lower between the TUG and the timed-walk tests in patients with EDSS scores of 4 or below (r = 0.76). Stratification by EDSS scores (≤4 and >4) also resulted in the observation that the smallest percentage difference required to detect a change varied for both the TUG and T30MW tests between these strata, with a smaller difference required to detect a change in patients with less disability. The TUG test is easy to administer in an office setting. The time needed to complete the test is only partially dependent on walking ability, which can be viewed as both an advantage (by broadening the assessment of mobility) and a limitation (when the focus is only on walking).
The Six Spot Step Test
The Six Spot Step Test (SSST)52 has been reported to be a more comprehensive assessment of lower-extremity function than other walking tests, such as the T25FW and the 6MW, because it evaluates various factors that contribute to ambulatory ability, including coordination and balance. In the SSST, the patient walks as quickly as possible from one end to the other of a delineated rectangular field that has been preset with cylindrical blocks, kicking the blocks out of their marked circles (Figure 1A).52 The test is performed four times, twice for each leg. The SSST appears to be less prone to floor effects than are other timed-walk tests. The test-retest reliability was found to be high, with no evidence of practice effects. The SSST demonstrated high correlation with the EDSS (r = 0.80). There was less correlation with the patient-reported MSWS-12 (r = 0.69), suggesting that patients may have a somewhat different perspective. However, the SSST demonstrated higher correlation with the T25FW (r = 0.92), and the trend lines for the relationship of the SSST and the T25FW with the EDSS were nearly parallel, although mean scores with the SSST were correspondingly higher than those with the T25FW because of the nature of the test (Figure 1B).52 The SSST was developed as a more comprehensive alternative to the T25FW by including balance and coordination, but the specific use of the test has not yet been defined, and its psychometric properties, particularly its sensitivity to change, still need to be established. Additionally, the SSST requiresa specifically defined test field with setup prior to each test.
The Six Spot Step Test (SSST)
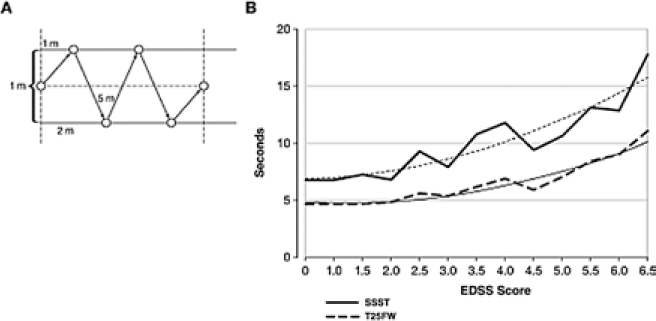
Quantitative Gait Analysis
The main drawback of most walking performance scales and tests is that they detect only a deviation from normal gait performance, such as decreased walking speed or walking distance, and its variation over time, without providing information on the underlying gait pattern. This is of particular relevance to the field of rehabilitation, in which interventions to improve gait performance are determined based on specific impairments. The RVGA is the only scale that provides such information, but its scoring is cumbersome and requires a well-trained evaluator.
Motion Analysis Systems
Computerized motion analysis systems, using markers placed on the body and detected by cameras, as well as force plates, represent the gold standard in terms of quantitative gait analysis. These systems provide a comprehensive three-dimensional assessment of gait kinetics and kinematics, and continuous surface electromyographic recording can be added for a more in-depth gait analysis. However, the space and time requirements, complexity of data analysis, and prohibitive cost of the equipment and its operation, combined with a comparatively low reimbursement rate, confines the clinical use of such gait analysis systems to large centers.
The computerized GAITRite (CIR System, Inc, Havertown, PA) system53 could have more widespread use in the clinical world. It consists of an instrumented walkway with sensors arranged in a grid-like pattern to identify footfall contacts, enabling quantitative assessment of temporal and spatial parameters of gait. Use of the GAITRite system has demonstrated that gait parameters were impaired in patients with MS, even among those with relatively short disease duration.54 However, data derived from this type of analysis need to be validated against more traditional functional outcomes. The burden in terms of cost, space, time, and need for trained personnel, while considerably less than with full-gait analysis systems, remains a limitation to use of the GAITRite system in routine clinical practice.
Energy Expenditure
One of the main benefits of the management of walking limitations may be improved walking efficiency, with decreased energy expenditure for the same amount of walking.24 This is not a trivial issue, considering the importance of fatigability in MS-related disability. Accurate measurement of energy expenditure is cumbersome. The Physiological Cost Index (PCI),55 which is based on the observed correlation between heart rate during sustained walking and maximum oxygen consumption, may serve as a proxy for walking efficiency. However, because of the presence of dysautonomia in some patients with MS,56 the PCI may not necessarily be a valid measure.
Measures of Walking Performance in Daily Life
None of the instruments described above rely on direct observation of patients' walking performance in their own environment and over an extended period of time. Therefore, at best, they represent a proxy for patients' “real” walking performance. Technological advances now make it possible to measure walking outside of the clinic or gait laboratory.
Pedometry/Accelerometry
Pedometry and accelerometry require the use of devices that detect movement, with the former detecting and counting the number of steps, and the latter monitoring movement in one or more dimensions. Both of these techniques have been evaluated or used in studies of patients with MS.57–61 Although these methods can provide objective quantitative assessments and have generally demonstrated reliability in the MS population, there is a need to determine exactly what is being assessed. An early study of these devices in patients with MS suggested that they provide a quantitative assessment of physical activity rather than walking per se.61 In that study, a moderate correlation was reported between the reliability of accelerometer readings with the 5-Minute Walk test, although overall measurement properties were less than optimal and appeared to be dependent on where the instrument was placed on the lower extremity. However, support for the potential use of accelerometry for measuring walking is available from other studies, with strong correlations observed between accelerometry data and patients' self-reports of walking ability.62 63 A more sophisticated, microprocessor-based accelerometer has been shown to provide a range of data outputs in patients with MS, including the counting of steps over a specified period and determining the number of steps per minute.24 57 58 A recent study has suggested that accelerometry may be used to differentiate between groups with different levels of activity.64 Of additional relevance is the observation that in a patient's normal ambulation environment, habitual walking performance, defined as the real amount of steps performed in the customary living environment and measured using accelerometry, was significantly predicted by walking capacity tests such as the 2MW test and the 6MW test.65 Interestingly, while the 6MW test was predictive in the overall population and in patients with mild MS, the 2MW test had greater predictive value among patients with moderate MS.
Despite the ability to provide quantitative data, and the suggestion that the type of data obtained from pedometry and accelerometry be considered the gold standard for assessment of walking activity,24 these devices have specific limitations that may make them impractical for widespread use in the clinical setting. Such factors as cost of the devices, the need for calibration, verification of accuracy, and the need for a high rate of patient compliance with wearing the device all affect the use of pedometers and accelerometers. Consequently, these methods may not be practical for use in the regular assessment of patients with MS as part of long-term disease management. In addition, the responsiveness of these methods to change has not been determined, and gait disturbance in patients with MS cannot be fully characterized via step counting.
Global Positioning System Odometry
Global Positioning System (GPS) odometry66 may represent the next technological advance after accelerometry. GPS odometry uses satellite technology to determine and track relative changes in position with respect to distance covered over time. This system allows objective measurement of walking distance in patients with MS using the maximum objective walking distance (MOWD) as the outcome variable of interest.
GPS odometry was determined to be a reliable assessment of walking that demonstrated a significant relationship with EDSS disability scores, as well as with subjective assessment using the MSWS-12 (Figure 2A) and the T10MW—an objective, quantitative assessment of walking speed (Figure 2B).66 The best correlation for MOWD was with walking speed based on the T10MW (Figure 2B). The authors suggest that these data may support evaluation of walking capacity as an adequate surrogate for evaluation of effort.
Global Positioning System (GPS) odometry
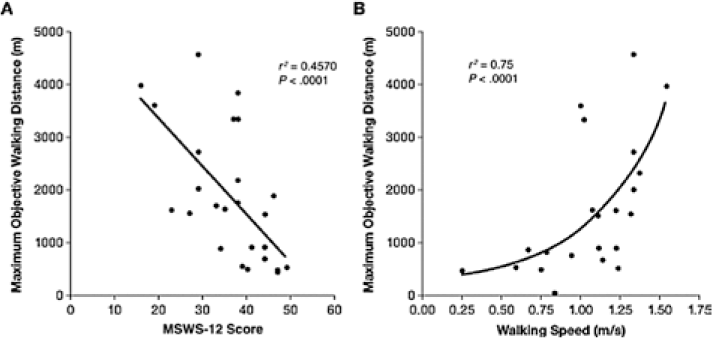
Despite the potential advantages of GPS odometry over pedometry and accelerometry, the practicality of this system for use in the clinical setting is uncertain, as its limitations are the same as those of other ambulatory activity monitors. In addition to the fact that its sensitivity to change has yet to be determined, there are costs associated with device acquisition and maintenance; moreover, the requirements for time, distance, and the continual need to receive signals from at least three satellites in order for the system to function reduce its practicality for routine use in clinical practice.
Patient-Reported Outcomes
The MSWS-12
The MSWS-1267 was designed as a disease-specific, patient-based instrument for use in clinical trials and clinical practice, to capture the complex impact of MS on walking ability. It contains 12 questions with Likert-type responses and has a recall period of 2 weeks. The psychometric properties of the MSWS-12 have been extensively evaluated in diverse MS populations in both community and hospital settings, with demonstration of internal consistency, high reliability and validity, and good generalizability.62 67 68 The MSWS-12 is less prone to floor and ceiling effects than are other tools, suggesting adequate assessment of the impact of walking impairment across the range of disability.
Comparison of MSWS-12 scores with other measures of physical, psychological, and cognitive domains showed stronger correlation with measures of physical domains that relate to mobility and lower-extremity function versus other domains.62 The overall strong correlation between the MSWS-12 and the EDSS (r = 0.80) appeared to be driven primarily by a strong correlation with EDSS scores of 1.0 to 4.5 (r = 0.71); EDSS scores of 5 to 8 showed only a weak correlation with the MSWS-12 (r = 0.33).62 A strong correlation between the MSWS-12 and accelerometer counts was also observed (Figure 3),62 suggesting a measurable relationship between objective mobility and a patient's perception of his or her walking ability. Although the correlation with the T25FW was also strong (r = 0.65), the variance in the MSWS-12 was not completely explained by walking speed.68 One of the most important attributes of the MSWS-12 appears to be its responsiveness to change. During psychometric validation, the MSWS-12 was shown to be more responsive than other walking-based measures, including the EDSS, the Functional Assessment of Multiple Sclerosis (FAMS) mobility subscale, and the T25FW.67
Bivariate plot of the relationship between the 12-Item Multiple Sclerosis Walking Scale (MSWS-12) score and accelerometer counts
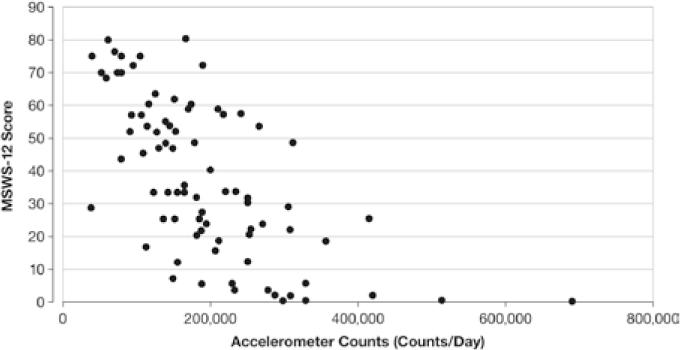
The combination of good psychometric properties, short administration time, and responsiveness to change makes the MSWS-12 a useful, practical instrument in clinical trials and in clinical practice. Its apparent correlation with several objective measures of mobility warrants further investigation of its use in a mutually complementary assessment of subjective and objective outcomes of walking and mobility.
Other Measures
The Patient-Determined Disease Steps (PDDS) is a self-report surrogate measure of the EDSS that is used in the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry (now the NARCOMS/Global Patient Registry). The PDDS is scored from 0 (normal) to 8 (bedridden), with scores between 3 and 7 specifically focused on patient-reported walking limitations.69 The PDDS correlates with the EDSS in both cross-sectional and longitudinal studies.69 70
Other self-report measures, such as the 29-Item Multiple Sclerosis Impact Scale (MSIS-29)5 or the FAMS mobility subscale,71 do not focus primarily on walking.
Conclusion
There is no ideal measure of walking for patients with MS. It is necessary to recognize the importance of walking and mobility from both the clinical and patient perspectives and to incorporate regular assessments of walking and mobility into the global evaluation of disease status and patient function. This would increase the ability to identify patients who might benefit from specific management of walking limitations, and to monitor treatment- or disease-progression–related changes over time. Such assessments should include both clinician-rated and patient-reported measures, and should have demonstrated psychometric qualities, clinical meaningfulness, and practicality.
After reviewing all the instruments discussed above, the T25FW (a performance-based test) and the MSWS-12 (a patient-rated questionnaire) appear to provide complementary assessments of a patient's walking limitations, while exhibiting appropriate qualities for use in the clinical setting. A recent consensus committee assembled by the Consortium of Multiple Sclerosis Centers found that the DGI and TUG instruments may also be practical for evaluating mobility-related function in the clinical setting.72 When the main focus is walking endurance, use of the 6MW test or 2MW test should be considered. Other tools show promising characteristics but need further validation and refinement before they can be considered for widespread clinical use.
PracticePoints
Walking is frequently affected by MS and has a significant impact on patients’ functional status and quality of life.
It is important to assess walking performance systematically in the clinical setting, using validated instruments.
Among all the tools available, the Timed 25-Foot Walk (observed performance) and the 12-Item Multiple Sclerosis Walking Scale (patient-reported) combine feasibility in clinical practice and satisfactory psychometric properties.
Acknowledgments
The authors wish to thank The Curry Rock-efeller Group, Tarrytown, NY, for assistance with manuscript development, and E. Jay Bienen, PhD, for editorial support, both of which were funded by Acorda Therapeutics, Hawthorne, NY.
References
National Multiple Sclerosis Society. About MS: who gets MS? http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx. Accessed April 2, 2010.
McCrory DC, Pompeii LA, Skeen MB, Criteria to Determine Disability Related to Multiple Sclerosis. Evidence Report/Technology Assessment No. 100. AHRQ Publication No. 04-E019-2. Rockville, MD: Agency for Healthcare Research and Quality, 2004.
World Health Organization. International Classification of Functioning, Disability, and Health, d450 Walking. http://apps.who.int/classifications/icfbrowser/. Accessed April 2, 2010.
Swingler RJ, Compston DA. The morbidity of multiple sclerosis. Q J Med. 1992; 83: 325–337.
Hobart JC, Lamping DL, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001; 124: 962–973.
Martin CL, Phillips BA, Kilpatrick TJ, Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006; 12: 620–628.
Johansson S, Ytterberg C, Claesson IM, High concurrent presence of disability in multiple sclerosis: associations with perceived health. J Neurol. 2007; 254: 767–773.
Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010; 26: 109–119.
Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther. 2009; 26: 1043–1057.
Scheinberg L, Holland N, Larocca NG, Laitin P, Bennett A, Hall H. Multiple sclerosis; earning a living. N Y State J Med. 1980; 80: 1395–1400.
Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008; 14: 988–991.
Datamonitor Healthcare Reports. Treatment Algorithms 1999: Segmenting the Multiple Sclerosis Patient Population. London: Datamonitor; 1999.
Paltamaa J, Sarasoja T, Leskinen E, Wikström J, Mälkiä E. Measures of physical functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil. 2007; 88: 1649–1657.
Whetten-Goldstein K, Sloan FA, Goldstein LB, Kulas ED. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler. 1998; 4: 419–425.
Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006; 66: 1696–1702.
Kobelt G, Berg J, Lindgren P, Fredrikson S, Jönsson B. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006; 77: 918–926.
Prescott JD, Factor S, Pill M, Levi GW. Descriptive analysis of the direct medical costs of multiple sclerosis in 2004 using administrative claims in a large nationwide database. J Manag Care Pharm. 2007; 13: 44–52.
Edgley K, Sullivan MJ, Dehoux E. A survey of multiple sclerosis, part II: determinants of employment status. Can J Rehabil. 1991; 4: 127–132.
Kornblith AB, La Rocca NG, Baum HM. Employment in individuals with multiple sclerosis. Int J Rehabil Res. 1986; 9: 155–165.
Albrecht H, Wötzel C, Erasmus LP, Kleinpeter M, König N, Pöllmann W. Day-to-day variability of maximum walking distance in MS patients can mislead to relevant changes in the Expanded Disability Status Scale (EDSS): average walking speed is a more constant parameter. Mult Scler. 2001; 7: 105–109.
Morris ME, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002; 72: 361–365.
Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler. 2006; 12: 613–619.
Paltamaa J, Sarasoja T, Leskinen E, Wikström J, Mälkiä E. Measuring deterioration in international classification of functioning domains of people with multiple sclerosis who are ambulatory. Phys Ther. 2008; 88: 176–190.
Pearson OR, Busse ME, van Deursen RWM, Wiles CM. Quantification of walking mobility in neurological disorders. QJM. 2004; 97: 463–475.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452.
Sharrack B, Hughes RA. Clinical scales for multiple sclerosis. J Neurol Sci. 1996; 135: 1–9.
Schwid SR, Goodman AD, Mattson DH, The measurement of ambulatory impairment in multiple sclerosis. Neurology. 1997; 49: 1419–1424.
Sharrack B, Hughes RAC, Soudain S, Dunn G. The psychometric properties of clinical rating scales used in multiple sclerosis. Brain. 1999; 122: 141–159.
Hobart JC, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain. 2000; 123: 1027–1040.
Hauser SL, Dawson DM, Lehrich JR, Intensive immunosuppression in progressive multiple sclerosis: a randomized, 3-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med. 1983; 308: 173–180.
Shumway-Cook A, Wollacott M. Motor Control: Theory and Practical Applications. Baltimore, MD: Williams and Wilkins; 1995.
McConvey J, Bennett SE. Reliability of the Dynamic Gait Index in individuals with multiple sclerosis. Arch Phys Med Rehabil. 2005; 86: 130–133.
Lord SE, Halligan PW, Wade DT. Visual gait analysis: the development of a clinical assessment and scale. Clin Rehabil. 1998; 12: 107–119.
Nilsagard Y, Lundholm C, Gunnarsson L-G, Denison E. Clinical relevance using timed walk tests and ‘timed up and go’ testing in persons with multiple sclerosis. Physiother Res Int. 2007; 12: 105–114.
Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999; 5: 244–250.
Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite; a new clinical outcome measure for multiple sclerosis trials. Mult Scler. 2002; 8: 359–365.
Kalkers NF, de Groot V, Lazeron RH, MS functional composite: relation to disease phenotype and disability strata. Neurology. 2000; 54: 1233–1239.
Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000; 6: 286–290.
Schwid SR, Goodman AD, McDermott MP, Bever CF, Cook SD. Quantitative functional measures in MS: what is a reliable change? Neurology. 2002; 58: 1294–1296.
Kragt JJ, van der Linden FA, Nielsen JM, Uitdehaag BM, Polman CH. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Mult Scler. 2006; 12: 594–598.
Hoogervorst EL, Kalkers NF, Cutter GR, Uitdehaag BM, Polman CH. The patient's perception of a (reliable) change in the Multiple Sclerosis Functional Composite. Mult Scler. 2004; 10: 55–60.
Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32:2–5.
Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008; 14: 552–562.
Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil. 2008; 89: 865–872.
Goldman M, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2007; 14: 383–390.
Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982; 284: 1607–1608.
Brooks D, Parsons J, Hunter JP, Devlin M, Walker J. The 2-minute walk test as a measure of functional improvement in persons with lower limb amputation. Arch Phys Med Rehabil. 2001; 82: 1478–1483.
Moreland JD, Goldsmith CH, Huijbregts MP, Progressive resistance strengthening exercises after stroke: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2003; 84: 1433–1440.
van den Berg M, Dawes H, Wade DT, Treadmill training for individuals with multiple sclerosis: a pilot randomised trial. J Neurol Neurosurg Psychiatry. 2006; 77: 531–533.
Leung AS, Chan KK, Sykes K, Chan KS. Reliability, validity, and responsiveness of a 2-min walk test to assess exercise capacity of COPD patients. Chest. 2006; 130: 119–125.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39: 142–148.
Nieuwenhuis MM, Van Tongeren H, Sørensen PS, Ravnborg M. The Six Spot Step Test: a new measurement for walking ability in multiple sclerosis. Mult Scler. 2006; 12: 495–500.
Cutlip RG, Mancinelli C, Huber F, DiPasquale J. Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture. 2000; 12: 134–138.
Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture. 2009; 29: 138–142.
MacGregor J. The evaluation of patient performance using long-term ambulatory monitoring technique in the domiciliary environment. Physiotherapy. 1981; 67: 30–33.
Merkelbach S, Haensch CA, Hemmer B, Koehler J, König NH, Ziemssen T. Multiple sclerosis and the autonomic nervous system. J Neurol. 2006;253(suppl 1):I/21–I/25.
Pearson OR, Busse ME, van Deursen R, Wiles CM. Quantification of walking mobility in multiple sclerosis (MS) using an ambulatory activity monitor—a pilot study [abstract]. J Neurol Neurosurg Psychiatry. 2003; 74:1450.
Busse ME, Pearson OR, Van Deursen R, Wiles CM. Quantified measurement of activity provides insight into motor function and recovery in neurological disease. J Neurol Neurosurg Psychiatry. 2004; 75: 884–888.
Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Community Health. 2007; 30: 144–150.
Motl RW, Zhu W, Park Y, McAuley E, Scott JA, Snook EM. Reliability of scores from physical activity monitors in adults with multiple sclerosis. Adapt Phys Activ Q. 2007; 24: 245–253.
Hale L, Williams K, Ashton C, Connole T, McDowell H, Taylor C. Reliability of RT3 accelerometer for measuring mobility in people with multiple sclerosis: pilot study. J Rehabil Res Dev. 2007; 44: 619–628.
Motl RW, Snook EM. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12). J Neurol Sci. 2008; 268: 69–73.
Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. 2010; 290: 6–11.
Klassen L, Schachter C, Scudds R. An exploratory study of 2 measures of free-living physical activity for people with multiple sclerosis. Clin Rehabil. 2008; 22: 260–271.
Gijbels D, Alders G, Van Hoof E, Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler. 2010; 16: 618–626.
Créange A, Serre I, Levasseur M, .; Réseau SINDEFI-SEP. Walking capacities in multiple sclerosis measured by global positioning system odometer. Mult Scler. 2007; 13: 220–223.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31–36.
McGuigan C, Hutchinson M. Confirming the validity and responsiveness of the Multiple Sclerosis Walking Scale-12 (MSWS-12). Neurology. 2004; 62: 2103–2105.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler. 1999;5:349–354.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995; 45: 251–255.
Cella DF, Dineen K, Arnason B, Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996; 47: 129–139.
Hutchinson B, Forwell SJ, Bennett S, Brown T, Karpatkin H, Miller D. Toward a Consensus on Rehabilitation Outcomes in MS: Gait & Fatigue CSMC Consensus Conference, November 28–29, 2007. Int J MS Care. 2009; 11: 67–78.
Financial Disclosures: Dr. Bethoux has received honoraria for consulting and/or speaking from Acorda Therapeutics, Allergan, Biogen Idec, IMPAX, and Medtronic Inc; honoraria for serving on a clinical advisory committee from Acorda Therapeutics, Allergan, and Medtronic Inc; and research funding from Acorda Therapeutics, Innovative Neurotronics, and Medtronic Inc. Dr. Bennett has received honoraria for speaking and/or consulting from Acorda Therapeutics, Biogen Idec, EMD Serono/Pfizer, Medtronic Inc, and Teva.
Funding/Support: This project was supported by Acorda Therapeutics, Hawthorne, NY.







