Publication
Research Article
International Journal of MS Care
Asymmetrical Hip Bone Density in Multiple Sclerosis
As multiple sclerosis (MS) may affect one leg more than the other, we predicted that bone mineral density (BMD) would be lower in the limb self-identified as more affected. Therefore, the purpose of this study was to determine whether ambulatory individuals with MS displaying moderate-to-severe lower-extremity paresis exhibit asymmetrical femoral neck BMD. Dual-energy x-ray absorptiometry was used to assess proximal femoral neck BMD. Lower BMD was observed in the proximal femoral neck of the more paretic limb (P = .001) in a group (N = 23) of ambulatory individuals with relapsing-remitting MS (RRMS). Our preliminary findings suggest that bilateral hip screening may be important in the early detection of compromised bone health in ambulatory individuals with RRMS. Further research is warranted to fully characterize the factors and mechanisms associated with bone loss and identify effective strategies for optimizing bone health in people with MS.
Multiple sclerosis (MS) is a chronic degenerative disease of the central nervous system with onset typically occurring during early adulthood. Among the common symptoms of the disease are excessive fatigue, muscle weakness, and poor balance, all of which can contribute to reduced mobility. In older adults, reduced mobility has been associated with an increased risk of developing osteoporosis.1 Low bone density may contribute to increased risk of bone fractures in people with MS.2 In addition to reduced weightbearing status, secondary to disability and inactivity, other factors such as prolonged high-dose glucocorticoid administration, low exposure to sunlight, vitamin D deficiency, and disease-related factors may affect bone health in individuals with MS.3 Disability, as measured by the Expanded Disability Status Scale (EDSS) score, has been shown to be negatively correlated with proximal femur bone mineral density (BMD) in people with MS.4 5 In addition, MS disease duration has been shown to correlate negatively with proximal femur BMD.6 Despite increasing evidence that poor bone density is a significant health problem in people with MS,5–8 screening, prevention, and treatment guidelines remain unclear.
Conventional practices for assessing lower-extremity BMD include determining the BMD of only one proximal femoral hip and using this measurement to represent the BMD of the contralateral hip as well.9 This practice is based on data revealing minimal variation between hips within an individual as well as the goals of minimizing cost and reducing exposure to radiation.9 10 However, some data indicate possible interfemur variations of BMD within nonclinical populations11 12 and among individuals with osteoarthritis, rheumatoid arthritis, and hemiplegia.13–15
In some individuals with MS, varying levels of lower-extremity paresis may contribute to altered bone loading and remodeling, bone health, and BMD symmetry of the proximal femurs. To expand our understanding of lower-extremity paresis and bone health, we designed a study to assess bilateral hip BMD in ambulatory individuals with MS exhibiting asymmetrical lower-extremity paresis. We hypothesized that BMD would be lower in the more paretic limb. This is a potentially important health concern given that the accepted bone health screening protocols include only single hip assessment (as well as spine evaluation). Therefore, in some instances, preventive measures to minimize bone loss may not be pursued because of a failure to detect clinical indices while using standard assessment protocols.
Methods
Study Participants
Participants were recruited from local MS support group meetings and through flyers posted at local neurologists' offices. Individuals were included in the study if they had a physician diagnosis of relapsing-remitting MS16 and an EDSS score (as determined by a physician) of less than 6.5 (ambulatory) and exhibited lower-extremity paresis, with one leg being more affected than the other. Paresis was defined as partial or mild paralysis or muscle weakness and was identified by the participant using a medical history and self-reports. Each study participant signed a consent form approved by the university institutional review board prior to enrollment. Participants who had ever used steroids for longer than 3 months at a given time and those with known metabolic disorders that could compromise bone density, including those taking bisphosphonates or similar medications to attenuate bone loss, were excluded. The final study sample consisted of 23 people (2 men and 21 women).
Bone Density
Right and left femoral neck BMD (g/cm2) was assessed using dual-energy x-ray absorptiometry (DXA; GE Lunar, GE Healthcare, Madison, WI). DXA scans were performed according to manufacturer guidelines. Daily calibration was performed by scanning the manufacturer's phantom of a known density, which achieved short- and long-term densitometer accuracy. The Lunar DXA performs a 6-point calibration with normal, osteopenic, and osteoporotic BMD values as well as lean, normal, and obese values. Participants were positioned according to conventional procedures.17 Bone density measurements are typically expressed as absolute (g/cm2) and relative (T- and Z-score) values. The T score represents the number of standard deviations above or below the mean in comparison with a young adult (healthy 20–40-year-old) of the same sex and ethnicity, while the Z score represents the number of standard deviations above or below the mean in comparison with the age-matched reference mean as supplied by the manufacturer (GE Healthcare, which uses the National Health and Nutrition Examination Survey [NHANES] database). Osteopenia, or low bone density, is defined as a T score less than or equal to –1.0 but greater than –2.5, whereas osteoporosis is defined as a T score less than or equal to –2.5.
Statistical Analysis
Data analysis was conducted using SPSS, version 16.0 (SPSS, Chicago, IL) using a paired t test with P ≤ .05 as the criterion for statistical significance.
Results
Participant Demographics
Table 1 indicates the demographics of the 23 study participants.
Participant characteristics
Femoral Neck BMD
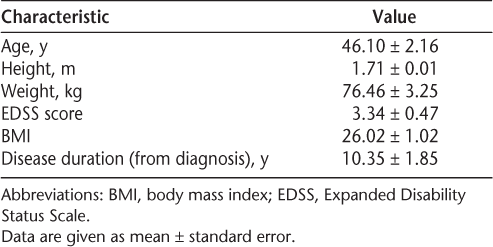
In comparison of aggregate group mean BMD between the left and right hips, no statistically significant differences were observed. However, in evaluating BMD based on participant-reported symptoms in each leg, the more affected leg displayed significantly lower femoral neck BMD (P = .001) (Figure 1). Mean ± SE T and Z scores for the more affected leg were –1.33 ± 0.30 and –0.94 ± 0.25, respectively. Mean T and Z scores for the less affected leg were –1.05 ± 0.27 and –0.66 ± 0.21, respectively. Negative scores indicate lower bone density.
Differences in femoral neck bone density between more and less affected limbs and between left and right limbs
Discussion
To our knowledge, this is the first report of bilateral differences in femoral neck BMD in ambulatory individuals with MS who report lower-extremity paresis. Our findings are similar to those observed in individuals with possible unilateral hip disorders such as osteoarthritis, rheumatoid arthritis, and hemiplegia.13–15 The difference in bone density observed between hips may be related to atypical bone remodeling associated with low or unusual load-bearing status, muscle weakness, and atrophy. Additionally, factors such as glucocorticoid use, disability, disease duration, low sunlight exposure, compromised dietary intake of calcium and vitamin D, and medication side effects may have contributed to the observed BMD differences in individuals with MS.3 Other factors resulting from the MS disease processes may also influence BMD. For example, common cytokines such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), IL-6, and IL-11, which play a role in the pathogenesis of MS, may also be involved in the development of osteoporosis.18 19
In the clinical setting, bone health and fracture risk screening procedures typically include lumbar spine and unilateral proximal femoral BMD assessments.20 The rationale for single hip assessment is based on data indicating a negligible difference in bone density between right and left or between dominant and nondominant hips.9,10,21,22 However, in a sample of 3012 white women who were over the age of 50 years, left-right differences between hips including the femoral neck were observed that could have resulted in 2% of participants being at risk for osteopathic misclassification (based on T-score classification).23 In the current study, nearly 13% of participants could have been misclassified or experienced undetected bone loss if the more paretic limb had not been scanned.
Conventional bone health screening guidelines established by the World Health Organization and the National Osteoporotic Foundation recommend that women over 65 years of age and people (men and women) at increased risk for bone loss undergo bone density testing.17 Individuals at increased risk include those with dementia, poor health, recent falls, prolonged immobilization, smoking, alcohol intake of 3 or more units per day, low body weight, overactive or underactive thyroid gland, fragility, fracture in a first-degree relative, early-onset estrogen deficiency (<45 years), physical inactivity, a disease or condition that can cause bone loss (such as rheumatoid arthritis or anorexia nervosa), and steroid use for more than 3 months.17 It is less clear whether lower-extremity paresis or having a neurologic disease is considered to raise the risk of bone loss in individuals who remain ambulatory.
It has been suggested that individuals who survive a hip fracture are at a two- to threefold increased risk of future fracture,24 with a 5% to 10% increased chance of another hip fracture within 1 year.25 Nilsagard and colleagues26 observed that 63% of ambulatory individuals with MS reported falling during normal activities of daily living and had a fracture rate of 2.6% during a 3-month study period. In a cohort of community-dwelling women aged 65 years or older with a recent hip fracture, the average loss of femoral neck BMD was 2.1% at 2 months, 2.5% at 6 months, and 4.6% at 12 months, with a 6% loss of lean mass and a 3.6% increase in body fat at 1 year. The loss of both bone density and muscle mass increases the risk of new fractures.27
Early screening for BMD remains underutilized in people with MS despite the evidence of lower BMD and elevated risk of falling in this population compared with the general population. Because of the lack of guidelines and consensus, Hearn and Silber3 proposed an algorithm for the screening and treatment of osteoporosis in people with MS, with the following recommendations: 1) individuals suspected to be at risk of vitamin D and calcium deficiency should have their status checked and supplements prescribed if necessary; 2) postmenopausal women should be routinely scanned and treated accordingly if low BMD is found; and 3) disability status scale (EDSS) scores should be used as an indicator for screening in all other individuals. Because the loss of BMD has been correlated to the loss of independent ambulation (EDSS scores of 7 or greater),5 28 Hearn and Silber3 proposed an EDSS score of 6 or greater as the threshold at which all patients should routinely receive a DXA scan. They did not, however, recommend bilateral hip scanning.
Implementing conventional bone health screening protocols may result in some individuals with compromised bone health being misclassified, possibly resulting in failure to take preventive measures to minimize bone loss. Considering the high personal and economic costs associated with osteoporosis and fractures, implementing strategies early in the disease course to minimize bone loss may have important long-term benefits. In addition to pharmacologic agents that may attenuate bone loss, nonpharmacologic therapeutic interventions with potential to improve BMD include physical activity and exercise, particularly weightbearing activities and resistance training. These approaches could play an important role in a comprehensive health plan for people with MS, reducing disability. Strength training has been shown to enhance bone density or limit bone density decline in both older and young individuals.29–33 In a study of older adults (aged 50–73 years) who engaged in high-impact exercises specifically designed to load the proximal femur, Welsh and Rutherford31 observed a 1.57% increase in femoral neck BMD along with a 5.4% increase in quadriceps strength, providing evidence that strength training has the potential to improve BMD. In contrast, some researchers have reported little or no impact of resistance training on bone density.34 35 For example, in a study involving pre-menopausal women who participated in a lower-body resistance training routine, increases were observed in lean muscle mass without associated changes in BMD.35 However, the lack of randomization and differences in body weight and body fat at the beginning of the study make the findings difficult to interpret. The impact of strength training on bone health in individuals with MS exhibiting lower-extremity paresis requires further investigation considering the highly variable symptomatology across individuals, particularly in those with conditions that may compromise skeletal muscle performance. In addition to rehabilitation and possible pharmacologic interventions, providing comprehensive information and guidance to people with MS and their families on BMD may help optimize long-term health and quality of life.
Although the results of this study provide new information related to BMD in people with MS, the study has some limitations. These include the relatively small sample size, involvement of only ambulatory individuals with relapsing-remitting disease, lack of data on nutrient intake and sunlight exposure, use of self-reports to identify paretic limbs, and absence of a matched control group for comparison. Lower-extremity strength data were not included in this study due to unanticipated equipment failure, limiting the amount and quality of those data. The ability to also analyze the relationship between muscular strength and BMD would have offered further insight into the phenomenon of bilateral asymmetry. Despite these limitations, the findings may facilitate the development of bone health screening and education guidelines for people with MS.
Conclusion
Our preliminary findings provide evidence that in some individuals with MS, scanning a single hip for diagnostic purposes might obscure changes in bone health. Scanning both hips may yield a more comprehensive diagnostic assessment of bone health and allow for earlier detection of low BMD. Earlier implementation of preventive measures might help minimize future hip fractures and their associated complications and economic costs. Further research is warranted to fully characterize the factors and mechanisms associated with bone loss and identify effective strategies for optimizing bone health in people with MS.
PracticePoints
Ambulatory individuals with MS may exhibit asymmetrical femoral neck bone mineral density (BMD).
To detect compromised hip bone health, practitioners should consider bilateral hip BMD screening in individuals with MS.
References
Lips P, van Ginkel FC, Netelenbos JC, Wiersinga A, van der Vijgh WJ. Lower mobility and markers of bone resorption in the elderly. Bone Miner. 1990; 9: 49–57.
Formica CA, Cosman F, Nieves J, Herbert J, Lindsay R. Reduced bone mass and fat-free mass in women with multiple sclerosis: effects of ambulatory status and glucocorticoid use. Calcif Tissue Int. 1997; 61: 129–133.
Hearn AP, Silber E. Osteoporosis in multiple sclerosis. Mult Scler. 2010; 16: 1031–1043.
Ozgocmen S, Bulut S, Ilhan N, Gulkesen A, Ardicoglu O, Ozkan Y. Vitamin D deficiency and reduced bone mineral density in multiple sclerosis: effect of ambulatory status and functional capacity. J Bone Miner Metab. 2005; 23: 309–313.
Weinstock-Guttman B, Gallagher E, Baier M, Risk of bone loss in men with multiple sclerosis. Mult Scler. 2004; 10: 170–175.
Tuzun S, Altintas A, Karacan I, Tangurek S, Saip S, Siva A. Bone status in multiple sclerosis: beyond corticosteroids. Mult Scler. 2003; 9: 600–604.
Cosman F, Nieves J, Komar L, Fracture history and bone loss in patients with MS. Neurology. 1998; 51: 1161–1165.
Marrie RA, Cutter G, Tyry T, Vollmer T. A cross-sectional study of bone health in multiple sclerosis. Neurology. 2009; 73: 1394–1398.
Lessig HJ, Meltzer MS, Siegel JA. The symmetry of hip bone mineral density: a dual photon absorptiometry approach. Clin Nucl Med. 1987; 12: 811–812.
Yang RS, Chieng PU, Tsai KS, Liu TK. Symmetry of bone mineral density in the hips is not affected by age. Nucl Med Commun. 1996; 17: 711–716.
Lilley J, Walters BG, Heath DA, Drolc Z. Comparison and investigation of bone mineral density in opposing femora by dual-energy X-ray absorptiometry. Osteoporos Int. 1992; 2: 274–278.
Hall ML, Heavens J, Ell PJ. Variation between femurs as measured by dual energy X-ray absorptiometry (DEXA). Eur J Nucl Med. 1991; 18: 38–40.
Takamoto S, Masuyama T, Nakajima M, Alterations of bone mineral density of the femurs in hemiplegia. Calcif Tissue Int. 1995; 56: 259–262.
Gotfredsen A, Riis BJ, Christiansen C, Rodbro P. Does a single local absorptiometric bone measurement indicate the overall skeletal status? Implications for osteoporosis and osteoarthritis of the hip. Clin Rheumatol. 1990; 9: 193–203.
LeBlanc AD, Evans HJ, Marsh C, Schneider V, Johnson PC, Jhingran SG. Precision of dual photon absorptiometry measurements. J Nucl Med. 1986; 27: 1362–1365.
Poser CM, Brinar VV. Diagnostic criteria for multiple sclerosis. Clin Neurol Neurosurg. 2001; 103: 1–11.
Baddoura R, Awada H, Okais J, An audit of bone densitometry practice with reference to ISCD, IOF and NOF guidelines. Osteoporos Int. 2006; 17: 1111–1115.
Rifas L. Bone and cytokines: beyond IL-1, IL-6 and TNF-alpha. Calcif Tissue Int. 1999; 64: 1–7.
Lucas K, Hohlfeld R. Differential aspects of cytokines in the immunopathology of multiple sclerosis. Neurology. 1995; 45: S4–5.
Leslie WD, Tsang JF, Caetano PA, Lix LM; Manitoba Bone Density Program. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007; 92: 77–81.
Kanis JA, Borgstrom F, De Laet C, Assessment of fracture risk. Osteoporos Int. 2005; 16: 581–589.
Yang R, Tsai K, Chieng P, Liu T. Symmetry of bone mineral density at the proximal femur with emphasis on the effect of side dominance. Calcif Tissue Int. 1997; 61: 189–191.
Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006; 17: 1772–1780.
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000; 15: 721–739.
Colon-Emeric CS, Sloane R, Hawkes WG, The risk of subsequent fractures in community-dwelling men and male veterans with hip fracture. Am J Med. 2000; 109: 324–326.
Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis—a longitudinal study. Clin Rehabil. 2009; 23: 259–269.
Fox KM, Magaziner J, Hawkes WG, Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000; 11: 31–35.
Nieves J, Cosman F, Herbert J, Shen V, Lindsay R. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology. 1994; 44: 1687–1692.
Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000; 55: M489–491.
Bemben DA, Bemben MG. Dose-response effect of 40 weeks of resistance training on bone mineral density in older adults. Osteoporos Int. 2010; Feb 27.
Welsh L, Rutherford OM. Hip bone mineral density is improved by high-impact aerobic exercise in postmenopausal women and men over 50 years. Eur J Appl Physiol Occup Physiol. 1996; 74: 511–517.
Gleeson PB, Protas EJ, LeBlanc AD, Schneider VS, Evans HJ. Effects of weight lifting on bone mineral density in premenopausal women. J Bone Miner Res. 1990; 5: 153–158.
Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992; 7: 761–769.
Rockwell JC, Sorensen AM, Baker S, Weight training decreases vertebral bone density in premenopausal women: a prospective study. J Clin Endocrinol Metab. 1990; 71: 988–993.
Vuori I, Heinonen A, Sievanen H, Kannus P, Pasanen M, Oja P. Effects of unilateral strength training and detraining on bone mineral density and content in young women: a study of mechanical loading and deloading on human bones. Calcif Tissue Int. 1994; 55: 59–67.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This research was partially funded by the National Multiple Sclerosis Society.







