Publication
Research Article
International Journal of MS Care
Effects of Functional Electrical Stimulation on Gait Function and Quality of Life for People with Multiple Sclerosis Taking Dalfampridine
Author(s):
Background: Multiple sclerosis (MS) can adversely affect gait, causing gait slowing, loss of balance, decreased functional mobility, and gait deficits, such as footdrop. Current treatments for gait dysfunction due to MS are pharmacologic, using dalfampridine, or orthotic, using an ankle-foot orthosis. Functional electrical stimulation (FES) to the fibular nerve stimulates active dorsiflexion and provides an alternative treatment for gait dysfunction caused by footdrop. The objective of this study was to determine the effect of FES on gait function and the impact of MS on walking and quality of life for people with MS taking a stable dalfampridine dose.
Methods: Participants demonstrating gait slowing and footdrop completed the Timed 25-Foot Walk (T25FW) test, 6-Minute Walk (6MW) test, GaitRite Functional Ambulation Profile, 12-item Multiple Sclerosis Walking Scale (MSWS-12), and 36-item Short Form Health Status Survey (SF-36) at screening without FES; the measures were repeated with FES at baseline, 1 month, and 3 months.
Results: Twenty participants (8 men and 12 women) completed this unblinded case series study. The mean age, duration of MS, and time taking dalfampridine were 51.7, 15.8, and 1.4 years, respectively. Changes from screening to baseline and screening to 3 months were analyzed. Significant improvement was noted from screening to baseline for the MSWS-12 (P = .024) and SF-36 Physical Function domain (P = .028) and from screening to 3 months for the T25FW (P = .015), MSWS-12 (P = .003), and SF-36 Physical Function (P = .032) and Role Limitation–Physical Health (P = .012) domains.
Conclusions: Improvements above those induced pharmacologically suggest that FES can augment pharmacologic intervention and significantly improve gait function, decrease the impact of MS on walking, and improve quality of life for people with MS.
Multiple sclerosis (MS) is a chronic immune-mediated disorder affecting movement, sensation, and bodily functions. It is caused by destruction of the myelin insulation covering nerve fibers (neurons) in the central nervous system (brain and spinal cord). MS has an unpredictable course, is progressive in nature, and affects nearly 2.5 million people worldwide.1 Up to 75% of people with MS have some difficulty with ambulation,2 and many depend on an assistive device or a wheelchair.3 Gait disturbances are reported to be among the most disabling effects of the disease, and maintaining mobility is one of the highest priorities for people with MS.4 Footdrop may be an early or late consequence of MS, and it leads to impaired gait. Footdrop is an inability to dorsiflex the foot during the swing phase of gait due to either dorsiflexor weakness, increased tone in the plantarflexor muscles, or disordered neural control causing co-contraction of agonist and antagonist muscles.5 This compromised neuromuscular control around the ankle typically leads to abnormal patterns of gait that negatively affect speed,6 7 endurance,7 energy expenditure,6 and balance.6 7 These gait deficits can restrict functional mobility and activities of daily living and can negatively affect quality of life (QOL).5
Currently, the treatments most commonly used to specifically address gait disorders due to MS are pharmacologic, using dalfampridine, or orthotic, using an ankle-foot orthosis (AFO). Dalfampridine therapy has been shown to increase gait speed by approximately 25% in approximately one-third of patients with MS.8 The medication is extended release; the recommended dosage is 10 mg every 12 hours, with peak concentrations noted within 3 to 4 hours of administration.9 Adverse effects, such as confusion, locomotor or balance problems, and seizures, have been associated with dalfampridine therapy.10 An AFO is a rigid or hinged plastic brace that keeps the foot in a neutral position during the swing phase, thus increasing toe clearance. The disadvantages of AFOs include restriction of movement,7 11 12 muscle atrophy,11 limited choice of footwear,11 poor cosmetic appearance,11 and user discomfort.12
An alternative treatment for the gait deviation footdrop is fibular nerve functional electrical stimulation (FES), which electrically stimulates the dorsiflexor muscles of the foot during swing and promotes active and effective toe clearance. The advantages of FES are that it facilitates a more natural gait pattern,11 provides muscle activation,11 13 increases blood circulation,11 12 and reduces the occurrence of muscle atrophy.11–13 Functional electrical stimulation has been shown to improve voluntary muscle control and to facilitate a therapeutic effect (improved function after the device is removed) in people with MS7 14 and in individuals who have sustained a cerebrovascular accident (CVA),7 14 15 suggesting that FES facilitates positive neuroplastic changes. The results of the study by Stein et al.7 showed significant increases in gait speed in individuals with MS and those post-CVA after 3 months of FES wear when the FES was turned off, thus demonstrating a therapeutic effect. In a study assessing the effects of 3 months of FES wear on gait function and cortical activity in people with MS and individuals post-CVA, Everaert et al.14 also found a significant increase in gait speed without FES as well as significant increases in maximum voluntary contraction and motor evoked potentials measured at the anterior tibialis. In previous studies evaluating the effects of FES in patients with MS and those post-CVA, FES of the fibular nerve has also been shown in both populations to increase gait velocity,7 11 16 decrease energy expenditure with gait,7 11 16 and improve voluntary muscle control.14 In addition, two studies designed to assess the effects of FES on the quality of gait found that FES improves gait symmetry in patients with chronic CVA.6 12
The purpose of this study was to investigate the effect of FES on gait speed, endurance, and quality in people with MS who have footdrop and who are taking a stable dose of dalfampridine. A second purpose was to determine the impact of MS on walking and on the physical components of QOL for people with MS. This study was designed to evaluate the effects of FES over a 3-month course of daily full-time wear.
Methods
Participants
Participants were individuals who exhibited leg weakness, slowing of gait, and footdrop as a result of their MS and who had been taking a stable dose of dalfampridine for at least 3 months. All the participants were recruited from the MS patient population of Central Texas Neurology Consultants in Round Rock, Texas. Individuals were included in the study if they 1) could walk at least 25 feet with or without assistance, 2) could complete the 25-foot distances in more than 8 and less than 45 seconds (gait assessment completed while the individual was taking a steady dose of dalfampridine), 3) demonstrated inadequate dorsiflexion during the swing phase of gait (defined as −5° of plantarflexion), 4) demonstrated adequate dorsiflexion of the ankle in response to fibular nerve stimulation, and 5) were not currently using FES for treatment of footdrop. The range of times for walking 25 feet included in the inclusion criteria was based on the functional ambulation categories of Perry et al.17 Validated in the stroke population, these categories classify functional ambulation based on gait speed as follows: 1) physiological ambulation (0.1–0.23 m/s), 2) limited household ambulation (0.23–0.27 m/s), 3) unlimited household ambulation (0.27–0.4 m/s), 4) most-limited community ambulation (0.4–0.6 m/s), 5) least-limited community ambulation (0.6–0.8 m/s), and 6) full community ambulation (>0.8 m/s).17 The ability to complete a 25-foot walk in less than 8 seconds equates to a gait speed of 0.9 m/s and would classify an individual as a full community ambulator, an ambulation level too high for an intervention to have an appreciable effect. Conversely, completing a 25-foot walk in greater than 45 seconds equates to a gait speed of 0.17 m/s. This speed would classify an individual as capable of physiological ambulation only; an individual at this level would likely not be able to tolerate a trial emphasizing gait.
Individuals were excluded from the study if they 1) had an MS exacerbation within 60 days; 2) required an AFO for stance control of the foot, ankle, or knee; 3) had a seizure disorder; 4) had an existing electrical stimulation device (implantable cardioverter defibrillator, pacemaker, or spinal stimulation); 5) had botulinum toxin (Botox; Allergan, Inc., Irvine, CA) injections in the lower extremity in the preceding 6 months; 6) had a baclofen pump with unstable dosing in the past 3 months; or 7) were diagnosed as having peripheral nerve injury in the involved lower extremity with symptoms that limited participation in study activities.
Procedure
All the participants signed an informed consent form approved by the Western Institutional Review Board. They participated in a screening visit at which demographic information was collected and they were screened for the inclusion and exclusion criteria. Screening for the inclusion and exclusion criteria included ambulation over a distance of 25 feet to assess baseline gait ability and a fibular nerve stimulation test. If all the eligibility criteria were met, the person was enrolled in the study.
The FES device used for this study was the WalkAide device (WA) (Innovative Neurotronics, Austin, TX). The WA is a battery-operated, single-channel electrical stimulator used for the treatment of footdrop of central nervous system origin. The device consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. The WA uses a tilt sensor and accelerometer to trigger ankle dorsiflexion and control the timing and duration of fibular nerve stimulation during the swing phase of the gait cycle. After the initial fitting, programming, and patient education performed by a trained clinician, participants were able to use the WA to facilitate walking in daily activities. All the measures in this study were collected by a licensed physical therapist (TW), and the WA was fit by a WA-certified orthotist. Measures were taken at screening without the WA device. The same measures were taken with the WA device at a baseline visit 1 to 2 weeks later and again at visits scheduled 1 and 3 months after baseline.
Outcome Measures
Measures used in this study to assess gait function were the Timed 25-Foot Walk (T25FW) test, the 6-Minute Walk (6MW) test, and the Functional Ambulation Profile (FAP) calculated by the GAITRite instrumented mat (CIR Systems Inc., Sparta, NJ). The T25FW test is considered a reliable, objective measure of walking disability18 and is one of the most frequently used measures to assess gait function in people with MS.19 Participants are instructed to walk 25 feet at their fastest safe speed; they may use assistive devices. The time begins at a command of start and ends when the participant crosses the 25-foot mark; the acceleration phase is included in the scoring. The participant completes two trials; the average of the trials is considered the final measure. The 6MW test measures the distance that a participant can walk indoors on a flat, hard surface in 6 minutes. The test is considered a reliable measure of functional exercise capacity in MS.20 The GAITRite FAP is a quantitative means of assessing gait function and quality in adults. The FAP scores are calculated from temporal and spatial gait measures collected as the participant walks over the GAITRite instrumented mat; scores are normalized to leg length. The FAP score, which ranges from 0 to 100 (with higher scores indicating better gait quality), is considered a reliable and valid means of assessing gait quality that has been validated in the MS population.21
The impact of MS on walking ability and on the physical components of QOL was measured by the 12-item Multiple Sclerosis Walking Scale (MSWS-12) and the 36-item Short Form Health Status Survey (SF-36). The MSWS-12, considered more responsive than other walking-based scales, is a reliable and valid patient-based measure of the impact of MS on walking.22 23 For this measure, the participant completes a questionnaire and rates the degree of limitation in walking due to MS experienced in the previous 2 weeks for each of 12 activities. Individual item responses are summed, and the total score is standardized to a scale with a range of 0 to 100. Higher scores reflect a greater limitation on walking abilities due to MS. The SF-36 is a reliable, valid measure shown to capture the broad effects of health problems in patients with MS.24 This study aimed to evaluate changes in QOL due to physical improvement; therefore, analysis was performed on scores for the individual domains of Physical Function and Role Limitation–Physical Health.
Analysis
This was an unblinded sequential case series, institutional review board–approved study. Measures in this study were analyzed via paired-samples t tests for changes in measures from screening to baseline to assess for immediate orthotic effects and from screening to 3 months to assess for effects due to use of the device over time.
Results
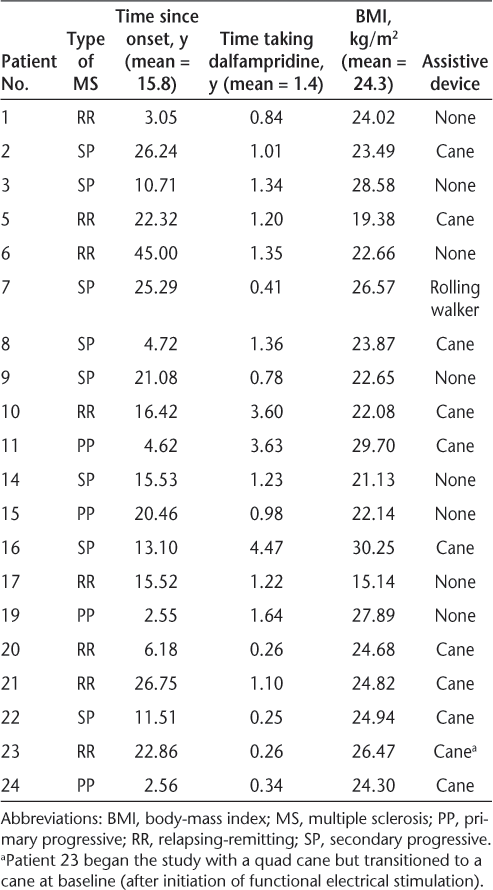
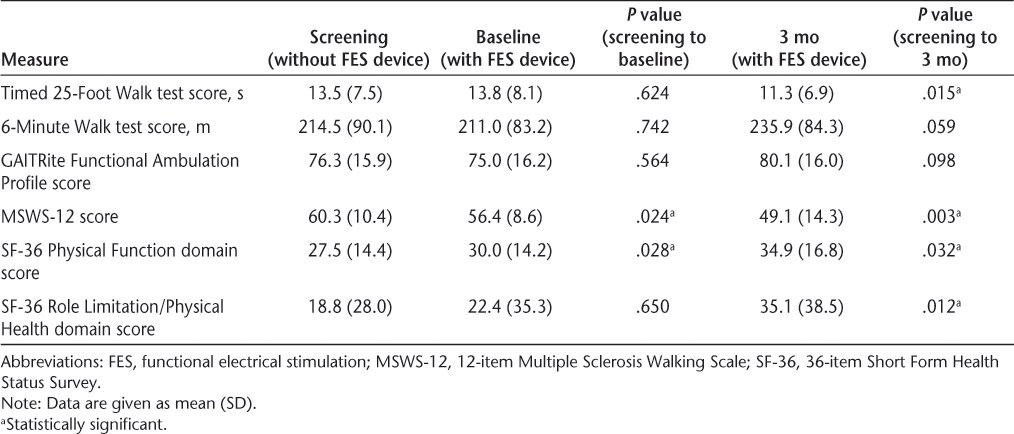
Twenty-four individuals were enrolled, and 20 completed the study (12 women and 8 men); 3 participants were withdrawn for noncompliance and 1 owing to an exclusion criterion that was discovered after enrollment. Participants ranged in age from 35.7 to 67.5 years, with a mean of 51.7 years. The characteristics of the participants in this study are further detailed in Table 1. Detailed results for all the outcome measures are shown in Table 2.
Characteristics of the 20 study participants
Outcome measures in the 20 study participants
Gait Performance
Participants demonstrated a significant decrease in time to complete the T25FW, from 13.5 seconds at screening to 11.3 seconds at 3 months (P = .015), a change of 16.3%. The distance walked during the 6MW test increased from 214.5 m at screening to 235.85 m at 3 months. Although this increase represented an improvement of 10% in distance walked, it was not significant (P = .059). There were no significant differences in T25FW or 6MW test measures between screening and baseline. There was no significant change in FAP measures between screening and baseline or between screening and 3 months.
Impact of MS on Walking and QOL
Use of FES resulted in a significant decrease in the impact of MS on walking ability, as demonstrated by a reduction in the MSWS-12 score. The MSWS-12 score decreased by 6.4% between screening and baseline (P = .024) and continued to decrease, resulting in an 18.5% reduction between screening and 3 months (P = .003). Significant changes were noted for the physical components of QOL as well. The Physical Function domain score was significantly decreased between screening and baseline (P = .028) and between screening and 3 months (P = .032). The Role Limitation–Physical Health domain score was also significantly decreased from baseline to 3 months (P = .012), but no significant difference was noted between screening and baseline for this measure.
Discussion
Gait dysfunction due to footdrop disrupts walking patterns and can have a significant negative effect on speed, endurance, balance, and community mobility.6 7 The results of this study demonstrate that FES can have a positive effect on gait function and on an individual's perception of the impact of MS on their disability, both of which can affect perception of QOL. The participants in this study, on average, began treatment with dalfampridine 1.4 years before the study. There is evidence in the literature demonstrating that treatment with dalfampridine can result in an approximate 25% increase in gait speed8 10 and that the effects are noticeable within 3 to 4 hours.9 Any improvement in participants' gait due to dalfampridine would be expected to have stabilized before enrollment in this study; therefore, the results seen in this study are thought to be attributable to the effects of FES.
Gait Performance
Participants in this study demonstrated a significant improvement in time to complete the T25FW at 3 months. Although there was no significant immediate effect (at baseline), these results indicate that the participants' gait performance improved significantly over time. The magnitude of decrease in the time to complete the T25FW test by participants in this study was 16.3%. The minimal clinically important difference for this measure has been noted in the literature to be approximately 20%.25 Coleman et al.,26 in their study of the effects of dalfampridine on gait speed for people with MS, suggested that a minimal clinically important difference of 17% was representative of a clinically relevant change. The results of that study also estimated a change in gait speed of 0.35 to 0.37 ft/s to be an appropriate estimate of minimal clinically important difference for this population.25 This study showed similar results, with a mean change in gait speed of 0.37 ft/s, suggesting that the changes noted for the participants in this study would be clinically relevant. The T25FW test has additional functional significance because the measure assesses not only steady-state gait speed but also the ability of the individual to accelerate to their preferred speed, making the test a more valid measure of overall ability with gait. This is important because the ability to change speed requires some degree of motor control and dynamic stability. Patients with MS have been shown to experience greater variability in gait.21 27 They walk more slowly, take shorter, slower steps, and spend more of their gait cycle in double support than do controls.28 Variability in gait patterns affect gait quality and can contribute to instability.28 A significant improvement over time on this measure suggests an improvement in speed as well as an improvement in control of gait, with quicker initiation of walking and better control and stability with acceleration.
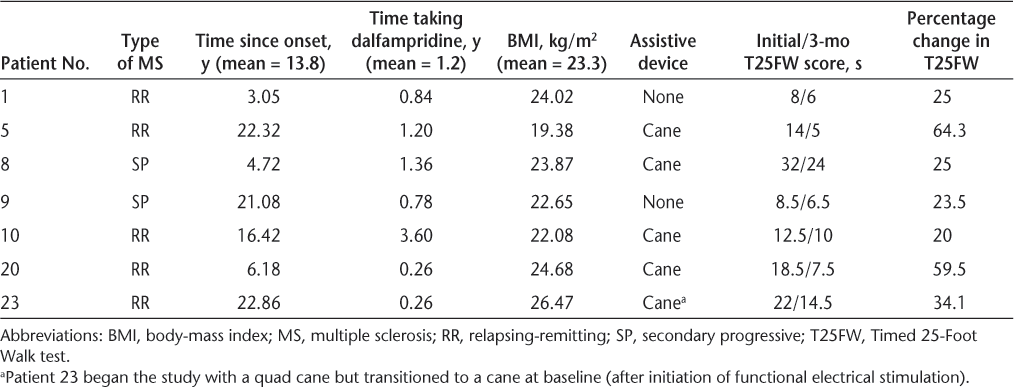
Evidence suggests that approximately one-third of people with MS respond to dalfampridine therapy, with an approximate 25% increase in gait speed.8 10 This study noted an increase in speed sufficient to decrease the time to complete the T25FW by 16.3% in participants already taking dalfampridine. This decrease was statistically and clinically relevant. A more detailed analysis of the data showed that 65% of the participants (13 of 20) decreased their T25FW time. The smallest improvement represented a 5% decrease and the largest a 64% decrease in time to complete the test. An analysis of change for the best responders, those showing an improvement in percentage change of at least 20%, shows that 35% of the participants (7 of 20) decreased their time to complete the T25FW by an average of 36%, more than twice that of the average of the whole group (13.75%). The mean age of the seven best responders (five men and two women) was 46.1 years, slightly lower than that of the entire group. The time since diagnosis, time taking dalfampridine, and body-mass index were similar between the seven best responders and the whole group. The characteristics of these seven best responders are outlined in Table 3.
Characteristics of study participants demonstrating at least 20% improvement in the T25FW
The change in distance walked between screening and 3 months neared significance (P = .059) for the participants in this study. This suggested that there was a trend toward improved endurance and an increase in the distance that the participants were able to walk in 6 minutes. Several studies have shown that FES decreases the effort required to walk in individuals with MS.7 11 16 The results of a study investigating the physiological cost of gait found on the basis of a single wear trial that the amount of oxygen required per unit distance for people with MS at their preferred walking speed decreased significantly when wearing FES.11 Two studies looking at long-term FES wear used the Physiological Cost Index, a measure of heart rate and respiratory rate, to measure the energy cost of gait for individuals with MS. In the first study, the subjects wore an FES device for 18 weeks and demonstrated a significant decrease in Physiological Cost Index of 25%16; in the second, subjects demonstrated a significant decrease in Physiological Cost Index of 6.5% after wearing the FES device for 3 months.7 The results of this study are in agreement with previous evidence and suggest that FES improves the ease of gait and allows individuals to demonstrate increased endurance.
Impact of MS on Walking and QOL
Perhaps the most significant effects of the FES intervention in this study were the improvements in the MSWS-12 and the physical domains of the SF-36. The scores for the MSWS-12 and the Physical Function domain of the SF-36 decreased significantly from screening to baseline. These results show that FES had immediate positive effects on the impact of MS on walking ability and on participants' perception of the impact of their physical limitations on their QOL. These measures were also significant from screening to 3 months, illustrating that FES continues to decrease the impact of MS on walking and QOL over time. The participants also reported an improvement in the domain of Role Limitation–Physical Health at 3 months, which is additional evidence that over time FES decreases the impact of MS on QOL as it pertains to physical function.
Because the MSWS-12 and SF-36 are multidimensional measures, many factors enter into the participant's response. One of the possible reasons that the results of these measures were significant at baseline and the gait performance measures (T25FW and 6MW tests) were not is that there were qualitative effects that were not immediately detected by clinical gait measures. One contributor to gait dysfunction for individuals with MS is spasticity. Spasticity negatively affects motor control and the quality of walking patterns11 and can affect energy expenditure even more than weakness.29 Functional electrical stimulation has been shown in the literature to decrease spasticity in subjects with hemiplegia from stroke.30–32 The improvement in biomechanics due to the restoration of active dorsiflexion combined with an inhibiting effect on muscle tone could well have translated to an improved perception of gait ability and a perceived lessening of the impact of MS on walking and the physical aspects of QOL for the participants in this study. This perception of improvement was apparent immediately on application of the FES and was strengthened over the course of 3 months. This sustained decrease in the impact of MS on walking ability and on the physical domains of QOL could very well result in increased activity, community mobility, and social participation for these individuals.
Limitations
This was a small study with a population of convenience. Although the sample size was sufficient to produce statistically significant results, these results may be limited in their generalizability to a larger population. More studies are needed to examine these effects with larger groups and across varied populations. Another possible limitation of this study is that the participants were all taking a stable dose of dalfampridine. The effects of this pharmacologic intervention enhanced the gait performance of the participants. Although the FES intervention produced significant additional improvement, it is impossible to know what the effect of FES would have been had the participants been at their true, premedication baseline for gait ability. Although a truly blinded trial of FES is not possible owing to the clear physiological effect that cannot be masked, a randomized head-to-head comparison of FES versus dalfampridine might yield additional valuable information.
Conclusion
The participants in this study showed significant improvement in gait ability as measured by the T25FW test and a significant decrease in the impact of MS on walking and on physical domains of QOL. A decrease in the impact of MS on walking and improvements in the Physical Function domain of the SF-36 were both seen at baseline, demonstrating that people with MS perceive an immediate positive effect with FES. These effects continued to be significant across 3 months; also significant at 3 months were the T25FW test and the Role Limitation–Physical Health domain of the SF-36, indicating that the positive effect of FES can be cumulative. In addition, these effects were above and beyond those seen with a pharmacologic intervention, suggesting that FES can augment a pharmacologic intervention and significantly improve gait function as well as decrease the impact of MS on walking and on the physical aspects of QOL for people with MS.
PracticePoints
Use of functional electrical stimulation demonstrates improvement in gait speed, perceived walking ability, and physical domains of quality of life for people with MS-related footdrop.
Improvements after use of functional electrical stimulation are seen over as short a period as 3 months, even in patients with long-standing MS (duration of disease >15 years).
Additional improvements in gait function and quality of life beyond those seen with pharmacologic intervention (dalfampridine) are possible in people with MS-related footdrop.
References
World Health Organization. Atlas: country resources for neurological disorders 2004: results of a collaborative study of the World Health Organization and the World Federation of Neurology. http://www.who.int/mental_health/neurology/epidemiology/en. Accessed April 23, 2013.
Lord SE, Halligan PW, Wade DT. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil. 1998; 12: 477–486.
Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Mult Scler. 1999; 5: 369–376.
Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010; 26: 109–119.
Barrett CL, Mann GE, Taylor PN, et al. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009; 15: 493–504.
Hausdorff JM, Ring H. Effects of a new radio frequency-controlled neuroprosthesis on gait symmetry and rhythmicity in patients with chronic hemiparesis. Am J Phys Med Rehabil. 2008; 87: 4–13.
Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010; 24: 152–167.
Blight AR. Treatment of walking impairment in multiple sclerosis with dalfampridine. Ther Adv Neurol Disord. 2011; 4: 99–109.
Acorda Therapeutics. AMPYRA medication guidelines and prescribing information. http://ampyra.com/local/files/PI.pdf. Accessed July 16, 2013.
Goodman AD, Brown TR, Cohen JA, et al. Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology. 2008; 71: 1134–1141.
Paul L, Rafferty D, Young S, et al. The effect of functional electrical stimulation on the physiological cost of gait in people with multiple sclerosis. Mult Scler. 2008; 14: 954–961.
Ring H, Treger I, Gruendlinger L, et al. Neuroprosthesis for footdrop compared with ankle-foot orthosis: effects on postural control during walking. J Stroke Cerebrovasc Dis. 2009; 18: 41–47.
Stein RB, Chong SL, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006; 20: 371–379.
Everaert DG, Thompson AK, Chong SL, et al. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010; 24: 168–177.
Kottink AI, Hermens HJ, Nene AV, et al. Therapeutic effects of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized clinical trial. Phys Ther. 2008; 88: 437–448.
Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999; 80: 1577–1583.
Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995; 26: 982–989.
Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. 2012; 18: 914–924.
Rasova K, Martinkova P, Vyskotova J, et al. Assessment set for evaluation of clinical outcomes in multiple sclerosis: psychometric properties. Patient Relat Outcome Meas. 2012; 3: 59–70.
Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008; 14: 383–390.
Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal–spatial parameters using GAITRite functional ambulation system. Gait Posture. 2009; 29: 138–142.
Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003; 60: 31–36.
Motl RW, Dlugonski D, Suh Y, et al. Multiple Sclerosis Walking Scale-12 and oxygen cost of walking. Gait Posture. 2010; 31: 506–510.
Isaksson AK, Ahlstrom G, Gunnarsson LG. Quality of life and impairment in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005; 76: 64–69.
Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-Foot Walk in the Multiple Sclerosis Functional Composite. Mult Scler. 2000; 6: 286–290.
Coleman CI, Sobieraj DM, Marinucci LN. Minimally important clinical difference of the Timed 25-Foot Walk Test: results from a randomized controlled trial in patients with multiple sclerosis. Curr Med Res Opin. 2012; 28: 49–56.
Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006; 12: 620–628.
Socie MJ, Sosnoff JJ. Gait variability and multiple sclerosis. Mult Scler Int. 2013; 2013:645197.
Olgiati R, Burgunder J, Mumenthaler M. Increased energy cost of walking in multiple sclerosis: effect of spasticity, ataxia and weakness. Arch Phys Med Rehabil. 1988; 69: 846–849.
Burridge JH, McLellan DL. Relation between abnormal patterns of muscle activation and response to common peroneal nerve stimulation in hemiplegia. J Neurol Neurosurg Psychiatry. 2000; 69: 353–361.
Sabut S, Sikdar C, Mondal R, et al. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010; 32: 1594–1603.
Sabut SK, Sikdar C, Kumar R, et al. Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation. 2011; 29: 393–400.
Financial Disclosures: At the time of the study, Dr. Rogers was employed by Innovative Neurotronics, the manufacturer of the WalkAide. Her role was administrative and consisted of assisting with the development of the protocol, providing guidance to the independent statisticians performing the analysis, and assisting in the writing and editing of the manuscript. Ms. Mayer, Ms. Warring, Ms. Agrella, and Dr. Fox have no conflicts of interest to disclose.
Funding/Support: This research was supported by Innovative Neurotronics, the International Organization of Multiple Sclerosis Nurses, and Round Rock Orthotics and Prosthetics.







