Publication
Research Article
International Journal of MS Care
Effects of Torso-Weighting on Standing Balance and Falls During the Sensory Organization Test in People with Multiple Sclerosis
Author(s):
Background:
In people with multiple sclerosis (MS), common gait and balance impairments can lead to falls, fear of falling, activity restriction, and social isolation. Sensory augmentation in the form of torso-weighting has resulted in improvement in gait and balance, but research on its effect on falls in MS is lacking.
Methods:
60 people with MS and 10 bin-matched controls completed the Sensory Organization Test (SOT) while nonweighted and again while weighted using the Balance-Based Torso-Weighting assessment method. This was a quasi-experimental pre-post intervention study. The SOT composite scores, equilibrium scores, and number of falls occurring across six SOT conditions were compared between and within groups using 2-way analysis of variance, α = .05 with planned t test analyses of weighting effects.
Results:
A significant increase in composite score of 9.14 points nonweighted to weighted occurred in the MS group (P < .001) but not in controls (P = .626). Equilibrium scores were significantly higher with weights in the MS group (P < .001) but not in controls (P = .5). Falls during the SOT were reduced by 35% with weights in the MS group versus without weights (P < .001), with the greatest number of falls occurring in the most challenging SOT conditions.
Conclusions:
During a single testing session, torso-weighting produced significant improvements in postural stability and fall reduction during the SOT for people with MS but no change in controls. Further research is needed to determine whether torso-weighting has the potential to reduce falls in MS during real-world activities.
Approximately 400,000 people in the United States live with the effects of multiple sclerosis (MS), an autoimmune disease of the central nervous system.1 MS is the most common cause of progressive disability in young adults in the United States, with most new diagnoses occurring between the ages of 20 and 40 years.1 Although medications can slow the accumulation of pathologic changes,2 dysfunction remains and tends to accrue over time. As the disease progresses, people with MS turn to rehabilitation to address movement impairments, activity limitations, and participation restrictions that affect their quality of life.
Impairments and activity restrictions of balance and mobility receive particular emphasis in rehabilitative assessment and intervention for this population because these dysfunctions have substantial effects on daily life.3 4 Imbalance occurs in 87% to 94% of people with MS.3 4 Balance problems can increase the chance of falling compared with controls.5 Between 52% and 71%6 7 of younger and middle-aged people with MS report having fallen recently, with up to 48% having multiple falls7; 41% of all falls and 50% of falls in those older than 55 years result in injury.3 7 Falls can have psychological consequences, including restricted activity because of concern about falling,8 limited social interaction, and decreased quality of life.9 For example, of 575 individuals with MS, 62% reported a concern about falling and 67% limited their activity because of these concerns.8 Activity limitation can ultimately result in physical deconditioning.10 Rehabilitative exercise can successfully improve strength and exercise tolerance,11 sometimes identified as factors affecting fall risk,12 if people are able and willing to participate regularly.
However, reviews of exercise therapy and physiotherapy interventions designed for people with MS have indicated that balance and fall rate may not change even if the intervention improves a factor associated with fall risk.11 13 The overall effect size for gait measures is moderate at best, the effect on balance measures is minimal,13 and few researchers have reported reduction in the number of falls as an outcome of their intervention.13 To date, the most successful programs for improving balance include those that target the sensorimotor and integration aspects of balance directly,12 possibly because the postural control deficits in people with MS are strongly associated with slowed somatosensory conduction and impaired central integration of sensory input.12 In one seminal pilot study comparing balance-specific motor, sensory plus motor, and non–balance-specific activities, Cattaneo et al.14 noted differences in effect on static and dynamic balance: augmenting motor interventions with specific sensory input resulted in no additional benefit to static balance measures but tended to improve dynamic balance measures. They also noted fall reduction during the 3-week inpatient rehabilitation program but reported no retention data to indicate carryover after discharge.14 In another study, neurotherapeutic interventions that included “facilitation of proprioceptive or sensory input” successfully improved dynamic balance measures, but the effects faded before a follow-up assessment 8 weeks after the 8-week supervised programs ended.15
Sensory augmentation using a variety of inputs (somatosensory, vestibular, visual)16–18 has shown success in populations with sensorimotor impairments similar to those in MS; however, results in people with MS have been variable.16 17
Providing somatosensory input via torso-weighting has resulted in gait and balance changes in MS when using the Balance-Based Torso-Weighting (Motion Therapeutics, Oxnard, CA) assessment method.19 20 Using this method, clinicians assess an individual's directional balance pattern. Based on assessment results, clinicians then augment somatosensory input with the strategic placement of light weights on a vest-like garment to improve response to static eyes-open and eyes-closed testing and balance perturbations. Torso-weighting differs from the traditional rehabilitative method of placing heavier weights at the trunk or limbs to help control movement21 because it uses light weights, generally less than 2% of body weight, strategically placed based on evidence of imbalance or asymmetry of response.19
Previous work has shown that immediately after torso-weighting, people with MS have improved static stability with decreased sway when standing with eyes open and closed,5 20 increased ability to resist rotational forces while standing,5 and more optimized variability of postural sway.22 Torso-weighting has resulted in improved dynamic stability in sit-to-stand transfers and walking20 and in increased gait velocity, cadence, and percentage of gait cycle spent in single-limb support in people with MS.23 However, to our knowledge, the effect of torso-weighting on falls in people with MS has not yet been studied.
The purpose of this study was to investigate the immediate effects of torso-weighting on stability and fall frequency as measured by the Sensory Organization Test (SOT) in people with MS. This study was conducted as part of a larger single-session investigation assessing the characteristics of people who responded positively to torso-weighting.
Methods
Participant Criteria and Overview
People with MS were recruited through advertisements in newsletters sent by the regional chapter of the National Multiple Sclerosis Society, fliers posted at MS walks hosted by the National Multiple Sclerosis Society, and local neurologists' offices. Potential participants were screened via a telephone call to ensure eligibility. All the participants self-reported the following: having an MS diagnosis; having problems with falling, walking, or balance; speaking English; aged 18 years or older; able to walk 35 ft with or without a cane; and able to endure 5 hours of testing, with rest breaks.
Individuals with a history of concurrent neurologic disorders, an MS exacerbation within the previous 2 months, or pain that could be increased during testing were excluded from this study. Because it is unknown how healthy individuals respond to torso-weighting, we included a comparison group of controls who were bin-matched for age and sex to participants with MS. Control participants were recruited through personal contacts and Internet advertisements. This was a quasi-experimental pre-post intervention study. All the participants gave informed consent as directed by the institutional review board of Samuel Merritt University (Oakland, CA).
Before testing, all the participants completed a medical questionnaire about their current health, MS symptoms (for those with MS), and fall history. Blood pressure and heart rate were measured, and participants' age, height, and weight were recorded.
Clinical testing occurred under two conditions presented in a set order: nonweighted followed by weighted. The nonweighted testing always preceded the weighted testing because people have shown carryover effects of the Balance-Based Torso-Weighting for hours after the weights have been removed.5 20 Disease Steps24 was rated by a single researcher (G.L.W.) during the first set of clinical tests.
Participants wore a safety harness while standing for the SOT, a type of computerized dynamic posturography. This was followed by three additional clinical tests performed in random order: the Timed Up and Go test,25 the Timed 25-Foot Walk test,26 and the Dynamic Gait Index.25 Participants then underwent torso-weighting using the Balance-Based Torso-Weighting method, followed by a rest break of at least 20 minutes before continuing testing. When weighted, the SOT was repeated, and clinical tests followed the same order as in nonweighted testing. Participants completed all the testing in a single session lasting 3 to 5 hours for people with MS or 1.5 to 3 hours for controls.
Sensory Organization Test
The SOT was performed using the EquiTest with NeuroCom System version 9.2 software (NeuroCom International Inc, Clackamas, OR). The SOT has been used previously to investigate imbalance and the effect of intervention in people with MS.27 28 The EquiTest machine consists of a square platform housing two force plates encased by a visual surround on three sides. The platform and visual surround can move in response to an individual's body sway. The safety harness allowed body sway but prevented a fall to the ground. In addition, a member of the research team guarded each participant. During testing, participants stood quietly with their arms at their sides with bare feet placed on the platform according to the manufacturer's instructions. The location of each participant's feet was marked by tape to ensure consistent foot positioning throughout the trials and in case of foot movement during testing.
The SOT consists of six conditions that progressively challenge the use of sensory information to maintain balance. For each of the six conditions, participants stood for three 20-second trials while the machine recorded body sway. In conditions 1 to 3, the platform remains stable while the visual settings vary: 1) eyes open, 2) eyes closed, and 3) eyes open with the visual surround moving according to the degree of anterior and posterior body sway (ie, visual sway referenced). In conditions 4 to 6, the platform moves according to body sway (ie, platform sway referenced): 4) eyes open, 5) eyes closed, and 6) eyes open with visual and platform sway referenced. All SOT trials were completed in the order recommended by the manufacturer. For each trial, the software program produces an equilibrium score ranging from 0% (representing a fall) to 100% (representing perfect stability or no body sway). Seeing the results of each of the three trials for each condition allows examination of the initial effects of encountering a novel task when the visual surround and platform are sway referenced. The software program also produces a composite score, calculated via a proprietary algorithm to average the equilibrium scores across the six SOT conditions, with the more challenging conditions 3 to 6 having a greater influence on the final score. In addition, the number of trials resulting in a fall (equilibrium score = 0) was counted. Falls were defined by the program as touching the surround, taking a step, or being caught by the harness.
Torso-Weighting Protocol
For the torso-weighting protocol,19 participants donned an adjustable vest-like garment (BW100; Motion Therapeutics, Oxnard, CA) to which light weights (0.25–0.5 lb) could be placed in any location via Velcro attachment. The assessor observed the direction, magnitude, and latency of body sway with feet together (toes and heels touching), first with eyes open and then with eyes closed. The assessor then applied anteroposterior and lateral perturbations at the shoulders and pelvis of participants while they were told to “stay strong, don't let me move you.” The assessor then applied rotational forces at the shoulders and then the pelvis to determine whether there was asymmetry in the participants' ability to resist rotational forces; participants were told to “hold, don't let me move you.” Balance loss was defined as tilt or lean of the trunk that required a stepping response, an opposing parachute reaction, or manual contact by a researcher to regain center of mass over the base of support. Direction, comparative magnitude, and latency of responses were recorded. After identification of asymmetries of response to perturbations and rotations, sensory input (weights) were strategically placed on the garment at locations customized to each individual to counteract balance deficiencies. Retests of perturbations and rotations were performed to confirm correct placement of weights.20 The protocol, including both pre and post assessments, required a minimum of 24 perturbations and eight resisted trunk rotations. One physical therapist (C.G.-H.) completed all the torso-weighting assessments. This assessor was not present during any of the nonweighted testing and did not have access to any participant data before weighting.
The mandatory rest break after the weighting protocol occurred with participants wearing the weighted garment. They were encouraged to rest in whichever position they preferred: sitting, or lying on a plinth with lights dimmed. Additional rest breaks were given throughout the testing sessions as dictated by participant fatigue. Rest breaks given during nonweighted testing were repeated during weighted testing. Once participants felt that they had sufficient rest to mimic the same energy level they had at the start of nonweighted testing, they repeated the tests while weighted.
Data Analysis
Data comparing the nonweighted and weighted conditions between and within the MS and control groups for each variable were analyzed via 2 × 2 two-factor repeated-measures analyses of variance (ANOVAs), with planned post hoc analyses to examine the weighting effects in each group (α = .05). Comparisons within the MS and control groups for composite scores, equilibrium scores, and fall frequency were made using dependent t tests (α = .05). We used one-way ANOVA to analyze changes in the composite score by level of disease severity as measured by Disease Steps in MS.
Results
Participants
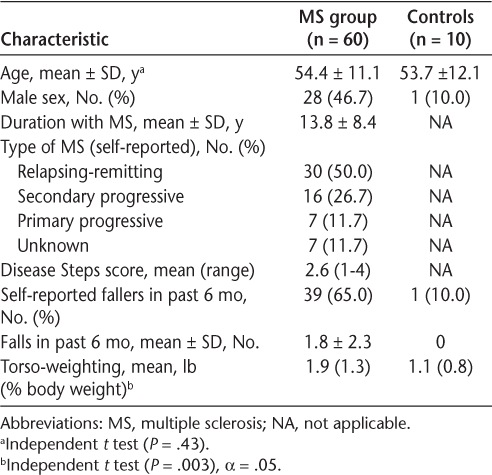
Sixty-four people with MS were enrolled in this study. Four participants did not complete testing: two stopped because of excessive fatigue and two had data marred by equipment malfunction. All ten control participants completed the testing. There were no significant differences in age between the MS and control groups (P = .43). Table 1 displays the self-reported MS subtypes, researcher-determined Disease Steps scores, and self-reported falls data in the sample.
Participant characteristics
Composite Scores
The ANOVA results indicated significant between (P < .001), within (P < .001), and interaction (P = .006) effects for composite scores. Planned post hoc analyses showed that mean (SD) composite scores increased from the nonweighted to the weighted condition, from 50.73 (14.6) to 60.10 (14.5) in people with MS (P < .001), a mean (SD) improvement of 9.37 (8.53) (range, −6 to 38) percentage points (Table 2). No significant change in the mean composite score occurred from nonweighted (73.9 [6.0]) to weighted (75.2 [9.5]) in controls (P = .626). The mean change in composite score in MS was greater than 7 for each level of disability on the Disease Steps scale; changes by level of disability were not statistically significantly different from each other (Table 3).
SOT analysis
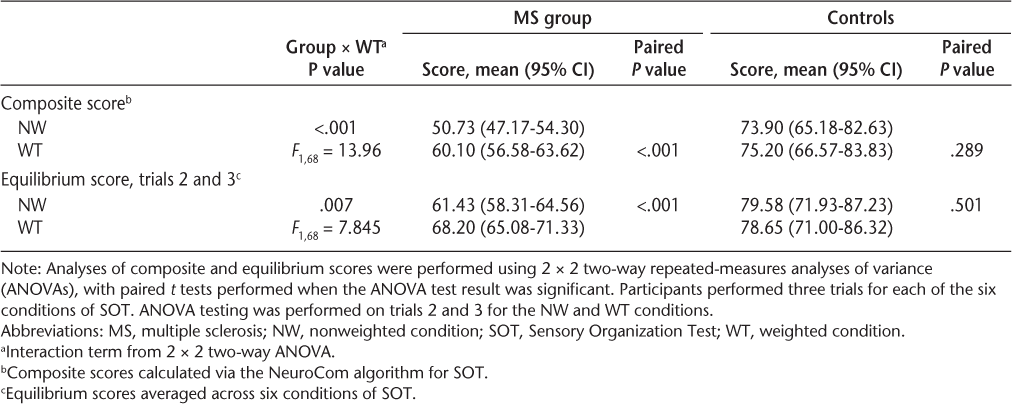
Composite score change in people with multiple sclerosis from nonweighted to weighted by Disease Steps score
Equilibrium Scores
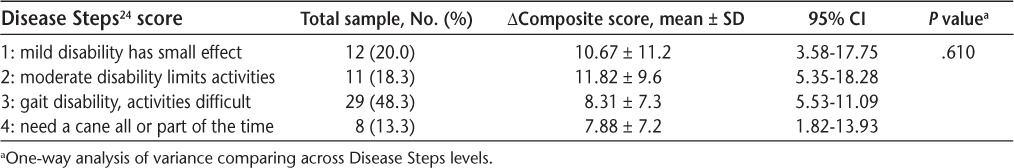
Observation of the equilibrium score data revealed that the first of three trials of the six SOT conditions consistently had a lower score than trials 2 and 3 (Figure 1). This observation was confirmed by post hoc statistical analysis (3 × 2 × 2 ANOVA, F 2,136 = 14.22, P < .001). Pairwise comparison of the trial factor showed a significant difference between trial 1 and trials 2 and 3 (P < .001) and no difference between trials 2 and 3 (P = .059). Table 2 shows the results of analyses of equilibrium scores when trial 1 was removed for the nonweighted and weighted conditions; we analyzed only trials 2 and 3 for comparison of the effect of weighting to reduce the impact of repeating the SOT twice in one session. Equilibrium scores were significantly different across weighting conditions in the MS group but, as expected, not in the control group (Figure 2).
Mean equilibrium scores for Sensory Organization Test (SOT) conditions 1 to 6 in people with multiple sclerosis
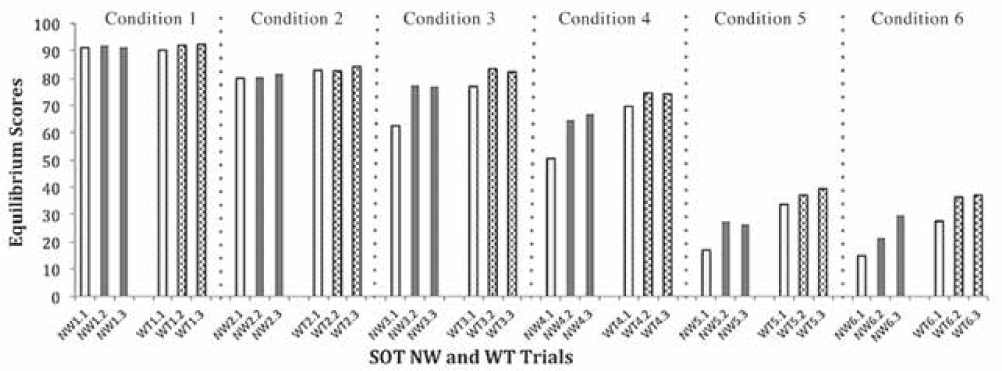
Mean (SD) equilibrium scores for trials 2 and 3 for nonweighted (NW) and weighted (WT) conditions in participants with multiple sclerosis (MS) and controls for each condition of Sensory Organization Test (SOT)

Falls
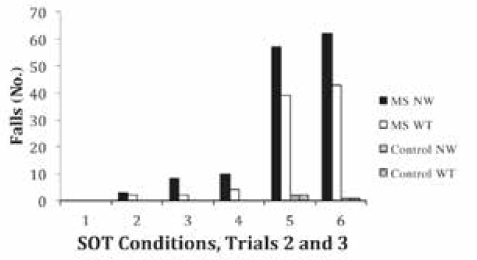
When analyzing falls, we observed that the greatest number of falls occurred during the first trial of each SOT condition, particularly conditions 3 to 6. When comparing falls across SOT conditions, we omitted falls that occurred in trial 1 from the nonweighted and weighted trials for all six SOT conditions. The ANOVA comparing fall occurrence across groups and weighting conditions showed a significant group × weighting interaction (F 1,68 = 4.291, P = .042). Planned post hoc analyses revealed that fall occurrence was significantly reduced during the weighted condition in people with MS (P < .001); the low fall occurrence at baseline in controls did not change with weighting (P = .5). Participants with MS had a mean 35% reduction from nonweighted to weighted in the total number of falls during SOT (Figure 3).
Total number of falls in trials 2 and 3 for each Sensory Organization Test (SOT) condition comparing nonweighted (NW) and weighted (WT) conditions in multiple sclerosis (MS) and control groups
Discussion
This study documents the immediate effects of torso-weighting on SOT performance in persons with MS and controls. The two groups were different at baseline, as expected. Participants with MS were able to stand during the SOT activities with improved composite and equilibrium scores and fewer falls, indicating greater stability under sensory challenge while weighted. In the small cohort of control participants, an effect of torso-weighting was not found, likely because of a ceiling effect. Torso-weighting resulted in changes in people with MS even though all the participants wore a relatively heavy SOT harness over the torso-weighting garment during SOT testing in the nonweighted and weighted conditions.
The improvements noted in people with MS occurred despite the fatigue observed in many participants while completing the 3- to 5-hour study protocol. Fatigue was surmised by asking participants if they were fatigued and recording the number of rest breaks that participants with MS required. Many participants with MS required multiple rest breaks during the testing session. None of the age- and sex-matched controls required additional rest breaks during the testing session. Despite people with MS typically noting fatigue as a limiting factor in daily function,4 the potential effect of fatigue on single-session performance is uncertain. Morris et al.29 reported no difference in gait parameters when repeating four 10-m gait trials later in the same day for people with MS. McLoughlin et al.30 reported that at the end of the 6-Minute Walk Test, people with MS had poorer spatiotemporal gait parameters and reported higher levels of fatigue. Age- and sex-matched control participants did not show these effects. Hebert and Corboy28 found a strong association between fatigue and SOT performance but not gait (measured using the 6-Minute Walk Test), particularly in people with MS who had pyramidal or cerebellar system involvement. The testing session in our study challenged participants much more than a single SOT, four 10-m gait trials, or a 6-Minute Walk Test. Seemingly no reports have yet been published of SOT performance when used twice in a single data collection session for people with MS, although Bernstein and Burkard31 repeated SOT twice on each of two separate days for healthy, young adults. Because the order of testing always had the nonweighted condition first, the improvements we noted 1 to 2 hours later in the weighted condition occurred when participants were potentially most fatigued.
Familiarity with the tests could have improved performance during the weighted condition, which always followed the nonweighted condition. If test familiarity was a major factor across weighting conditions, however, we would expect to see an average improvement across all trials, not just between weighting conditions. Instead, examination of the equilibrium scores from the separate trials on each of the six SOT conditions showed lower scores consistently for trial 1 of the SOT conditions, with no difference between trials 2 and 3. Because this effect was more prominent for SOT conditions 3 to 6 (Figure 1), we speculate that this intertrial difference is related to participants' surprise when the visual surround or platform first moved. When we omitted trial 1 from the equilibrium score analyses (Figure 2), we controlled for this initial impact of a novel task and thereby mitigated much of the influence of test familiarization in this single session. Removing trial 1 from the nonweighted and weighted conditions and comparing equilibrium scores from trials 2 to 3 demonstrated the robustness of the results, with significant interaction effects and weighting effects on the equilibrium scores (Table 2). Removal of trial 1 from the nonweighted and weighted conditions helped mitigate the confounder of test familiarity and the element of surprise from the analysis, a potential concern because this seems to be the first study in MS in which the SOT was performed twice in the same session. When comparing fall data, we also omitted the first trial under both weighting conditions to standardize the number of trials in which falls could be counted and, again, to reduce the factor of surprise on fall incidence.
People with MS increased composite scores during the weighted condition over nonweighted by a mean of 9.14 points, which is higher than the minimal detectable change (MDC) determined by Wrisley et al.32 of 8 composite score points in healthy young adults. In the present study, 34 people with MS (57%) and three controls (30%) increased their composite score by at least 8 points. Because no MDC has been published for people with MS, we calculated the MDC to be 6.14 points based on the standard error of measurement for participants with MS on the SOT. We considered a composite score change of 7 points or greater to be a reasonable cutoff score for meaningful change. Using this cutoff score, 38 participants (63%) had a composite score change of 7 points or more. Hebert et al.27 used a composite score change of 8 points to indicate a meaningful change in SOT scores but suggested the use of 7 points for people with MS. Our calculated MDC concurs with their recommendation. Analysis of composite score changes across Disease Steps disability levels 1 to 4 indicated that the mean change was greater than the 7-point cutoff score for all disability levels (Table 3).
The potential clinical importance of the change in composite score is supported by the change in numbers of falls during the SOT performance when participants were weighted. We believe this is the first study to show an effect of torso weighting on falls during the SOT. Decreased falls while performing the SOT may translate to fewer falls in the home and community. However, the number of falls that participants self-reported occurring in the past 6 months did not relate to the falls experienced on the SOT with nonweighting or weighting. Of the 21 individuals with MS who reported not falling in the past 6 months, 18 fell during the SOT. This increase in falling incidence during testing may be because the SOT forces people to respond to constraints placed on sensory inputs (eg, with eyes closed or platform or surround moving to confuse sensory inputs) or because people who have gait or balance difficulties often limit their ambulation to avoid falling. A prospective, longer-term study might help clarify the relationship between fewer falls on the SOT and fall rate in daily life.
The SOT data (Table 3) support findings in other studies. Similar to the present study, Hebert and Corboy28 report that equilibrium scores for participants with MS were lower in conditions 5 and 6 than in the other SOT conditions (Figure 1). In another study, Hebert et al.27 had people with MS participate in 14 weeks of a vestibular exercise program and reported improvements in SOT performance by 18.5 composite score points, whereas the exercise control group changed by 5.4 points and the waitlist control group changed by 6.4 points during the same period. In contrast, in the present study, the mean improvement of more than 9 points occurred in a single session. Future studies might evaluate long-term torso-weighting combined with other targeted exercise programs to see whether the effects can be compounded.
Using another mode of sensory augmentation, whole-body vibration, Schuhfried et al.16 found a mean change in composite score of 5.8 and 7.0 immediately and 1 week later, respectively, in six people with MS. They calculated a clinically meaningful difference of 6.7 points on the SOT composite score. Their mean change in composite score was less than the immediate effects noted in the present study of 9.14 points.
This study has limitations. The nonweighted testing always preceded the weighted testing. Future studies are planned in which nonweighted and weighted testing will occur in separate sessions to mitigate for the potential carryover effects lasting up to several hours after weights are removed.5 20 In this study, we reduced the impact of test familiarity on immediate performance by eliminating trial 1 when analyzing equilibrium scores and fall frequency. Another limitation was the absence of an MS control group, the inclusion of which may have helped clarify the effects of torso-weighting on fatigue and the potential practice effects of the SOT. Although this study was designed to evaluate the effects of torso-weighting during a single session, longitudinal studies will help discover whether this intervention can affect balance and posture in people with MS during real-world activity.
Improvements with torso-weighting for people with MS did not reach the performance of controls. Future research is planned for examining the long-term effects of this targeted sensory input (torso-weighting) in real-world situations to evaluate the effects on fatigue, falls, and quality of life. Falls are more likely to be related to everyday activities or when walking in varied environments,33 such as those experienced by people with MS in their homes and communities. However, the immediate findings from this study demonstrate the potential of this intervention to improve postural stability and reduce falls, thus improving quality of life in people with MS.
PRACTICE POINTS
Torso-weighting augments somatosensation through the strategic placement of light weights on a vest-like garment based on directional imbalance.
Torso-weighting, using the Balance-Based Torso-Weighting assessment method, significantly reduced the number of falls and improved balance on the Sensory Organization Test during a single session in people with MS.
Financial Disclosures:
Ms. Gibson-Horn is the chief technology officer, part owner, and co-founder of Motion Therapeutics. Ms. Horn and Drs. Allen and Widener have no conflicts of interest to disclose.
References
Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(suppl 2):15S–20S.
Finkelsztejn A. Multiple sclerosis: overview of disease-modifying agents. Perspect Medicin Chem. 2014;6:65–72.
Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil. 2008;89:1031–1037.
Trojan DA, Arnold D, Collet JP, et al. Fatigue in multiple sclerosis: association with disease-related, behavioural and psychosocial factors. Mult Scler. 2007;13:985–995.
Crittendon A, O'Neill D, Widener GL, Allen DD. Standing data disproves biomechanical mechanism for balance-based torso-weighting. Arch Phys Med Rehabil. 2014;95:43–49.
Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:1274–1279.
Cameron M, Mazumder R, Murchison C, King L. Mini Balance Evaluation Systems Test in people with multiple sclerosis: reflects imbalance but may not predict falls. Gait Posture. 2014;39:669.
Matsuda PN, Shumway-Cook A, Ciol MA, Bombardier CH, Kartin DA. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther. 2012;92:407–415.
Coote S, Gallagher S, Msetfi R, et al. A randomised controlled trial of an exercise plus behaviour change intervention in people with multiple sclerosis: the step it up study protocol. BMC Neurol. 2014;14:241.
Rampello A, Franceschini M, Piepoli M, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007;87:545–555.
Rietberg MB, Brooks D, Uitdehaag BM, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2011(1):CD003980.
Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010;10:407–412.
Paltamaa J, Sjogren T, Peurala SH, Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med. 2012;44:811–823.
Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil. 2007;21:771–781.
Wiles CM, Newcombe RG, Fuller KJ, Furnival-Doran J, Pickersgill TP, Morgan A. Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70:174–179.
Schuhfried O, Mittermaier C, Jovanovic T, Pieber K, Paternostro-Sluga T. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil. 2005;19:834–842.
Armutlu K, Karabudak R, Nurlu G. Physiotherapy approaches in the treatment of ataxic multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2001;15:203–211.
Broekmans T, Roelants M, Alders G, Feys P, Thijs H, Eijnde BO. Exploring the effects of a 20-week whole-body vibration training programme on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med. 2010;42:866–872.
Gibson-Horn C. Balance-based torso-weighting in a patient with ataxia and multiple sclerosis: a case report. J Neurol Phys Ther. 2008;32:139–146.
Widener GL, Allen DD, Gibson-Horn C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair. 2009;23:784–791.
Clopton N, Schultz D, Boren C, Porter J, Brillhart T. Effects of axial loading on gait for subjects with cerebellar ataxia: preliminary findings. Neurol Report. 2003;27:15–21.
Hunt CM, Widener GL, Allen DD. Variability in postural control with and without balance-based torso-weighting in people with multiple sclerosis and healthy controls. Phys Ther. 2014;94:1489–1498.
Gorgas AM, Widener GL, Gibson-Horn C, Allen DD. Gait changes with balance-based torso-weighting in people with multiple sclerosis. Physiother Res Int. 2015;20:45–53.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255.
Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006;28:789–795.
Rasova K, Martinkova P, Vyskotova J, Sedova M. Assessment set for evaluation of clinical outcomes in multiple sclerosis: psychometric properties. Patient Relat Outcome Meas. 2012;3:59–70.
Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91:1166–1183.
Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture. 2013;38:37–42.
Morris ME, Cantwell C, Vowels L, Dodd K. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:361–365.
McLoughlin JV, Barr CJ, Patritti B, Crotty M, Lord SR, Sturnieks DL. Fatigue induced changes to kinematic and kinetic gait parameters following six minutes of walking in people with multiple sclerosis. Disabil Rehabil. 2016;38:535–543.
Bernstein J, Burkard R. Test order effects of computerized dynamic posturography and calorics. Am J Audiol. 2009;18:34–44.
Wrisley DM, Stephens MJ, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning effects of repetitive administrations of the Sensory Organization Test in healthy young adults. Arch Phys Med Rehabil. 2007;88:1049–1054.
Cattaneo D, Jonsdottir J, Coote S. Targeting dynamic balance in falls-prevention interventions in multiple sclerosis: recommendations from the International MS Falls Prevention Research Network. Int J MS Care. 2014;16:198–202.







