Publication
Research Article
International Journal of MS Care
Effect of Comorbidities on Outcomes of Neurorehabilitation Interventions in Multiple Sclerosis
Author(s):
Background: Interest in comorbidities has increased in the past few years, but the effect of comorbidities on outcomes of multiple sclerosis (MS) neurorehabilitation interventions is unclear. The aim of this review was to identify and summarize the existing evidence regarding the effect of comorbidities on outcomes of neurorehabilitation interventions targeting people with MS.
Methods: Five databases (Embase, MEDLINE through Ovid, PubMed Central, Cumulative Index to Nursing and Allied Health Literature, and Web of Science) were searched using index terms and keywords relating to MS and a wide range of rehabilitation interventions. Studies screened were limited to English-language randomized controlled trials. Information related to included and excluded comorbidities and how they were reported and described was extracted from the included studies.
Results: Fifty-four neurorehabilitation randomized controlled trials were included and were grouped into categories: robotics/technology-enhanced (n = 7), task-oriented training/neurorehabilitation principles (n = 7), electrical stimulation (n = 12), temperature regulation (n = 6), magnetic field therapy (n = 5), vibration (n = 9), and miscellaneous (n = 8). Although the issue of comorbidity was considered in 40 studies, it was limited to excluding individuals from participating in the trials. Only two studies reported on comorbidity, but neither examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Conclusions: This review documents important knowledge gaps about the effect of comorbidity on neurorehabilitation outcomes and identifies a critical need for future studies to address this issue. Without this information, we limit our understanding of the mechanisms of comorbidity and its effects on relevant clinical and research outcomes specific to neurorehabilitation.
Approximately 50% of people with multiple sclerosis (MS) may become moderately to severely disabled within 15 years of disease diagnosis1; this proportion increases to 75% after 45 years.2 An increasing level of disability has substantial consequences for the ability of people with MS to engage in daily activities and maintain social relationships.3 When MS occurs in the presence of comorbidities, the combined effect of and interactions between conditions often result in a more rapid progression of disability, a reduction in quality of life, and an increase in mortality.4
Comorbidity, which refers broadly to physical or mental conditions that exist at the time of diagnosis of MS or later but that are not a consequence of MS, occurs in up to 50% of individuals with this disease.5 The most common physical comorbidities in people with MS include hypertension, hyperlipidemia, and chronic lung disease. Mental conditions, such as depression and anxiety, are also commonly reported.6
Despite advances in disease-modifying therapies that may reduce disease progression, the residual level of disability remains unchanged.7 Consequently, neurorehabilitation interventions are needed to manage the consequences of MS.8 For the purposes of this study, we defined neurorehabilitation interventions as any treatment activities or services aimed at reducing the effect of disability resulting from MS using the principles of neuroplasticity.9
Researchers have shown that neurorehabilitation interventions can improve physical function, increase activity and participation, and optimize the quality of life of people with MS.10 Given the high prevalence of comorbidities in MS and the desire of clinicians to apply evidence-based practices, it is critical to understand the extent to which neurorehabilitation researchers have considered the presence of comorbidities in the course of their studies. This knowledge would guide the application of evidence in real-life settings.
Scoping reviews provide a synthesis of research on a particular topic by describing emerging trends and identifying gaps in knowledge.11 Given the breadth of rehabilitation interventions targeting people with MS, we conducted this scoping review as part of an ongoing study aimed at documenting the effect of comorbidities on rehabilitation intervention outcomes. In particular, this review focuses on identifying gaps and summarizing existing evidence regarding the effect of comorbidities on neurorehabilitation outcomes. Two research questions guided this review: 1) Do neurorehabilitation interventions targeting people with MS address participant comorbidities? If so, how? 2) What are the gaps in current knowledge about the effect of comorbidities on neurorehabilitation intervention outcomes?
Methods
We used the framework put forth by Arksey and O'Malley.11
Search Strategy
Five databases were searched to locate articles published from inception to January 8, 2016 (Embase, MEDLINE through Ovid, PubMed Central, Cumulative Index to Nursing and Allied Health Literature, and Web of Science). First, we searched multiple sclerosis as an index term and a keyword, and then we combined the results using the operator OR in each database. Next, we added database-specific terms for randomized controlled trials (RCTs) to limit the results to intervention research. We focused on RCTs because they are the gold standard for evaluating intervention efficacy and effectiveness.
Next, we conducted a series of searches using database-specific index terms (eg, Medical Subject Headings) that captured the focus or content of a wide range of rehabilitation interventions. These terms included rehabilitation, exercise, physical activity, motor activity, fitness, self-care, self-management, health promotion, health education, patient education, health behavior, assistive technology, assistive device, self-help, behavior modification, environment, home environment, and modification. Each term was also searched as a keyword with a truncation operator when appropriate (eg, behavio*). Medical Subject Headings and keyword searches were conducted across all the databases except for Web of Science, where only keywords were used as topic terms.
Finally, we combined the results of previous searches, for example, multiple sclerosis AND RCT AND motor activity. All the searches were limited to articles in English. In total, 3903 citations were identified.
Screening for Inclusion in the Full-Text Review
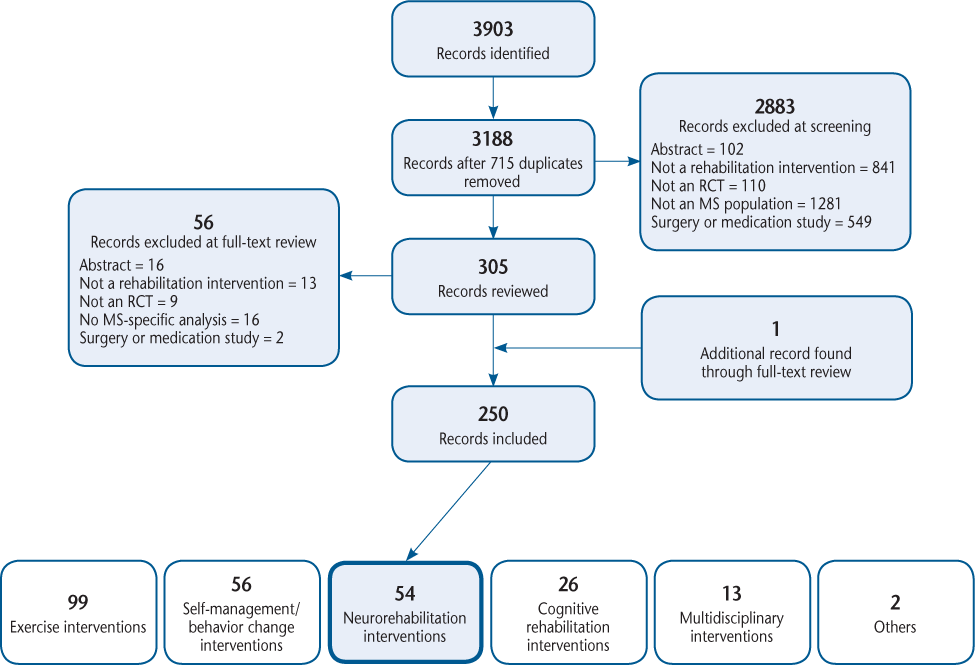
The full citations and abstracts for all 3903 studies were downloaded into EndNote, and duplicates were deleted (n = 715). Three reviewers (AF, EJB, and JP) screened the titles and abstracts of the remaining 3188 studies to identify citations for full-text review. We included studies of humans with MS and studies evaluating rehabilitation interventions using an RCT design. We excluded conference abstracts, dissertations, letters to the editor, protocols, studies reporting results of MS combined with other populations (eg, stroke), and studies in which the rehabilitation intervention was secondary or adjunctive to a medical intervention (eg, surgery or pharmaceutical treatment). All the citations were reviewed by at least two reviewers. When disagreements about inclusion/exclusion were encountered, discussions with the third reviewer occurred. If consensus could not be reached, the senior author (MF) made the final decision. Figure 1 is a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) chart showing the search and screening processes.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) chart showing the results of the search and screening processes
Full-Text Review, Data Extraction, and Analysis
One of two authors (JP or JL) completed the full-text review and data extraction. Another author (AF or EJB) then verified the extraction. General study information was extracted from the articles: research questions/objectives, intervention description, sample size, age, percentage of females, and level of disability.
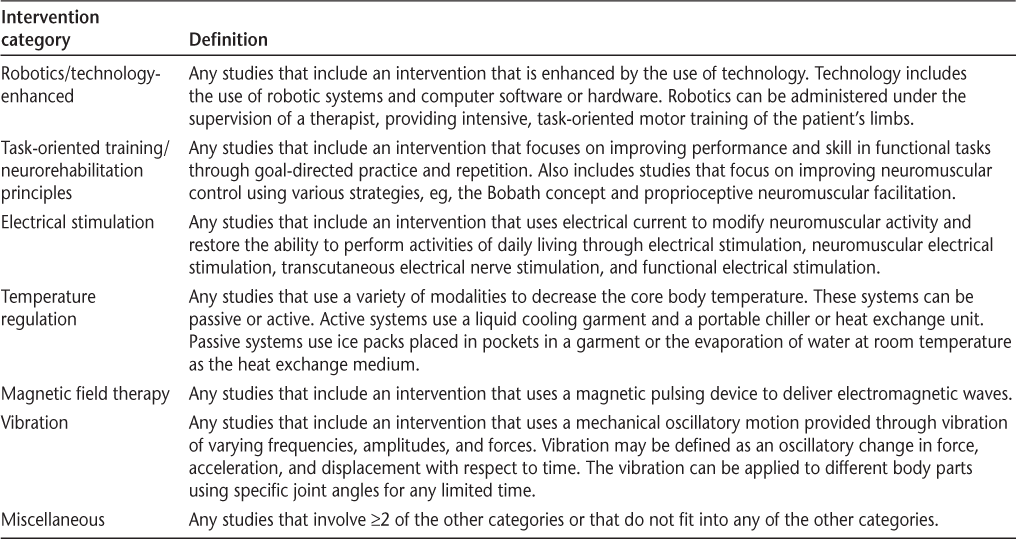
Two rehabilitation clinicians with experience delivering rehabilitation services to people with MS (AF and MF) grouped articles by type of intervention. These groupings were defined a priori by one clinician (MF) and then were confirmed and validated by the other clinician (AF). Groupings were determined by mechanism of the intervention rather than by outcomes based on the intervention description in the article. Six intervention groupings were identified: exercise (n = 99), self-management/behavior change (n = 56), cognitive rehabilitation (n = 26), multidisciplinary (n = 13), others (n = 2), and neurorehabilitation (n = 54). This article focuses on articles with neurorehabilitation interventions, which included six broad categories: robotics/technology-enhanced (eg, robot-assisted gait training) (n = 7), task-oriented training/neurorehabilitation principles (eg, the Bobath concept, task-oriented exercises) (n = 7), electrical stimulation (eg, functional electrical stimulation/neuromuscular electrical stimulation/noninvasive brain stimulation) (n = 12), temperature regulation (n = 6), magnetic field therapy (n = 5), and vibration (n = 9). In addition, we had a miscellaneous category of interventions (n = 8) that did not fit any of the other categories. Table 1 provides the definition of each category.
Definitions of neurorehabilitation intervention categories
The included articles were scrutinized for information about comorbidities: if they were excluded/included, how they were reported and described, and whether their effect on the outcomes was tested.
Results
Of the 54 RCTs, 40 excluded individuals with comorbidities, and only 2 reported on the comorbidity of enrolled participants, and neither of these examined comorbidity as a moderator or mediator of intervention outcomes. A summary of the reviewed studies follows, and Supplementary Table 1 (published in the online version of this article at ijmsc.org) provides a summary of the study characteristics.
Robotics/Technology-Enhanced Interventions
Three of seven RCTs had active comparison groups, and the remaining four had true control groups.12–18 The number of intervention sessions ranged from 618 to 1512 over 312,14,18 to 616,17 weeks. The duration of each session ranged from 3012,13,15 to 6016 minutes.
The sample size (per group) ranged from 618 to 2613 participants. The mean age of the groups ranged from 4715 to 6113 years. Participants were mostly females (range, 43%–90%) with relapsing-remitting MS (RRMS) and minimal to severe disability measured using the Expanded Disability Status Scale (EDSS).
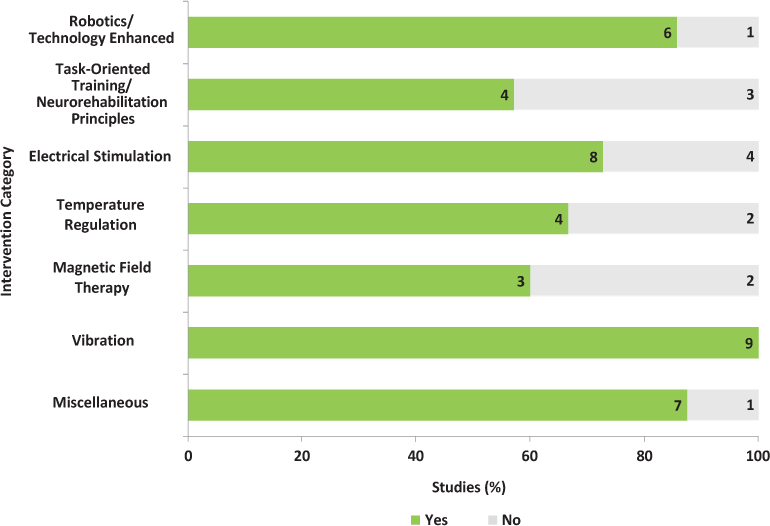
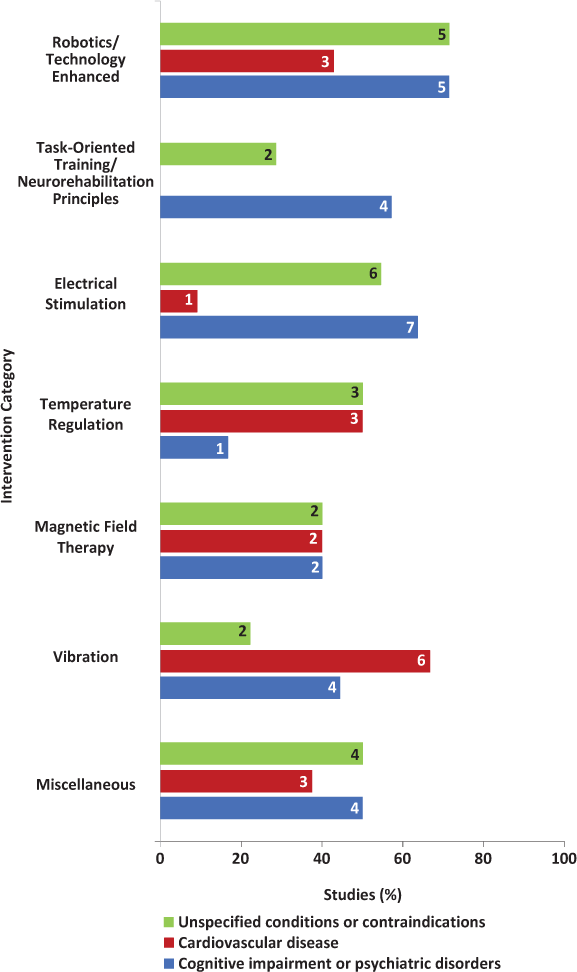
Six of seven studies (86%) reported the comorbidities of excluded individuals (Figure 2).12–14,16–18 The most commonly excluded comorbidities were cognitive impairment (n = 5, 71%)12,14,16–18 and any orthopedic or joint problems that limited range of motion (n = 4, 57%)12–14,18 (Figure 3). Of the five studies that excluded cognitive impairment, only two provided information on the measurement tools and cutoff scores used to determine eligibility.16,17 No studies provided details on the number of individuals excluded because of comorbidity. Furthermore, no studies reported on the comorbidities of enrolled participants or examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Studies excluding at least one comorbidity by intervention category
Most common comorbidities excluded by intervention category
Task-Oriented Training/Neurorehabilitation Principles
Four of seven studies (57%) had active comparison groups,19–22 and the remaining three had usual care23,24 or no intervention control groups.25 The number of intervention sessions ranged from 1025 to 2424 over 225 to 824 weeks. The duration of each session ranged from 3021 to 12025 minutes.
The sample size (per group) ranged from 921 to 2823 participants. The mean age of the groups ranged from 3319 to 5525 years. Participants were mostly females (range, 54%–83%) with RRMS and minimal to severe disability measured using the EDSS.
Four of seven studies (57%) reported the comorbidities of excluded individuals (Figure 2).20,21,24,25 The most commonly excluded comorbidities were cognitive impairment (n = 4, 57%)20,21,24,25 and psychiatric disorders (n = 3, 43%)20,21,24 (Figure 3). These psychiatric disorders were not identified. Of the four studies that excluded cognitive impairment, only one provided information on the measurement tools and cutoff scores used to determine eligibility.25
No studies provided details on the number of individuals excluded because of comorbidity. Furthermore, no studies reported on the comorbidities of enrolled participants or examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Electrical Stimulation Interventions
Of the 12 RCTs, 3 (25%) had active comparison groups26–28 and 9 (75%) had sham treatment29–36 or no intervention control groups.37 The number of intervention sessions ranged from 10 to 11233 over 5 days29,32,36 to 24 weeks.28 The duration of each session ranged from 15 minutes32,36 to 24 hours.27
The sample size (per group) ranged from 929 to 3631 participants. The mean age of the groups ranged from 3834 to 5726–28 years. Participants were mostly females (range, 30%–84%) with RRMS and minimal to severe disability as measured using the EDSS.
Eight of 12 RCTs (67%) reported the comorbidities of excluded individuals (Figure 2).27,28,31–33,35–37 The most commonly excluded comorbidities were cognitive and psychiatric disorders,27,28,31,32,35,37 and any other neurologic problems27,32,33,36 (Figure 3). These psychiatric disorders and neurologic problems were generally not identified. No studies provided details on the number of individuals excluded because of comorbidity. Only one study reported the comorbidities of enrolled participants.29 These comorbidities were anxiety and depression measured using a visual analogue scale and the Beck Depression Inventory, respectively. However, the possible mediating or moderating effect of comorbidities on intervention outcomes was not examined.
Temperature Regulation Interventions
Four of six RCTs (67%) had no intervention or sham treatment control groups,38–41 and the remaining two had both no intervention and no sham treatment groups.42,43 The number of intervention sessions ranged from one40 to three42 over 1 day40 to 4 weeks.43 The duration of each session ranged from 2038 to 6042,43 minutes.
The sample size (per group) ranged from 642 to 2240 participants. Most of the studies (n = 5, 83%) provided the mean age of the overall sample, ranging from 4142 to 5239 years. Participants were mostly females (range, 40%–86%) with minimal to severe disability as commonly measured using the EDSS.
Four of six RCTs (67%) reported the comorbidity of excluded individuals (Figure 2).38,40,42,43 The most commonly excluded comorbidities were cardiovascular and pulmonary diseases (n = 3, 50%)38,40,43 and other unspecified conditions (n = 3, 50%)40,42,43 (Figure 3). No studies provided details on the number of individuals excluded because of comorbidity. Furthermore, no studies reported the comorbidities of enrolled participants or examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Magnetic Field Therapy Interventions
All five RCTs had sham treatment groups rather than active comparison groups.44–48 The number of intervention sessions ranged from 445 to 4046 over 147 to 844,48 weeks. The duration of each session ranged from 16 minutes46 to 24 hours.48
The sample size (per group) ranged from 1548 to 3445 participants. The mean age of the groups ranged from 4446,47 to 5344 years. Participants were mostly females (range, 53%–73%) with RRMS and moderate to severe disability as measured using the EDSS.
Three of five RCTs (60%) reported the comorbidities of excluded individuals (Figure 2).44,46,47 Across these studies, participants using pacemakers and those with cognitive impairment and other unspecified conditions were commonly excluded (Figure 3). No studies provided details on the number of individuals excluded because of comorbidity. Furthermore, no studies reported the comorbidities of enrolled participants or examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Vibration Interventions
Three of nine RCTs (33%) had active comparison groups,49–51 and the remaining six (67%) had control groups (ie, no intervention, including delayed entry or sham treatment).52–57 The number of intervention sessions ranged from 954 to 2450,51,53 over 354,57 to 2052 weeks.
The sample size (per group) ranged from 550 to 3054 participants. The mean age of the groups ranged from 3453 to 5549,55 years. Participants were mostly females (range, 57%–100%) with RRMS and minimal to severe disability as measured using the EDSS.
All nine studies reported the comorbidities of excluded individuals (Figure 2). The most commonly excluded comorbidities were cardiovascular problems (eg, thrombosis and the use of pacemakers),50,51,53–56 epilepsy,50,51,55,56 and psychiatric disorders49,51,52 (Figure 3). These psychiatric disorders were not identified. No studies provided details on the number of individuals excluded because of comorbidity. Of the nine RCTs, only one reported the comorbidities of enrolled participants.49 In this study, 4 of 42 participants were taking an antiepileptic medication. However, this study did not examine the possible mediating or moderating effect of comorbidities on intervention outcomes.
Miscellaneous Interventions
Studies (n = 8) were included in this category if they did not fit any of the other categories (balance and gait, n = 1; massage, n = 3; and vestibular interventions, n = 1) or involved a combination of two or more categories (n = 3). Four of eight RCTs (50%) had active comparison groups58–61; two studies had active comparison and waitlist or usual care control groups,62,63 and the remaining two had no intervention or sham treatment control groups.64,65 The number of intervention sessions ranged from 161 to 2859 over 161 to 864 weeks. The duration of each session ranged from 1559 to 6060–62,64 minutes.
The sample size (per group) ranged from 860 to 2565 participants. The mean age of the groups ranged from 3663 to 5959 years. Participants were mostly females (range, 33%–89%) with RRMS and minimal to severe disability as measured using the EDSS.
Seven of eight studies (88%) reported the comorbidities of excluded individuals (Figure 2). The most commonly excluded comorbidities were unspecified conditions or contraindications to participating in the intervention (n = 4, 50%)62–65 and cognitive impairment and psychiatric disorders (n = 4, 50%)58,60,64,65 (Figure 3). Of the four studies excluding participants with cognitive impairment and psychiatric disorders, only two provided information on the measurement tools and cutoff scores used to determine eligibility.60,64 No studies provided details on the number of individuals excluded because of comorbidity. Furthermore, no studies reported the comorbidities of enrolled participants or examined the possible mediating or moderating effect of comorbidities on intervention outcomes.
Discussion
Given the wide range of MS-related problems and the comprehensive rehabilitation approaches that are available to address them, it was not surprising to find 54 studies examining the effectiveness or efficacy of neurorehabilitation interventions. Although the issue of comorbidity was considered in most of these studies (n = 40, 74%), it was limited to excluding individuals from participating in the trials. Of concern is the finding that most of the commonly excluded comorbidities represent some of the most prevalent ones experienced by people with MS (eg, cognitive and psychiatric disorders and cardiovascular diseases).6 Furthermore, in some studies, the descriptions provided for excluded comorbidities were vague, for example, “other illnesses that may affect motor function.”16
Most studies did not provide any information about measurement tools and cutoff scores used to determine eligibility, particularly for cognitive impairment. Therefore, it is unclear what the nature and potential cause of the excluded impairments are (eg, executive functioning, attention, memory, dementia, and MS). This lack of detail means that we may be misclassifying some studies in this review (ie, classifying as comorbidity when it is MS related), although all readers would have the same challenge. The lack of detail is also a challenge for future replication and reduces reader confidence in knowing to whom the intervention results can be generalized.
Researchers in MS tend to exclude individuals with comorbidities in neurorehabilitation trials for several reasons. First, researchers usually attempt to maximize participant safety and reduce the risk of adverse events during trials. Although participant safety is a critical concern, excluding individuals with comorbidities may lead to an inaccurate safety evaluation of some interventions, which may have implications for clinical practice. The practice of excluding people with comorbidities from neurorehabilitation trials means that we have limited knowledge about the clinical safety of these interventions for a large proportion of people with MS.
Second, researchers usually work to maximize the internal validity of their studies by creating homogeneous samples and reducing the risk of confounding. Excluding individuals with comorbidities helps in these efforts. However, in achieving greater internal validity, external validity is compromised, and the applicability of results to routine clinical settings becomes questionable. In clinical practice, rehabilitation care is provided to people with MS who typically have comorbidities that could influence their clinical presentation and management. Furthermore, the exclusion of people with comorbidities limits our understanding of how these conditions may be contributing to differential adherence or treatment effects and may also be limiting our ability to tailor interventions for the “whole” person. Studies in people with other chronic neurologic conditions, such as stroke, have shown that comorbidity can affect treatment outcome, adherence, and maintenance of the treatment effect.66,67
To find a balance between the advantages and disadvantages of including people with comorbidities in neurorehabilitation trials, MS researchers need to start moving toward large multisite pragmatic trials that include a wider range of participants. Despite the complexity of these studies, such a design will maximize applicability and generalizability and provide opportunities for conducting subgroup analyses involving people with MS with comorbidities.68
Despite the growing interest in comorbidities in people with MS, knowledge of their effect on common MS treatments remains in its infancy, even among more traditional pharmacologic interventions.68 This review found that knowledge about the effect of comorbidity on neurorehabilitation outcomes in people with MS is almost nonexistent. Despite reviewing 54 RCTs, we found only two studies that reported the comorbidities of enrolled participants. However, neither of these studies examined the possible mediating or moderating effect of comorbidities on intervention outcomes. Recent systematic reviews have identified common comorbidities experienced by people with MS.6 Together with the gaps identified by the present review, the body of evidence suggests that MS neurorehabilitation researchers should prioritize the inclusion of participants with comorbidities such as cognitive impairment and psychiatric and cardiovascular conditions. This work also suggests that investigating the effect of these comorbidities on intervention outcomes is clearly warranted.
This review has some limitations that warrant consideration. First, scoping reviews usually include a wider spectrum of evidence gathered from various sources, for example, electronic databases and gray literature. The search strategy for this review was limited to electronic databases and peer-reviewed journal articles. Second, we focused on RCTs without including other designs. Although RCTs are considered the gold standard, the strategy reduced the number of included studies for this review.
Conclusion
Despite the limitations, this review documents important gaps in knowledge about the effect of comorbidity on neurorehabilitation outcomes and identifies a critical need for future studies to address this issue. This knowledge is needed so that clinicians can make evidence-based treatment decisions. Without this information, we will be limiting our understanding of the mechanisms of comorbidity and its effect on relevant clinical and research outcomes specific to neurorehabilitation.
PracticePoints
Investigations of neurorehabilitation interventions in MS tend to exclude individuals with comorbidities, making it difficult to generalize the findings to large segments of the MS population.
Without information about the comorbidities experienced by participants with MS, the effect on study outcomes cannot be determined.
Although including people with MS who have comorbid conditions will create more heterogeneous samples, taking this methodological risk is likely to provide more generalizable knowledge about the efficacy and effectiveness of neurorehabilitation interventions.
References
Weinshenker BG. The natural history of multiple sclerosis. Neurol Clin. 1995;13:119–146.
Kister I, Chamot E, Salter AR, Cutter GR, Bacon TE, Herbert J. Disability in multiple sclerosis: a reference for patients and clinicians. Neurology. 2013;80:1018–1024.
Johnson KL, Bamer AM, Fraser RT. Disease and demographic characteristics associated with unemployment among working-age adults with multiple sclerosis. Int J MS Care. 2009;11:137–143.
Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85:240–247.
Marrie RA, Hanwell H. General health issues in multiple sclerosis: comorbidities, secondary conditions, and health behaviors. Mult Scler. 2013;19:1046–1057.
Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21:263–281.
Williams UE, Oparah SK, Philip-Ephraim EE. Disease modifying therapy in multiple sclerosis. Int Sch Res Notices. 2014;2014:307064.
Stanescu I, Dogaru G. Neurorehabilitation strategies in multiple sclerosis. Balneo Res J. 2014;5:180–187.
Kesselring J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol. 2005;4:643–652.
Khan F, Turner-Stokes L, Ng L, Kilpatrick T. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev. 2007;(2):CD006036.
Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Beer S, Aschbacher B, Manoglou D, Gamper E, Kool J, Kesselring J. Robot-assisted gait training in multiple sclerosis: a pilot randomized trial. Mult Scler. 2008;14:231–236.
Vaney C, Gattlen B, Lugon-Moulin V, et al. Robotic-assisted step training (Lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil Neural Repair. 2012;26:212–221.
Wier LM, Hatcher MS, Triche EW, Lo AC. Effect of robot-assisted versus conventional body-weight-supported treadmill training on quality of life for people with multiple sclerosis. J Rehabil Res Dev. 2011;48:483–492.
Schwartz I, Sajin A, Moreh E, et al. Robot-assisted gait training in multiple sclerosis patients: a randomized trial. Mult Scler. 2012;18:881–890.
Straudi S, Benedetti MG, Venturini E, Manca M, Foti C, Basaglia N. Does robot-assisted gait training ameliorate gait abnormalities in multiple sclerosis? a pilot randomized-control trial. NeuroRehabilitation. 2013;33:555–563.
Gandolfi M, Geroin C, Picelli A, et al. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: a randomized controlled trial. Front Hum Neurosci. 2014;8:318.
Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008;22:661–671.
Armutlu K, Karabudak R, Nurlu G. Physiotherapy approaches in the treatment of ataxic multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2001;15:203–211.
Bonzano L, Tacchino A, Brichetto G, et al. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. Neuroimage. 2014;90:107–116.
Gatti R, Tettamanti A, Lambiase S, Rossi P, Comola M. Improving hand functional use in subjects with multiple sclerosis using a musical keyboard: a randomized controlled trial. Physiother Res Int. 2015;20:100–107.
Lord S, Wade D, Halligan P. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil. 1998;12:477–486.
Cattaneo D, Jonsdottir J, Regola A, Carabalona R. Stabilometric assessment of context dependent balance recovery in persons with multiple sclerosis: a randomized controlled study. J Neuroeng Rehabil. 2014;11:100.
Keser I, Kirdi N, Meric A, Kurne AT, Karabudak R. Comparing routine neurorehabilitation program with trunk exercises based on Bobath concept in multiple sclerosis: pilot study. J Rehabil Res Dev. 2013;50:133–140.
Straudi S, Martinuzzi C, Pavarelli C, et al. A task-oriented circuit training in multiple sclerosis: a feasibility study. BMC Neurol. 2014;14:124.
Esnouf JE, Taylor PN, Mann GE, Barrett CL. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Mult Scler. 2010;16:1141–1147.
Barrett C, Mann G, Taylor P, Strike P. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009;15:493–504.
Taylor P, Barrett C, Mann G, Wareham W, Swain I. A feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis. Neuromodulation. 2014;17:75–84.
Mori F, Codeca C, Kusayanagi H, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010;11:436–442.
Lúcio AC, D'Ancona CA, Lopes MH, Perissinotto MC, Damasceno BP. The effect of pelvic floor muscle training alone or in combination with electrostimulation in the treatment of sexual dysfunction in women with multiple sclerosis. Mult Scler. 2014;20:1761–1768.
McClurg D, Ashe R, Lowe-Strong A. Neuromuscular electrical stimulation and the treatment of lower urinary tract dysfunction in multiple sclerosis: a double blind, placebo controlled, randomised clinical trial. Neurourol Urodyn. 2008;27:231–237.
Ferrucci R, Vergari M, Cogiamanian F, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 2014;34:121–127.
Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, Danilov YP. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. J Neuroeng Rehabil. 2014;11:79.
Mori F, Ljoka C, Magni E, et al. Transcranial magnetic stimulation primes the effects of exercise therapy in multiple sclerosis. J Neurol. 2011;258:1281–1287.
Mori F, Nicoletti CG, Kusayanagi H, et al. Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain Stimul. 2013;6:654–659.
Tecchio F, Cancelli A, Cottone C, et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol. 2014;261:1552–1558.
Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler. 2011;17:468–477.
Chiara T, Carlos J Jr, Martin D, Miller R, Nadeau S. Cold effect on oxygen uptake, perceived exertion, and spasticity in patients with multiple sclerosis. Arch Phys Med Rehabil. 1998;79:523–528.
Grahn DA, Murray JV, Heller HC. Cooling via one hand improves physical performance in heat-sensitive individuals with multiple sclerosis: a preliminary study. BMC Neurol. 2008;8:14.
Nilsagård Y, Denison E, Gunnarsson L-G. Evaluation of a single session with cooling garment for persons with multiple sclerosis: a randomized trial. Disabil Rehabil Assist Technol. 2006;1:225–233.
Meyer-Heim A, Rothmaier M, Weder M, Kool J, Schenk P, Kesselring J. Advanced lightweight cooling-garment technology: functional improvements in thermosensitive patients with multiple sclerosis. Mult Scler. 2007;13:232–237.
Reynolds LF, Short CA, Westwood DA, Cheung SS. Head pre-cooling improves symptoms of heat-sensitive multiple sclerosis patients. Can J Neurol Sci. 2011;38:106–111.
Schwid SR, Petrie MD, Murray R, et al. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology. 2003;60:1955–1960.
de Carvalho ML, Motta R, Konrad G, Battaglia MA, Brichetto G. A randomized placebo-controlled cross-over study using a low frequency magnetic field in the treatment of fatigue in multiple sclerosis. Mult Scler. 2012;18:82–89.
Marinelli L, Mori L, Solaro C, et al. Effect of radial shock wave therapy on pain and muscle hypertonia: a double-blind study in patients with multiple sclerosis. Mult Scler. 2015;21:622–629.
Mostert S, Kesselring J. Effect of pulsed magnetic field therapy on the level of fatigue in patients with multiple sclerosis: a randomized controlled trial. Mult Scler. 2005;11:302–305.
Nielsen JF, Sinkjaer T, Jakobsen J. Treatment of spasticity with repetitive magnetic stimulation: a double-blind placebo-controlled study. Mult Scler. 1996;2:227–232.
Richards T, Lappin M, Acosta-Urquidi J, et al. Double-blind study of pulsing magnetic field effects on multiple sclerosis. J Altern Complement Med. 1997;3:21–29.
Paoloni M, Giovannelli M, Mangone M, et al. Does giving segmental muscle vibration alter the response to botulinum toxin injections in the treatment of spasticity in people with multiple sclerosis? a single-blind randomized controlled trial. Clin Rehabil. 2013;27:803–812.
Schyns F, Paul L, Finlay K, Ferguson C, Noble E. Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil. 2009;23:771–781.
Uszynski M, Purtill H, Donnelly A, et al. The feasibility of comparing whole body vibration intervention to the same duration and dose of exercise for people with multiple sclerosis. Physother Pract Res. 2014;35:75–86.
Broekmans T, Roelants M, Alders G, Feys P, Thijs H, Eijnde BO. Exploring the effects of a 20-week whole-body vibration training programme on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med. 2010;42:866–872.
Eftekhari E, Mostahfezian M, Etemadifar M, Zafari A. Resistance training and vibration improve muscle strength and functional capacity in female patients with multiple sclerosis. Asian J Sports Med. 2012;3:279–284.
Hilgers C, Mundermann A, Riehle H, Dettmers C. Effects of whole-body vibration training on physical function in patients with multiple sclerosis. NeuroRehabilitation. 2013;32:655–663.
Jackson KJ, Merriman HL, Vanderburgh PM, Brahler CJ. Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis. J Neurol Phys Ther. 2008;32:171–176.
Schuhfried O, Mittermaier C, Jovanovic T, Pieber K, Paternostro-Sluga T. Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil. 2005;19:834–842.
Wolfsegger T, Assar H, Topakian R. 3-week whole body vibration does not improve gait function in mildly affected multiple sclerosis patients: a randomized controlled trial. J Neurol Sci. 2014;347:119–123.
Jensen MP, Gianas A, George HR, Sherlin LH, Kraft GH, Ehde DM. Use of neurofeedback to enhance response to hypnotic analgesia in individuals with multiple sclerosis. Int J Clin Exp Hypn. 2016;64:1–23.
McClurg D, Hagen S, Hawkins S, Lowe-Strong A. Abdominal massage for the alleviation of constipation symptoms in people with multiple sclerosis: a randomized controlled feasibility study. Mult Scler. 2011;17:223–233.
Vergaro E, Squeri V, Brichetto G, et al. Adaptive robot training for the treatment of incoordination in multiple sclerosis. J Neuroeng Rehabil. 2010;7:37.
Widener GL, Allen DD, Gibson-Horn C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair. 2009;23:784–791.
Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91:1166–1183.
Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. 2013;27:1126–1136.
Miller L, McIntee E, Mattison P. Evaluation of the effects of reflexology on quality of life and symptomatic relief in multiple sclerosis patients with moderate to severe disability: a pilot study. Clin Rehabil. 2013;27:591–598.
Vahtera T, Haaranen M, Viramo-Koskela A, et al. Pelvic floor rehabilitation is effective in patients with multiple sclerosis. Clin Rehabil. 1997;11:211–219.
Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation. 2009;24:321–326.
Ferriero G, Franchignoni F, Benevolo E, Ottonello M, Scocchi M, Xanthi M. The influence of comorbidities and complications on discharge function in stroke rehabilitation inpatients. Eura Medicophys. 2006;42:91–96.
Marrie RA, Miller A, Sormani MP, et al. The challenge of comorbidity in clinical trials for multiple sclerosis. Neurology. 2016;88:1437–1445.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: Ms. Fakolade was supported in part by a Multiple Sclerosis Society of Canada Doctoral Studentship. Dr. Bisson was supported by a National Multiple Sclerosis Society Mentor-Based Postdoctoral Fellowship.







