Publication
Research Article
International Journal of MS Care
Effectiveness of Fatigue Management Interventions in Reducing Severity and Impact of Fatigue in People with Progressive Multiple Sclerosis
Author(s):
CME/CNE Information
Activity Available Online:
To access the article, post-test, and evaluation online, go to http://www.cmscscholar.org.
Target Audience:
The target audience for this activity is physicians, physician assistants, nursing professionals, and other health care providers involved in the management of patients with multiple sclerosis (MS).
Learning Objectives:
1) Identify different methods of managing fatigue in people with progressive MS and the effectiveness of these interventions.
2) Analyze the limitations of the current available evidence.
Accreditation Statement:

In support of improving patient care, this activity has been planned and implemented by the Consortium of Multiple Sclerosis Centers (CMSC) and Delaware Media Group. The CMSC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Credit
The CMSC designates this journal-based activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurse Credit
The CMSC designates this enduring material for 1.0 contact hour (none in the area of pharmacology).
Disclosures:
Editor in Chief of the International Journal of MS Care (IJMSC), has served as Physician Planner for this activity. He has disclosed relationships with Springer Publishing (royalty), Biogen (speakers' bureau), and Adamas Pharmaceuticals (contracted research).Francois Bethoux, MD,
has served as reviewer for this activity. She has disclosed no relevant financial relationships.Laurie Scudder, DNP, NP,
has disclosed no relevant financial relationships.Scott Rooney, BSc (Hons),
has disclosed no relevant financial relationships.Fiona Moffat, PhD,
has disclosed no relevant financial relationships.Les Wood, PhD,
has disclosed no relevant financial relationships.Lorna Paul, PhD,
The peer reviewers for IJMSC have disclosed no relevant financial relationships.
The staff at IJMSC, CMSC, and Delaware Media Group who are in a position to influence content have disclosed no relevant financial relationships.
Note: Financial relationships for some authors may have changed in the interval between listing these disclosures and publication of the article.
Method of Participation:
Release Date: February 1, 2019
Valid for Credit Through: February 1, 2020
In order to receive CME/CNE credit, participants must:
1) Review the continuing education information, including learning objectives and author disclosures.
2) Study the educational content.
3) Complete the post-test and evaluation, which are available at http://www.cmscscholar.org.
Statements of Credit are awarded upon successful completion of the post-test with a passing score of >70% and the evaluation.
There is no fee to participate in this activity.
Disclosure of Unlabeled Use:
This educational activity may contain discussion of published and/or investigational uses of agents that are not approved by the FDA. CMSC and Delaware Media Group do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of CMSC or Delaware Media Group.
Disclaimer:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any medications, diagnostic procedures, or treatments discussed in this publication should not be used by clinicians or other health-care professionals without first evaluating their patients' conditions, considering possible contraindications or risks, reviewing any applicable manufacturer's product information, and comparing any therapeutic approach with the recommendations of other authorities.
Abstract
Background:
Rehabilitation interventions are recommended to manage multiple sclerosis (MS)–related fatigue. However, existing research has largely been generalized to those with relapsing-remitting MS, making it difficult to determine the effectiveness of these interventions in people with progressive MS. Therefore, this study aimed to systematically review the evidence related to the effectiveness of fatigue management interventions in reducing the severity and impact of fatigue in people with progressive MS.
Methods:
Six electronic databases (CINAHL, Cochrane Library, MEDLINE, PEDro, ProQuest, and Web of Science Core Collections) were searched for relevant articles up until November 2017. Randomized controlled trials and quasi-experimental studies that examined the effects of exercise, behavioral interventions, and rehabilitation on fatigue in people with progressive MS using self-reported fatigue outcome measures were included in this review.
Results:
Eight exercise, two rehabilitation, and two behavioral interventions were investigated in the 13 articles included in this review. Heterogeneous effects were reported between studies, with only two exercise, one behavioral, and two rehabilitation interventions recording significant improvements in postintervention fatigue severity or impact. However, most studies were underpowered, only two used fatigue as the primary outcome, and only one specifically recruited participants with predefined levels of fatigue.
Conclusions:
Evidence from this review is inconclusive regarding the effectiveness of nonpharmacologic interventions in reducing the severity and impact of fatigue in progressive MS populations. Adequately powered randomized controlled trials are required to evaluate fatigue management interventions in people with progressive MS experiencing high levels of fatigue and using fatigue as the primary outcome.
Fatigue is a common symptom of multiple sclerosis (MS), reported in more than 70% of the population.1–3 Fatigue related to MS is often perceived as the most debilitating symptom, which significantly affects activities of daily living, social participation, and quality of life4,5 and is associated with changes in employment.6 Fatigue is a highly complex and multifactorial symptom that may be defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities.”7(p2) Subjectively, this may be described as exhaustion, a lack of energy, or overwhelming tiredness that is pervasive and can occur at rest.8
Although fatigue can be experienced throughout the course of MS, it has a higher prevalence in people with progressive forms of the disease.1,9,10 Primary pathologic disease processes involving structural and functional central nervous system changes, and secondary factors independent of MS pathology, are associated with fatigue pathogenesis.11–13 However, because the pathophysiologic mechanisms underlying fatigue in MS are not well understood,11–13 current treatment strategies are focused on symptom management through nonpharmacologic interventions.14
Rehabilitation interventions are recommended to manage MS-related fatigue,14 and several studies have demonstrated that interventions such as exercise, energy conservation management, and cognitive behavioral therapy have moderate, positive short-term effects on fatigue outcomes.15–18 However, results have largely been generalized to those with relapsing-remitting MS (RRMS), with few studies making a distinction between RRMS and progressive MS populations. Therefore, in line with the International Progressive MS Alliance research priorities,19 there is a need to determine the effectiveness of fatigue management interventions in people with progressive MS owing to the high prevalence and impact of fatigue in this population. Hence, the aim of this work was to systematically review the evidence related to the effectiveness of fatigue management interventions in reducing the severity and impact of fatigue in people with progressive MS. To achieve this aim, the following objectives were met: 1) to summarize the details of fatigue management interventions for people with progressive MS, 2) to critically evaluate the effectiveness of fatigue management interventions in reducing the severity and impact of fatigue in people with progressive MS, and 3) to identify limitations of the current evidence to inform the direction of future study.
Methods
A review protocol was developed and registered with the PROSPERO database in December 2017 (number: CRD42017082203).
Search Strategy
Searches of the following databases were conducted from inception to November 2017: CINAHL (via EBSCOhost), Cochrane Library, MEDLINE (via Ovid), PEDro, ProQuest (Health & Medical Collection, Nursing & Allied Health Database, and PsycINFO), and Web of Science Core Collections. Search strategies included a combination of keywords and subject headings related to MS, exercise, behavioral therapy, rehabilitation, and fatigue and were adapted for use in each different database (Table S1, which is published in the online version of this article at ijmsc.org). Reference lists of relevant review articles were also hand searched to identify any additional articles. After each database was searched, results were exported to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) and duplicates were removed before screening. The primary reviewer (S.R.) initially screened all articles by title and then by abstract against the inclusion and exclusion criteria. Subsequently, two reviewers (S.R. and L.P.) independently screened full texts of the remaining articles for eligibility. Disagreements were resolved through consensus in consultation with a third reviewer (F.M.) if required.
Inclusion and Exclusion Criteria
To be included in this review studies had to have 1) recruited adults with a definite diagnosis of MS and a progressive form of the disease (secondary or primary progressive), 2) evaluated nonpharmacologic interventions in accordance with the definitions provided in Table 1, 3) used a self-reported measure of fatigue impact or severity as either a primary or secondary outcome (including subscales of questionnaires), 4) used a randomized controlled trial (RCT) or quasi-experimental design, and 5) been published in English. Studies that included a combination of types of MS were included only when specific results for those with progressive MS could be identified. Nonhuman studies, pharmacologic studies, and conference proceedings and abstracts were excluded from this review.
Definitions of included interventions

Data Extraction
Data extraction was completed independently by one reviewer (S.R.) using a standardized data extraction form. The data extraction form was developed based on CONSORT (Consolidated Standards of Reporting Trials) and TIDieR (Template for Intervention Description and Replication) guidelines.22,23
Quality Assessment
Quality of evidence was assessed using the Downs and Black checklist, a 32-point scale developed for quality assessment of RCTs and non-RCTs.24,25 An initial quality assessment was conducted in which each of the three reviewers (S.R., F.M., and L.P.) independently scored an article to ensure consistency in assessment between reviewers. After this quality assessment, question 27 of the checklist was modified such that an article was assigned 1 point for including a sample size calculation and zero if the article did not, resulting in a total possible score of 28. This modification was implemented in keeping with two systematic reviews of exercise interventions in MS.26,27 Quality assessment was completed independently by two reviewers. When discrepancies arose, agreement was reached through consensus in consultation with a third reviewer.
Data Synthesis
Owing to the inclusion of quasi-experimental studies and the heterogeneity in study design, it was not feasible to conduct a meta-analysis; therefore, results were generated through narrative synthesis. Preliminary synthesis involved a descriptive summary of key information extracted from all articles. Individual study estimates of treatment effects were presented under each mode of intervention and explored within and between studies considering moderator variables to explain differences in results. Where available, results for the relevant fatigue outcome measures were compared with minimal clinically important difference (MCID).
Results
Search Results
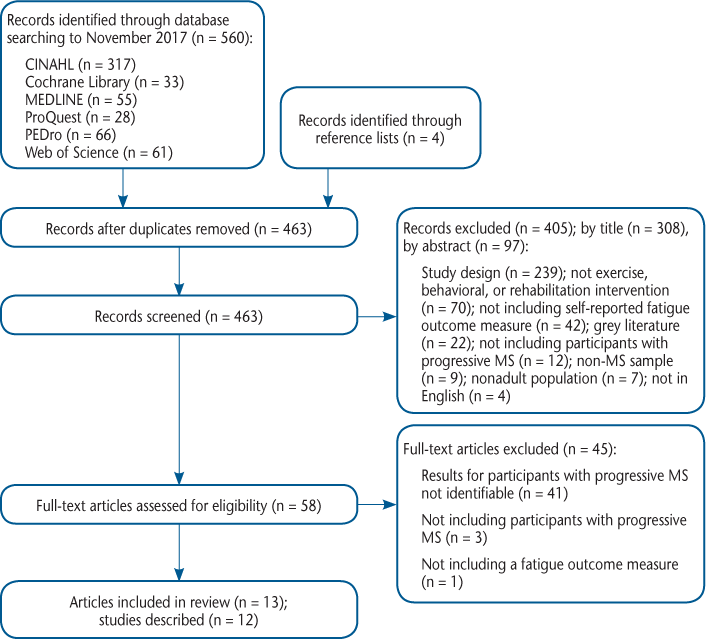
Through searching the selected electronic databases, 560 articles were identified, and an additional four articles were added from reference lists of relevant studies (Figure 1). After removing duplicates, 463 articles remained for title and abstract screening, of which 308 were excluded by title and 97 by abstract. The remaining 58 articles were included for full-text screening. After screening full texts, 45 articles were excluded because the results of those with progressive MS were not identifiable in 41 studies (either MS type was not reported or results for those with progressive MS were not presented separately), three studies did not include participants with progressive MS, and one study did not include a fatigue outcome measure. Two articles described the same study but reported different outcome measures29,30; therefore, 13 articles29–41 from 12 studies were included (Table 2).
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram28
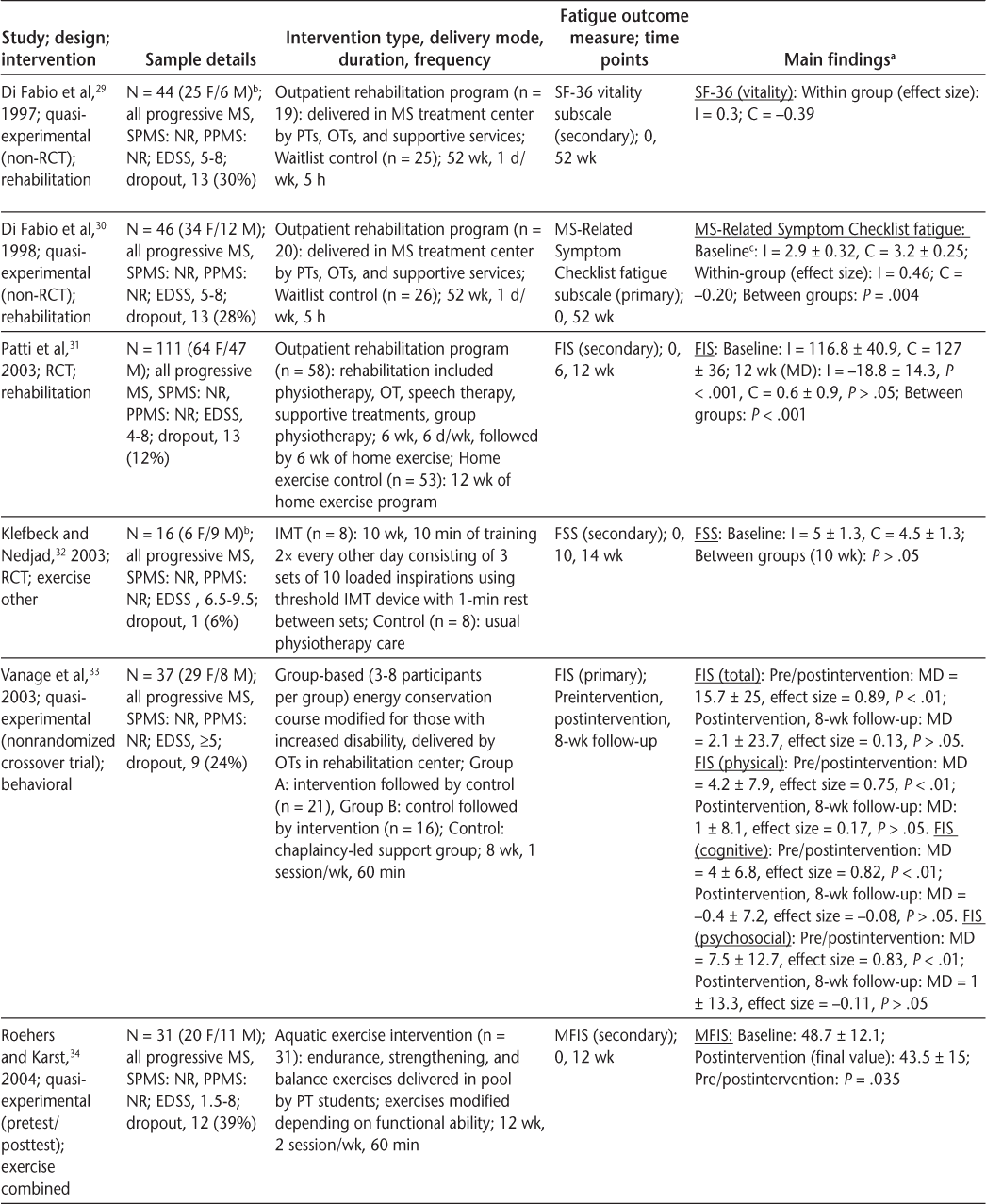
Characteristics of included articles (page 1 of 3)
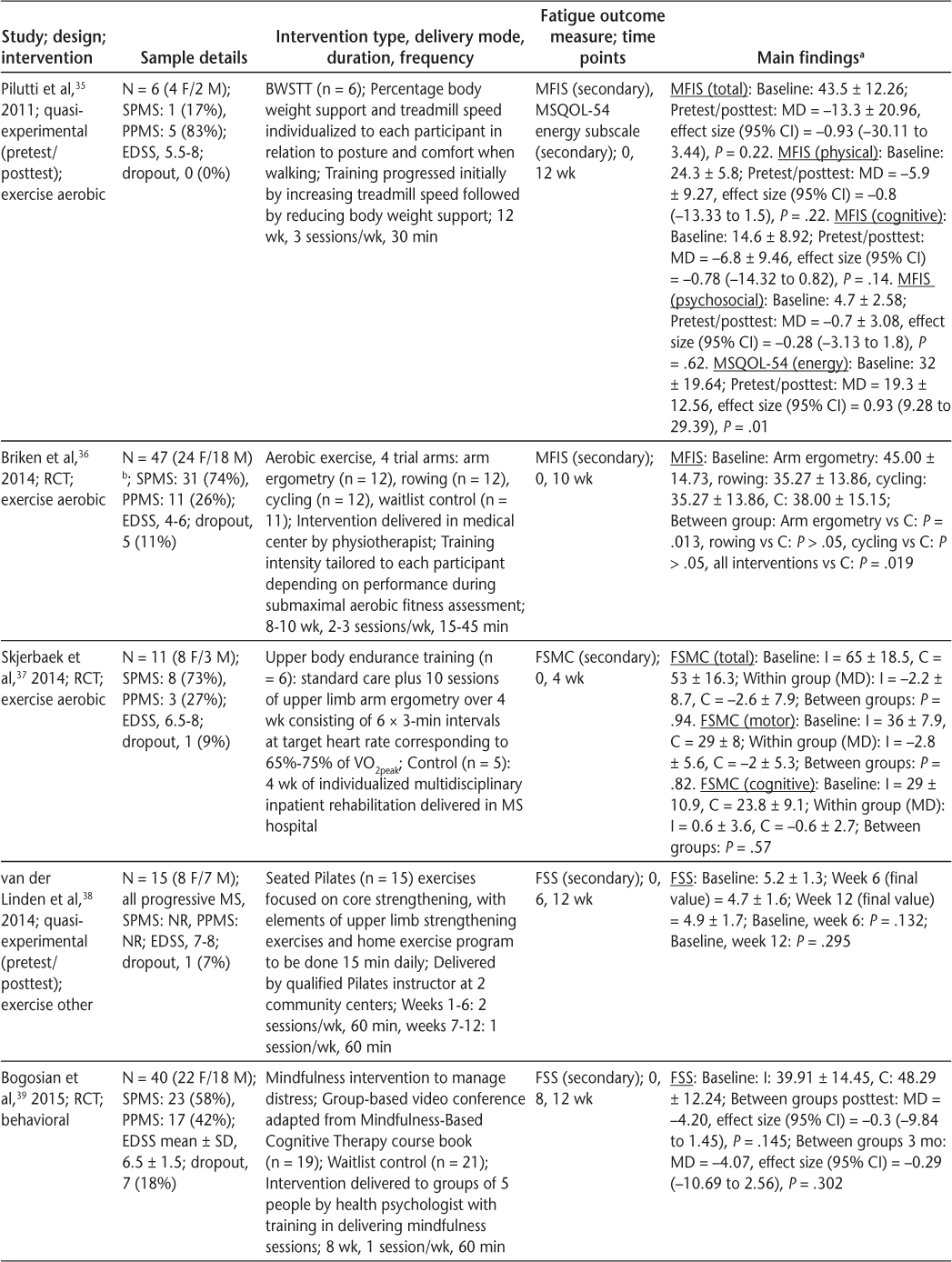
Characteristics of included articles (page 2 of 3)
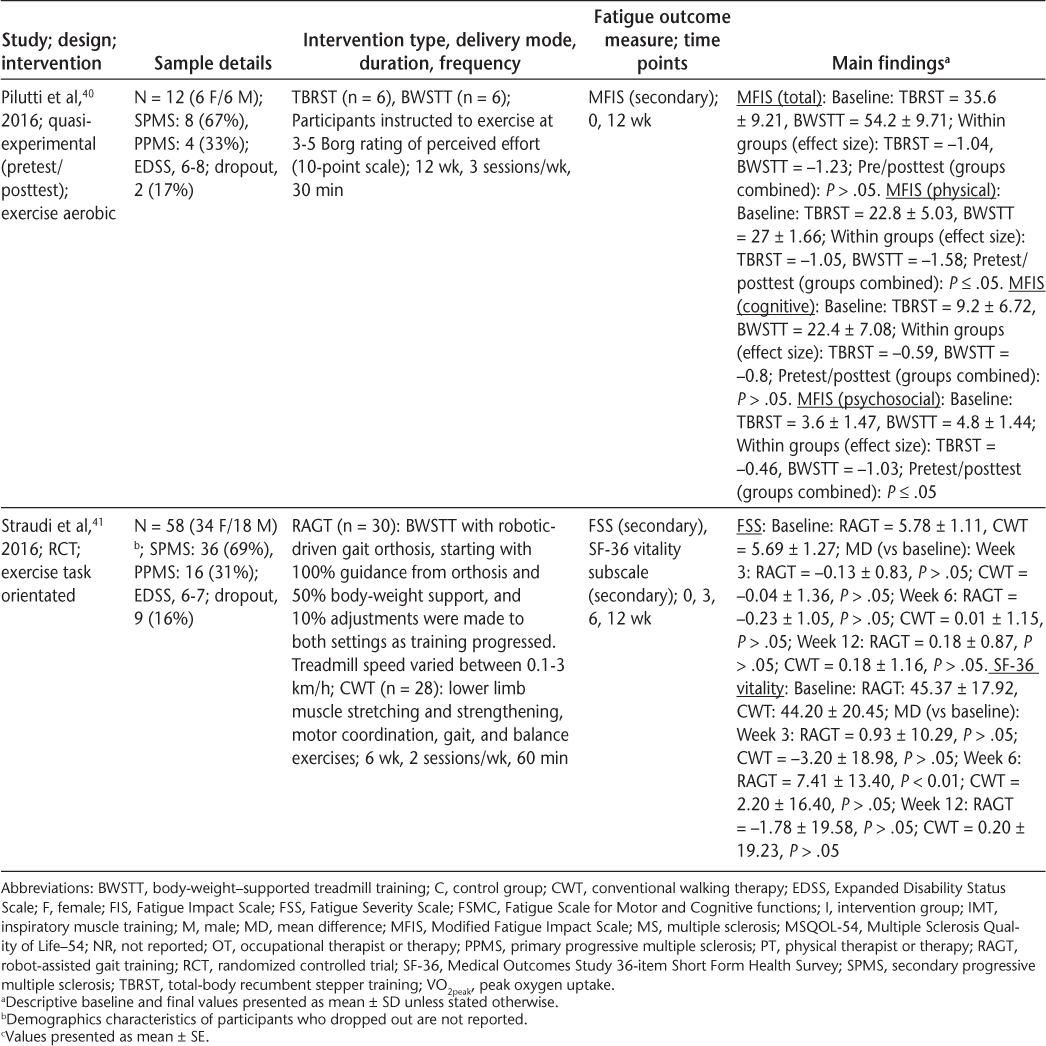
Characteristics of included articles (page 3 of 3)
Study Design
Of the included articles, six were RCTs31,32,36,37,39,41 and seven were quasi-experimental studies (pretest/posttest design [n = 4],34,35,38,40 non-RCT design [n = 2],29,30 or nonrandomized crossover trial design [n = 1]33). All but one RCT included two trial arms (control and intervention); the study by Briken et al36 involved three intervention conditions in addition to the control group. The length of the intervention period ranged from 4 to 52 weeks; however, most studies delivered interventions for 12 weeks or less (n = 11), with only one rehabilitation intervention lasting 52 weeks.29,30 Four articles reported follow-up outcome assessments conducted 4,32,39 6,41 or 833 weeks after the intervention.
Quality Assessment
Total quality assessment scores ranged from 15 to 25 (Table 3), and no study was excluded based on the results of the quality assessment. Only seven articles reported adverse events,31,33,34,37,38,40,41 seven adjusted for confounding variables and loss to follow-up,29,30,35–37,39,41 six reported compliance with interventions,35–40 and one included a power calculation to determine sample size.41 Due to the nature of the interventions, none of the studies blinded participants to treatment allocation.
Downs and Black24 checklist scores for included articles

Sample Characteristics
Study sample sizes ranged from 6 to 111 participants, and overall 474 participants were included, 325 of whom were allocated to receive an intervention and 149 to a control condition. Expanded Disability Status Scale (EDSS) scores of study samples ranged from 1.5 to 9, and 12 articles reported participants with EDSS scores greater than 6.29–35,37–41 Only one study used a predefined level for moderate-severe fatigue (Fatigue Severity Scale [FSS] score ≥4) as an inclusion criterion for participant recruitment.33
Outcome Measures
There were seven self-reported outcome measures used across the included articles to measure the severity or impact of fatigue, and the most commonly used were the FSS (n = 4)32,38,39,41 and the Modified Fatigue Impact Scale (MFIS) (n = 4).34–36,40 In addition, studies also used the Fatigue Impact Scale (FIS),31,33 the MS-Related Symptom Checklist (fatigue subscale),30 the Fatigue Scale for Motor and Cognitive functions,37 the 36-item Short Form Health Survey (SF-36) vitality subscale,29,41 and the Multiple Sclerosis Quality of Life–54 energy subscale.35 Of the 13 included articles, two stated that fatigue was the primary outcome of investigation,30,33 and in the remaining 11 fatigue was a secondary outcome and the primary outcomes were quality of life,29,31,34 aerobic fitness,36,37 global measures of physical function,35 distress,39 temporal measures of gait,41 lung function,32 exercise safety,40 and sitting balance.38
Intervention Types
In accordance with the definitions of interventions for this review, eight exercise,32,34–38,40,41 two rehabilitation,29–31 and two behavioral interventions33,39 were described by the 13 included articles.
Of the eight exercise interventions, four were classified as aerobic exercise,35–37,40 one as combined exercise,34 one as task-orientated exercise,41 and two as other exercise.32,38 Various modes of exercise were used across the four trials of aerobic exercise: one used arm ergometry,37 two used body-weight–supported treadmill training,35,40 one used recumbent stepping,40 and Briken et al36 used arm ergometry, cycling, and rowing. Most interventions were performed at moderate intensity and were progressed through increasing the duration of training; however, the study by Skjerbaek et al37 implemented a high-intensity interval training protocol involving 3-minute intervals working at a heart rate corresponding to 65% to 75% peak oxygen uptake.37 In addition to aerobic exercise, the combined exercise intervention described by Roehrs and Karst34 incorporated elements of upper and lower limb resistance exercises and was delivered in a pool by physical therapy students.
The study by Straudi et al41 was characterized as task-orientated exercise because the intervention aimed to improve temporal gait parameters by using a robotic-assisted gait orthosis in conjunction with body-weight–supported treadmill training. The two other exercise interventions involved seated Pilates38 and inspiratory muscle training.32 The seated Pilates intervention was delivered by a qualified Pilates instructor and incorporated elements of core and upper limb strengthening with a daily home exercise program.38 Inspiratory muscle training followed a self-management program of inspiratory muscle resistance exercises that consisted of three sets of ten loaded inspirations using a threshold inspiratory muscle training device.32
The two behavioral intervention studies involved mindfulness39 and energy conservation management.33 The mindfulness intervention was delivered via a group-based video conference by a health psychologist. The content involved components of the Mindfulness-based Stress Reduction program with additional cognitive therapy exercises and “homework” tasks. The energy conservation intervention was delivered face-to-face in a group by occupational therapists and involved education regarding optimum energy use to minimize the impact of fatigue through restructuring or altering activities of daily living in accordance with Packer's energy conservation course.
Rehabilitation interventions were delivered by a multidisciplinary team consisting of physiotherapists, occupational therapists, and support services in an outpatient setting, and treatments were individualized to each participant.29–31 In the study by Di Fabio et al,29,30 participants received 5 hours of rehabilitation 1 day per week that consisted of physiotherapy (gait, transfer, and balance training; endurance training; range of movement exercises), occupational therapy to maintain upper limb use during activities of daily living and enhance communication skills, and support services (support groups, social work, recreation activities, falls prevention programs, seating clinics, and nutritional information). The intervention delivered by Patti et al31 consisted of 1 hour of physiotherapy treatment 5 days per week, 30 minutes of occupational therapy and speech therapy twice per week, and support sessions on symptom self-management and goal setting. In addition to outpatient rehabilitation, Patti et al31 included the prescription of a daily home exercise program.
Effectiveness of Interventions
Exercise
Of the studies investigating aerobic exercise interventions, Skjerbaek et al37 reported that although Fatigue Scale for Motor and Cognitive functions scores improved in the exercise group after the intervention (mean ± SD difference, −2.2 ± 8.7), there was no significant difference between the exercise and control groups over time. Similarly, Pilutti et al35,40 reported nonsignificant improvements in MFIS scores after the intervention (effect sizes, −0.93 and −1.04, respectively). However, Pilutti et al35 found statistically significant changes in Multiple Sclerosis Quality of Life–54 energy subscale scores after the intervention (P = .01). The studies by Pilutti et al35,40 and Skjerbaek et al37 had small samples (n = 6–12) and included participants with severe disability (EDSS scores, 5.5–8). In contrast, Briken et al36 investigated three aerobic exercise interventions in a larger population (n = 47) of participants with moderate disability (EDSS scores, 4–6) and reported that exercise significantly improved fatigue from baseline (P = .019); however, only arm ergometry demonstrated significant improvements compared with the control group (P = .013).
Of the remaining exercise interventions, no significant changes were noted in fatigue after combined exercise,34 Pilates,38 or inspiratory muscle training.32 In addition, there were no significant improvements in FSS scores after the intervention or at 6-week follow-up for those receiving task-orientated exercise interventions; however, SF-36 vitality subscale scores improved after the intervention for the group receiving robot-assisted gait training (P < .01) but returned to baseline at 6-week follow-up.41
Behavioral
In a nonrandomized crossover trial, Vanage et al33 investigated the use of an energy conservation course and reported a significant improvement in FIS total and subscale scores after the intervention (effect size, 0.89; P < .01) that was maintained at 8-week follow-up. However, Bogosian et al39 reported no significant difference in fatigue scores after the intervention and at 6-week follow-up between the group receiving a mindfulness intervention and a waitlist control group. In addition to the mode of intervention, differences in results between studies may be explained by study design because Vanage et al33 recruited participants with a clinically significant level of fatigue and used fatigue as a primary outcome, whereas Bogosian et al39 did neither.
Rehabilitation
Di Fabio et al30 reported that fatigue scores (MS-Related Symptom Checklist) for those receiving 52-week multidisciplinary rehabilitation were significantly different after the intervention compared with those of waitlist controls (effect sizes, 0.46 and −0.2, respectively). From the same study, Di Fabio et al29 also reported that SF-36 vitality subscale scores improved after the intervention for the group receiving rehabilitation (effect size, 0.3) and that fatigue in the waitlist control group increased in severity (effect size, −0.39). In the study by Patti et al,31 those receiving 12 weeks of outpatient rehabilitation demonstrated a significant improvement in postintervention fatigue scores (P < .001).
Clinical Significance of Fatigue Changes
Of the outcome measures reported, MCID has been determined only for the FIS in MS populations. When anchored to measures of health-related quality of life, FIS demonstrates an MCID of 10 to 20 points.42 Of the two included studies that used the FIS, both reported significant improvements in fatigue after the intervention (mean ± SD differences of 18.8 ± 14.3 [P < .001]31 and 15.7 ± 25 [P < .01]33). However, although the mean change in FIS scores recorded by both studies is within the range of MCID estimates reported for the FIS, both studies reported large SDs, suggesting that these interventions may be clinically significant for only some participants.
Discussion
Overall, the evidence presented in this review is inconclusive regarding the use of exercise, behavioral, and rehabilitation interventions to manage the severity and impact of fatigue in progressive MS populations. However, the quality of evidence is generally weak due to the small number of underpowered studies with limited methodological designs.
Exercise Interventions
The evidence is inconclusive regarding the effectiveness of exercise as an intervention to reduce the severity and impact of fatigue in people with progressive MS. However, of the four studies that investigated aerobic exercise, all demonstrated improvement in fatigue impact after the intervention,35–37,40 although only Briken et al36 reported that changes in fatigue impact were statistically significant. The result of this review including studies of people with progressive MS is comparable with a similar review that reported that aerobic exercise improves fatigue in those with RRMS.17 However, the studies included in this current review had small sample sizes and were underpowered to detect significant changes in fatigue. In addition, three of the studies included participants with high levels of disability (EDSS scores ≥6), which may have further influenced results as, to date, the positive evidence for the effect of exercise on fatigue has been demonstrated only in those with mild-moderate disability (EDSS scores ≤5.5),17,43 whereas varied effects are reported in those with higher levels of disability.27
Comparing the effectiveness of aerobic exercise with other modes of exercise is limited by the small number of heterogeneous studies. Only four studies investigated forms of exercise other than aerobic—including aquatic therapy34 and inspiratory muscle training32—and the evidence generally does not support the effectiveness of these interventions for reducing fatigue in progressive MS populations. Furthermore, none of the included studies investigated the use of resistance training, which has been demonstrated to improve fatigue in people with RRMS.43 Consequently, although this review highlights the potential effectiveness of aerobic exercise in fatigue management for people with progressive MS, there is insufficient evidence to determine whether this is the most effective mode of exercise.
The mechanisms through which exercise may attenuate fatigue symptoms are unknown. It is hypothesized that exercise may have a neuroprotective and neuroregenerative benefit through increasing neural growth factors that modulate structural and functional central nervous system changes associated with primary MS-related fatigue.13 In addition, exercise training may influence secondary fatigue mechanisms caused by deconditioning, sleep disorders, and depression through increasing aerobic capacity, improving sleep quality, and managing depression.13 The immunologic biomarkers interferon γ, tumor necrosis factor α, and interleukin 1 have also been associated with fatigue in MS44 but may have limited relevance to those with progressive MS due to the absence of a marked inflammatory response.45
Of the aerobic exercise interventions included, three were performed at moderate intensity for durations of 30 to 45 minutes two to three times per week.35,36,40 Although this dose of exercise is recommended for people with mild-moderate MS,46 there was no evidence of a dose-response relationship to suggest that this prescription is most effective in managing fatigue, particularly in progressive MS populations. Indeed, one trial investigated shorter-duration, high-intensity aerobic exercise,37 which may hold potential in fatigue management through inducing greater improvements in aerobic capacity over a shorter time.47 Therefore, no conclusions regarding the optimum dose of exercise to manage fatigue in people with progressive MS can be generated from the evidence in this review.
There is also limited evidence for the long-term effectiveness of exercise interventions. Only two studies conducted follow-up measurement, neither of which reported a significant long-term change in fatigue severity compared with the baseline assessment.32,41 Consequently, there is a need to evaluate the long-term effectiveness of exercise interventions to determine whether improvements in fatigue are sustained after the intervention period.
Despite the limited evidence for the effectiveness of exercise intervention, most studies reported low attrition rates indicating acceptability of exercise interventions in progressive MS populations. In addition, some studies confirmed that exercise interventions were feasible in populations with higher levels of disability associated with progressive MS, which is in line with evidence from a previously published review.27
Behavioral Interventions
Because only two studies of behavioral interventions were included in this review, it is not possible to reach any conclusions regarding their effectiveness in reducing the severity or impact of fatigue. Both studies investigated different forms of behavioral therapy interventions and reported contrasting results regarding short- and long-term effectiveness. Vanage et al33 reported that an 8-week energy conservation course significantly reduced fatigue impact immediately after the intervention period and at 8-week follow-up, which is comparable with previous evidence from predominantly RRMS populations.15
In contrast, Bogosian et al39 reported no significant difference in fatigue severity after the intervention or at 4 weeks of follow-up between those receiving a mindfulness intervention and a waitlist control group. Mindfulness is used in MS to manage somatic symptoms and improve health-related quality of life48 and is recommended in the National Institute for Health and Care Excellence guidelines as a strategy to manage fatigue.14 However, the mindfulness intervention implemented by Bogosian et al39 was designed to manage distress not fatigue. Therefore, despite the association between mood disorders and fatigue,9,49–51 the applicability of these findings to fatigue management is limited. In addition, the mindfulness sessions were delivered via video conference, which, while accommodating those with severe mobility disabilities, may limit the social benefits reported during group-based interventions delivered face-to-face.33,52
Rehabilitation Interventions
Although evidence from this review is positive regarding the effects of rehabilitation on fatigue, only two studies of rehabilitation interventions were included. Generally, rehabilitation interventions were individualized to each participant, goal-orientated, addressed functional performance, and were delivered by a multidisciplinary team. In both articles, changes in fatigue severity after 52 weeks of multidisciplinary rehabilitation were statistically significant, with moderate effect sizes reported for those receiving rehabilitation and worsening fatigue in the waitlist control group.29,30 However, because this study included only two points of outcome assessment (baseline and 52 weeks), the rate at which improvements in fatigue were accumulated cannot be observed. Patti et al31 implemented a shorter duration, higher-intensity intervention that demonstrated clinically significant improvements in fatigue impact for some participants after the intervention. Therefore, there is a need to determine the most effective duration of rehabilitation interventions.
It is acknowledged that exercise or behavioral interventions can be delivered as components of rehabilitation. However, the rehabilitation interventions included in this review were multidisciplinary and were differentiated from exercise and behavioral interventions alone because they contained additional treatment strategies, such as physiotherapy and occupational therapy, to maintain physical function. Consequently, it was not possible to identify the effectiveness of each component part of rehabilitation, for example, the effectiveness of exercise delivered as part of rehabilitation. This information is essential to constructing rehabilitation programs that are best designed to manage fatigue.
Limitations of the Evidence
There were several important limitations that affect the overall quality of evidence. First, only two studies used fatigue as a primary outcome measure,30,33 and of these studies, only one recruited participants with clinically significant levels of fatigue (FSS score, ≥4).33 Therefore, there is limited evidence of the effect of interventions specifically designed to manage fatigue in people with clinically significant levels of fatigue.
In addition, seven different fatigue outcome measures were used in this review, limiting the ability to directly compare results between studies. Although a meta-analysis of exercise interventions demonstrated that the selection of fatigue outcome measures did not moderate the effect of interventions,17 there is a need for core fatigue outcome measures to enable pooling of statistical data for meta-analysis and comparison of effects between studies. In addition, MCID has been determined only for the FIS. Therefore, the MCID of the MFIS and FSS should be determined to establish the clinical significance of changes in fatigue severity and impact.
Finally, most studies were underpowered to detect significant changes in fatigue. In addition, due to the inclusion of quasi-experimental studies, several studies were unable to control for confounding variables, which may have accounted for the heterogeneous treatment response reported within and between studies. Furthermore, adverse events and compliance with interventions were poorly reported across studies, limiting the ability to determine the safety and efficacy of interventions in clinical practice.
Limitations of the Review
There were many other studies that investigated the effectiveness of fatigue management interventions in people with progressive MS; however, these studies were excluded because the results for people with progressive MS could not be specifically identified. In addition, the overall quality of evidence in this review is limited by the inclusion of quasi-experimental studies, which are less methodologically rigorous and introduce risk of selection bias. Furthermore, due to the inclusion of quasi-experimental studies and the heterogeneity in outcome measures and interventions used between studies, it was not feasible to conduct a meta-analysis, and results were generated by narrative synthesis.
Conclusion
There is insufficient evidence regarding the effectiveness of nonpharmacologic interventions in reducing the impact and severity of fatigue in people with progressive MS. This review suggests that exercise, behavioral interventions, and rehabilitation may have the potential to manage fatigue. However, future, adequately powered, rigorous trials of interventions to manage fatigue in populations with severe levels of fatigue are required. In addition, future studies should clearly identify the specific results for people with progressive MS due to the limited available evidence for this population.
PRACTICE POINTS
Exercise, behavioral interventions, and rehabilitation demonstrate the potential to manage fatigue in progressive MS populations.
Evidence in this review suggests that aerobic exercise can improve fatigue in people with progressive MS; however, the optimal dose was not determined.
Further evidence is required to determine the effectiveness of these interventions in studies that use fatigue as the primary outcome and recruit people who have high levels of fatigue.
Financial Disclosures:
The authors declare no conflicts of interest.
References
Lerdal A, Celius EG, Krupp L, Dahl AA. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol. 2007;14:1338–1343.
Hadjimichael O, Vollmer T, Oleen-Burkey M; North American Research Committee on Multiple Sclerosis. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:1–11.
Zajicek JP, Ingram WM, Vickery J, Creanor S, Wright DE, Hobart JC. Patient-orientated longitudinal study of multiple sclerosis in south west England (the South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort. BMC Neurol. 2010;10:1–11.
Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. 2002;205:51–58.
Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler. 2003;9:219–227.
Moore P, Harding KE, Clarkson H, Pickersgill TP, Wardle M, Robertson NP. Demographic and clinical factors associated with changes in employment in multiple sclerosis. Mult Scler. 2013;19:1647–1654.
Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
Mills RJ, Young CA. A medical definition of fatigue in multiple sclerosis. QJM. 2008;101:49–60.
Johansson S, Ytterberg C, Hillert J, Widen Holmqvist L, von Koch L. A longitudinal study of variations in and predictors of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:454–457.
Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011;17:604–612.
Kos D, Kerckhofs E, Nagels G, D'hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair. 2008;22:91–100.
Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis: a brief review. J Neurol Sci. 2012;323:9–15.
Langeskov-Christensen M, Bisson EJ, Finlayson ML, Dalgas U. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: a scoping review. J Neurol Sci. 2017;373:307–320.
National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. https://www.nice.org.uk/guidance/cg186. Accessed July 18, 2018.
Blikman LJ, Huisstede BM, Kooijmans H, Stam HJ, Bussmann JB, van Meeteren J. Effectiveness of energy conservation treatment in reducing fatigue in multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94:1360–1376.
Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int. 2014;2014:798285.
Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015;9:CD009956.
van den Akker LE, Beckerman H, Collette EH, Eijssen IC, Dekker J, de Groot V. Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Psychosom Res. 2016;90:33–42.
Fox R, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler. 2012;18:1534–1540.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131.
World Health Organization. World report on disability 2011. http://www.who.int/disabilities/world_report/2011/en/. Accessed July 18, 2018.
Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:1–12.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377.
Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:1–186.
Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94:1800–1828.
Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe mobility disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31–39.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:1–6.
Di Fabio RP, Choi T, Soderberg J, Hansen CR. Health-related quality of life for patients with progressive multiple sclerosis: influence of rehabilitation. Phys Ther. 1997;77:1704–1716.
Di Fabio RP, Soderberg J, Choi T, Hansen CR, Schapiro RT. Extended outpatient rehabilitation: its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis. Arch Phys Med Rehabil. 1998;79:141–146.
Patti F, Ciancio MR, Cacopardo M, et al. Effects of a short outpatient rehabilitation treatment on disability of multiple sclerosis patients: a randomised controlled trial. J Neurol. 2003;250:861–866.
Klefbeck B, Nedjad JH. Effect of inspiratory muscle training in patients with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:994–999.
Vanage S, Gilbertson K, Mathiowetz V. Effects of an energy conservation course on fatigue impact for persons with progressive multiple sclerosis. Am J Occup Ther. 2003;57:315–323.
Roehrs TG, Karst GM. Effects of an aquatics exercise program on quality of life measures for individuals with progressive multiple sclerosis. J Neurol Phys Ther. 2004;28:63–71.
Pilutti LA, Lelli DA, Paulseth JE, et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011;92:31–36.
Briken S, Gold SM, Patra S, et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult Scler. 2014;20:382–390.
Skjerbaek AG, Naesby M, Lutzen K, et al. Endurance training is feasible in severely disabled patients with progressive multiple sclerosis. Mult Scler. 2014;20:627–630.
van der Linden ML, Bulley C, Geneen LJ, Hooper JE, Cowan P, Mercer TH. Pilates for people with multiple sclerosis who use a wheelchair: feasibility, efficacy and participant experiences. Disabil Rehabil. 2014;36:932–939.
Bogosian A, Chadwick P, Windgassen S, et al. Distress improves after mindfulness training for progressive MS: a pilot randomised trial. Mult Scler. 2015;21:1184–1194.
Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18:221–229.
Straudi S, Fanciullacci C, Martinuzzi C, et al. The effects of robot-assisted gait training in progressive multiple sclerosis: a randomized controlled trial. Mult Scler. 2016;22:373–384.
Rendas-Baum R, Yang M, Cattelin F, Wallenstein GV, Fisk JD. A novel approach to estimate the minimally important difference for the Fatigue Impact Scale in multiple sclerosis patients. Qual Life Res. 2010;19:1349–1358.
Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med. 2013;75:575–580.
Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15:210–220.
Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366.
Latimer-Cheung AE, Ginis KAM, Hicks AL, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:1829–1836.
Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler. 2008;14:35–53.
Simpson R, Booth J, Lawrence M, Byrne S, Mair F, Mercer S. Mindfulness based interventions in multiple sclerosis: a systematic review. BMC Neurol. 2014;14:1–9.
Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Fatigue in multiple sclerosis persists over time: a longitudinal study. J Neurol. 2006;253:1466–1470.
Brown RF, Valpiani EM, Tennant CC, et al. Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother. 2009;82:41–56.
Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19:217–224.
Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler. 2005;11:592–601.







