Publication
Research Article
International Journal of MS Care
Does the Effect of a Physical Activity Behavioral Intervention Vary by Characteristics of People with Multiple Sclerosis?
Author(s):
Background: Behavioral interventions have significantly increased physical activity in people with multiple sclerosis (MS). Nevertheless, there has been interindividual variability in the pattern and magnitude of change. This study documented the efficacy and variability of a behavioral intervention for changing physical activity and examined the possibility that efficacy varied by the characteristics of individuals with MS.
Methods: Eighty-two people with MS were randomly assigned to one of two conditions: behavioral intervention (n = 41) or waitlist control (n = 41). We collected information before the study on MS type, disability status, weight status based on body-mass index, and current medications. Furthermore, all participants completed the Godin Leisure Time Exercise Questionnaire and the abbreviated International Physical Activity Questionnaire and wore an accelerometer for 1 week to measure minutes of moderate-to-vigorous physical activity before and after the 6-month intervention period.
Results: Analysis of covariance (ANCOVA) indicated that participants in the behavioral intervention had significantly higher levels of physical activity than control participants after the 6-month period (P < .001). There was substantial interindividual variability in the magnitude of change, and ANCOVA indicated that MS type (relapsing vs. progressive) (P < .01), disability status (mild vs. moderate) (P < .01), and weight status (normal weight vs. overweight/obese) (P < .05) moderated the efficacy of the behavioral intervention.
Conclusions: The behavioral intervention was associated with improvements in physical activity, particularly for those with mild disability, relapsing-remitting MS, or normal weight status.
Physical activity has considerable benefits for symptom management and rehabilitation of function in individuals with multiple sclerosis (MS).1 2 However, people with this immune-mediated disease of the central nervous system are often physically inactive compared with the general population.3 Researchers have identified variables from social-cognitive theory (SCT)4 as correlates of physical activity5 that have informed the design of behavioral interventions delivered through the Internet for successfully increasing this health behavior in people with MS.6–8 Inspection of the data from such behavioral interventions reveals the presence of interindividual variability in the pattern and amount of change in physical activity.7 This suggests that some people are benefiting from the behavioral intervention more than others, and this variability might be explained by the characteristics of the participants. For example, people with mild disability, relapsing-remitting MS (RRMS), or normal weight status might be more capable of accumulating additional ambulatory-based physical activity (ie, the primary focus of the behavioral interventions) than those with moderate disability, progressive forms of MS, or overweight or obese status who have substantial burden of disease and limited capacity for accumulating additional ambulatory-based physical activity.3 There might be an interaction between behavioral interventions and the use of disease-modifying or symptomatic medications regarding change in physical activity behavior9 such that those taking medications might demonstrate larger changes in physical activity.
This article presents a secondary analysis of data from a recently completed randomized controlled trial (RCT) testing the efficacy of an Internet-delivered behavioral intervention based on SCT for increasing physical activity and managing symptoms of MS.8 This secondary analysis examines variability in intervention efficacy and the characteristics of people with MS (ie, clinical course or type of MS, disability status, weight status, and use of disease-modifying or symptomatic medications) as factors that might explain the variation in the efficacy of the intervention for increasing physical activity. The examination of moderator variables is important for 1) understanding who is most likely to benefit from the currently designed behavioral interventions and 2) suggesting the need for refining behavioral interventions for those who demonstrate little or no change in physical activity.
Methods
Sample
We previously reported on the recruitment, inclusion criteria, and enrollment of the sample in the RCT.8 Participants were recruited through flyers distributed by the North American Research Committee on Multiple Sclerosis and through our laboratory database. Of 511 individuals who initially expressed interest in participating in the study, 230 were contacted for description of the study and screening of the inclusion criteria. The inclusion criteria were as follows: age 18 to 64 years, physician's confirmation of MS diagnosis, relapse-free status in the past 30 days, Internet access, ability to walk with or without an assistive device, physician's approval for study participation, minimal risk when engaging in physical activity (ie, reported “yes” to fewer than two questions on the Physical Activity Readiness Questionnaire), willingness to travel to the research site and complete assessments, and being physically inactive (ie, <30 minutes of moderate-to-vigorous physical activity [MVPA] per day on 2 days per week). Ninety-seven individuals did not meet the inclusion criteria, 39 did not return documentation for study inclusion, and 12 were unable to complete the pretrial assessments. The final sample included 82 participants who were randomly assigned to one of the two conditions: intervention (n = 41) or waitlist control (n = 41).
Measures
Physical activity was measured using the Godin Leisure Time Exercise Questionnaire (GLTEQ),10 the abbreviated version of the International Physical Activity Questionnaire (IPAQ),11 and minutes per day of MVPA based on accelerometry.12 All three measures have been validated in people with MS.13 The GLTEQ includes three items that measure the frequencies of strenuous, moderate, and mild physical activity over a 7-day period. The frequencies (ie, times per week) of strenuous, moderate, and mild physical activity were multiplied by 9, 5, and 3 metabolic equivalents, respectively, and then were summed to a total score (0–119). The IPAQ measures the frequencies of vigorous, moderate, and walking physical activity over a 7-day period. The frequencies of vigorous, moderate, and walking physical activity were multiplied by 8, 4, and 3.3 metabolic equivalents, respectively, and were summed to a total score (0–117). ActiGraph model GT3X accelerometers (ActiGraph, Pensacola, FL) provided an objective measurement of minutes spent in MVPA per day during the previous week. This accelerometer was worn on an elastic belt around the waist during the waking hours of a 7-day period. The accelerometer data were downloaded, processed into 1-minute epochs, and then scored for wear time and minutes per day spent in MVPA using validated cut-points for MS.12 Only days with sufficient wear time (ie, ≥600 minutes) were included in the analyses, and we averaged the minutes per day of MVPA across 2 or more available valid days.14
We generated a composite physical activity measure for this study by 1) transforming the scale scores for each measure into z scores (ie, comparable units) and then 2) averaging the z scores for each measure per time point. This provided a composite score for examining change in physical activity that can be interpreted in standardized units (ie, z scores) and avoided the compound error rate associated with analysis on multiple individual outcomes. The composite further provided a comprehensive, multidimensional assessment of physical activity given that there is no gold standard measure of this behavior.
We collected data on the use of disease-modifying medications (0 = no, 1 = yes) and symptomatic medications (0 = no, 1 = yes) using a standard laboratory questionnaire. The same questionnaire provided information on the clinical course of MS (0 = RRMS, 1 = progressive MS) based on definitions consistent with the Lublin and Reingold criteria.15 We collected data on disability status using the validated Patient-Determined Disease Steps (PDDS) scale,16 forming groups of mild (PDDS score ≤2) and moderate (PDDS score of 3−6) disability (0 = mild, 1 = moderate) consistent with previous research.17 We collected data on height and weight using a scale stadiometer for computing body-mass index (BMI; calculated as weight in kilograms divided by height in meters squared) and formed groups of normal weight (BMI <25) and overweight/obese (BMI ≥25) status, coded as 0 and 1, respectively.
Intervention
The physical activity behavioral intervention has been well tested and refined in previous research.6–8 Over a 6-month period, patients visited a study website, wore a Yamax SW-401 Digi-Walker pedometer (Yamax Health & Sports Inc, San Antonio, TX), recorded physical activity in a logbook and in a spreadsheet called Goal Tracker, and participated in one-on-one video coaching sessions. The website provided content based on SCT4 for increasing ambulatory physical activity. For example, the website content, in part, focused on teaching behavioral strategies of self-monitoring and goal setting by providing information, instructions, and examples for using the Yamax SW-401 Digi-Walker pedometer, a logbook for recording daily steps, and Goal Tracker spreadsheet for setting specific, measurable, attainable, action-oriented, result-oriented, and time-phased goals and monitoring progress toward goal attainment. The other content on the website focused on outcome expectations, self-efficacy, and facilitators/barriers for physical activity consistent with SCT. The website materials were delivered in a titrated manner over the 6-month period such that new content became available seven times during the first 2 months, four times during the second 2 months, and twice during the final 2 months of the intervention.
The behavioral intervention further involved weekly one-on-one behavioral coaching sessions via Skype technology (Skype Communications SARL, a division of Microsoft Corp, Luxembourg City, Luxembourg). The sessions were semi-scripted and based on principles of supportive accountability (ie, encouraging participants to wear the pedometer daily and monitor behavior change and goal attainment throughout the 6-month intervention). The coaching sessions each consisted of a weekly review of goal setting and progress toward goal attainment as well as discussion of strategies and facilitators of behavior change based on SCT and current website content. During the 6-month intervention, there were 15 scheduled Skype coaching sessions, which decreased in frequency during the intervention; seven sessions occurred in the first 2 months, six in the second 2 months, and only two in the final 2 months.
Control Condition
We used a waitlist control because this RCT was a phase 2 efficacy trial for increasing physical activity and managing symptoms. Participants in this condition completed the study measures before and after the 6-month period and then received the intervention as described previously once the study reached completion.
Procedures
The study procedures were approved by the University of Illinois at Urbana-Champaign institutional review board, and all the participants provided written informed consent and physician's clearance before enrollment into the study. Participants initially provided demographic and clinical information, completed the PDDS scale as part of a survey battery, and underwent measurement of height and weight during a 1-hour testing session in the laboratory. The participants were then given verbal instructions and demonstration for wearing the accelerometer. Participants were asked to maintain their usual activities while wearing the accelerometer on a belt around the waist above the nondominant hip for 7 full days during all waking hours, except when engaging in water activities. Participants completed the GLTEQ and the IPAQ after wearing the accelerometer and returned the materials in a prestamped and pre-addressed envelope through the US Postal Service.
Participants were grouped into matched pairs based on physical activity data (ie, average steps per day) and level of disability and then were randomly assigned to the intervention or waitlist control condition using a random number generator and allocation by a person who was uninvolved in data collection and intervention delivery. All the participants received notification of group assignment and instructions for participation through e-mail and the US Postal Service. The intervention group further received study materials, including a Yamax SW-401 Digi-Walker pedometer, a logbook for recording daily pedometer steps, an electronic spreadsheet for tracking progress toward step count goals (ie, Goal Tracker software), a webcam, and study website log-in information. The participants completed the same assessments immediately after the 6-month intervention period and received a $50 remuneration for completing each testing session. The payment was not linked to completion of the intervention.
Data Analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 21 (IBM Corp, Armonk, NY). We initially created z scores for the baseline and follow-up physical activity measures using the save standardized values feature in the descriptive statistics option of the analyze menu. We then used the compute variable function in the transformation menu and created composite scores as a mean of the z scores per measure for baseline and follow-up. This composite score can be directly interpreted in standard deviation (SD) units. Regarding data analysis, we provide descriptive statistics as mean (SD), unless otherwise noted (eg, median and interquartile range or frequency).
The effect of the intervention was initially verified using one-way (ie, condition) analysis of covariance (ANCOVA) on follow-up composite physical activity scores controlling for baseline physical activity values; this was necessary considering the composite measure of physical activity and the interest in examining moderators of intervention efficacy. We expressed variation in physical activity change per condition using histograms of the distribution of individual change scores on the composite measure (posttest minus pretest z scores), and we compared the differential distribution of change between groups using a χ2 test. We then performed a series of two-way ANCOVAs on follow-up composite physical activity scores controlling for baseline physical activity values, with condition as one factor and baseline characteristics as the other factor (eg, MS clinical course, disability status, weight status, or medication status). We directly examined the interaction term for identifying the possibility of characteristics modifying the intervention effect on physical activity. We reported partial eta-squared (ηρ 2) values for estimating the overall magnitude of effects, with a value of 0.04 representing a practically meaningful effect size.18
Results
Sample Characteristics
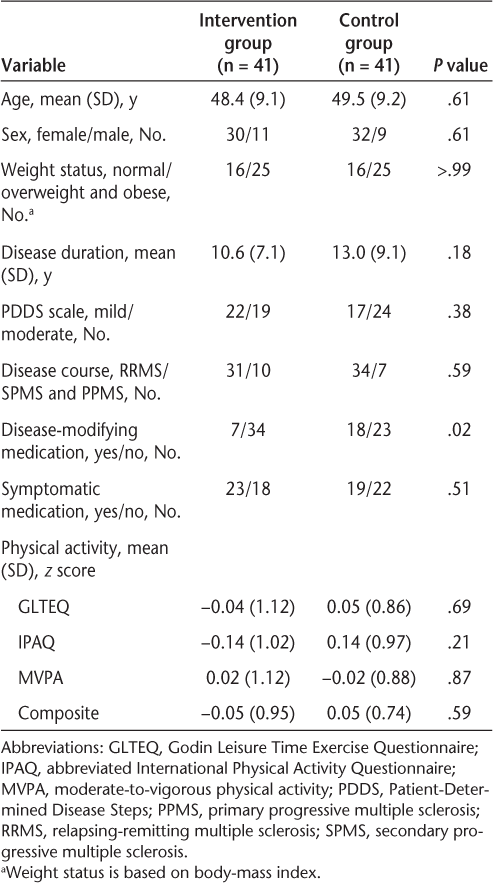
The baseline characteristics of participants in the intervention and waitlist control conditions are presented in Table 1. Overall, the sample primarily was female (76%), had an RRMS disease course (79%), and was overweight or obese (61%). The level of disability of the sample was moderate (median PDDS score, 3.0; interquartile range, 3.0). There were no statistically significant differences between groups in demographic, biometric, or clinical characteristics and physical activity levels, except for use of disease-modifying medications (Table 1); there were fewer people using a disease-modifying medication in the intervention group than in the control group. Of the 82 participants randomized to the control and intervention conditions, 37 completed the intervention condition and 39 completed the control condition.8 The subsequent analyses are based on those who completed the study.
Baseline participant characteristics for the intervention and control groups
Overall Intervention Efficacy
The one-way ANCOVA, controlling for baseline physical activity scores, indicated that participants in the behavioral intervention group had significantly higher levels of physical activity compared with individuals in the waitlist control group after the 6-month intervention (F 1,73 = 11.26, P < .001, ηρ 2 = 0.13). This finding was expected given the results reported in a primary outcome article8 and confirms the efficacy before examining variability and moderators. The ηρ 2 value indicated that this was a practically meaningful effect, and the mean composite physical activity z scores were 0.27 (SD 0.69) and −0.27 (SD 0.69) for the intervention and waitlist control conditions, respectively (overall difference of 0.54 SD for the intervention group vs. the control group).
Interindividual Variability in Intervention Efficacy
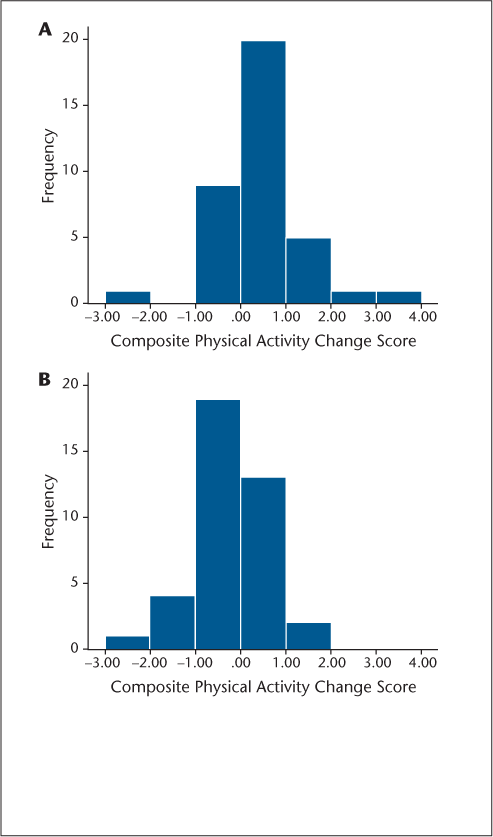
The histograms in Figure 1 indicate that there was substantial variability in the magnitude of change for participants in the intervention and waitlist control conditions. For example, 27 participants in the intervention condition demonstrated increases in physical activity (ie, a positive z score), with seven participants demonstrating large increases in physical activity (ie, a z score of ≥1 SD improvement). By comparison, ten participants in the intervention condition demonstrated decreases in physical activity (ie, a negative z score). Interestingly, 15 people in the waitlist control condition demonstrated an increase in physical activity without a behavioral intervention, whereas 24 demonstrated a reduction in physical activity. The differential distribution of change classification between groups was significant (χ2 = 10.46, P < .05).
Histograms of composite physical activity change scores (posttest minus pretest z scores) for the intervention (A) and control (B) groups
Intervention Efficacy by Characteristic
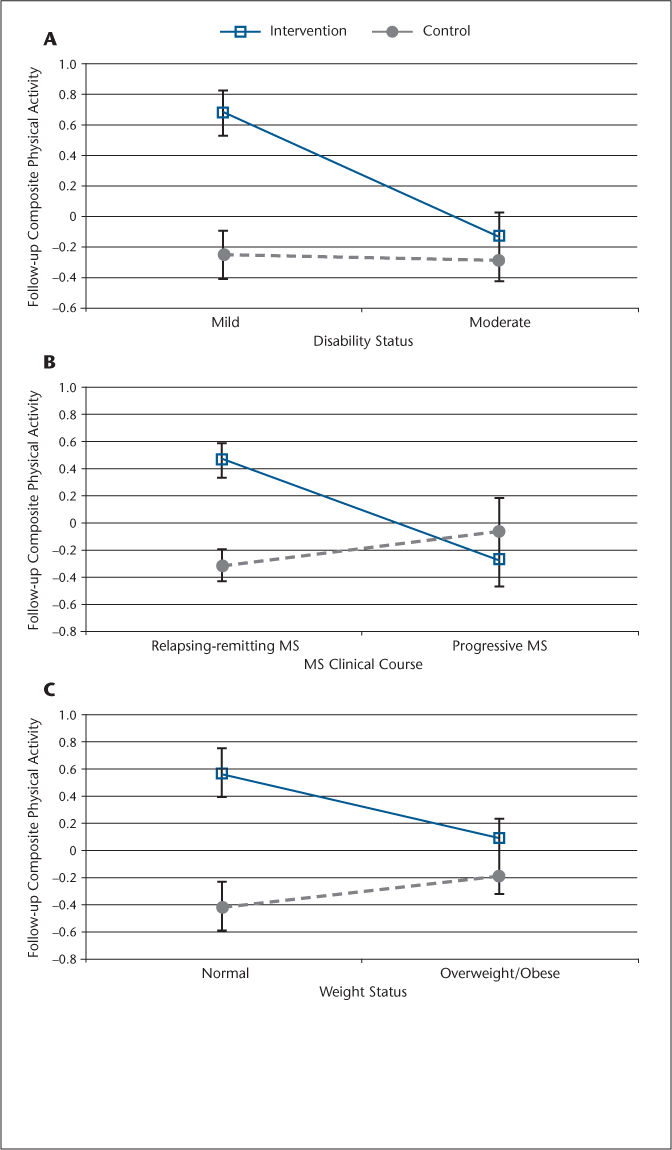
The two-way ANCOVAs indicated significant interactions for condition × disability status (ie, mild vs. moderate disability) (F 1,71 = 6.82, P < .01, ηρ 2 = 0.09), condition × MS type (ie, RRMS vs. progressive MS) (F 1,71= 6.99, P < .01, ηρ 2 = 0.09), and condition × weight status (ie, normal weight vs. overweight/obese) (F 1,71= 4.74, P < .05, ηρ 2 = 0.06); the main effect for condition was still significant in the analyses with disability status (F 1,71= 7.00, P < .01) and weight status (F 1,71= 15.18, P < .0001) but not MS type (F 1,71= 2.48, P = .12). The ηρ 2 values indicated that the interactions represented practically meaningful effects. Figure 2 illustrates that the behavioral intervention was more effective for increasing physical activity in those with mild disability (PDDS score ≤2) versus moderate disability (PDDS score of 3−6), RRMS versus progressive MS, or normal weight versus overweight/obese. There were neither statistically nor practically significant interactions for use of disease-modifying medications (F 1,71= 1.96, P = .17, ηρ 2 = 0.03) and symptomatic medications (F 1,71= 0.01, P = .87, ηρ 2 = 0.00); the main effect for condition was significant in the analyses with disease-modifying medications (F 1,71= 7.05, P < .01) and symptomatic medications (F 1,71= 11.26, P < .001). Of note, sex, age, and MS disease duration did not modify the effect of the behavioral intervention on physical activity based on an exploratory ANCOVA (ie, sex) and exploratory bivariate correlation analyses in the intervention and control groups separately (ie, age and MS disease duration).
Effect of the behavioral intervention on physical activity based on disability status (A), clinical course of multiple sclerosis (MS) (B), and weight status (C)
Discussion
We noted interindividual variability in the efficacy of behavioral interventions for increasing physical activity behavior in people with MS.6–8 This motivated a secondary analysis of data from a recent RCT8 for further expressing the degree of variability and understanding the clinical characteristics of participants that might explain this variability in physical activity change after the behavioral intervention. To that end, the behavioral intervention resulted in an overall improvement in physical activity, but there was heterogeneity in its efficacy. The improvement was largest in people with RRMS, minimal disability, or normal weight status. There was no significant difference in the efficacy of the intervention for physical activity by use of disease-modifying or symptomatic medications. Such results might suggest that the current behavioral intervention is most beneficial for those with the least burden of MS or normal weight status. The behavioral intervention likely requires reconsideration and retooling for those with progressive courses of MS that typically bring about greater disability.
We created a composite measure of physical activity for initially verifying the efficacy of the behavioral intervention delivered over a 6-month period before undertaking the moderator analyses. This composite measure was based on z scores and provided a simple, comprehensive metric for quantifying change in overall physical activity. To that end, the results indicated that the behavioral intervention yielded a practically meaningful change in physical activity (based on ηρ 2), with a mean standardized effect size of 0.54 SD units. This effect size is consistent with the magnitude of change averaged across measures in a previous study of the behavioral intervention delivered across a 3-month period in people with MS (d = 0.51).19 We note that some studies of the behavioral intervention have reported larger changes in physical activity (d = 0.98) (eg, Dlugonski et al.6), but those studies included only a single, self-reported measure of physical activity. Overall, the results of the present study and previous research suggest that behavioral interventions are beneficial for increasing physical activity in people with MS, and the overall effect is large enough to be considered practically meaningful.18 This is important considering the significant evidence for benefits of physical activity in MS1 2 and yet the alarming rate of physical inactivity in this population compared with healthy individuals from the general population.3 20
One observation based on further examination of data from previously published RCTs of behavioral interventions is that there is interindividual variability in the magnitude and pattern of change in physical activity.7 Overall, most people demonstrated an increase in physical activity levels with behavioral intervention, but some changes are exceptionally larger, whereas a small proportion of individuals demonstrated a reduction in physical activity with the behavioral intervention. This pattern of interindividual change was confirmed in the present study (Figure 1), as was the possibility that the magnitude and pattern of change might, in part, be a product of the clinical characteristics of the people with MS. Indeed, the present results indicate that the behavioral intervention was most effective for increasing physical activity in those with RRMS, mild disability status, or normal body weight. This is logical considering that such individuals have less accumulative burden of disease and, in turn, likely have the largest capacity for undertaking additional ambulatory-based physical activity (ie, the primary focus of the behavioral intervention involves walking-based physical activity, although there is a smaller focus on other types of physical activity). This suggests that the behavioral intervention requires refinement for those with progressive courses of MS, moderate disability, or overweight/obese weight status. Such refinement could be accomplished through qualitative and mixed-methods research. These research methods would provide new insight on the relevant targets of behavioral interventions or ways of changing the existing behavioral intervention for promoting physical activity in people with progressive courses of MS or those who are moderately disabled or overweight/obese.
The interindividual variability in physical activity change with the behavioral intervention was not accounted for by the use of disease-modifying or symptomatic medications. This might suggest that the behavioral intervention can facilitate physical activity behavior change across therapeutic regimens. We did not collect data regarding physical therapy or other types of rehabilitation that might have moderated the effect of the behavioral intervention. Furthermore, we did not have sufficient cases for examining changes in physical activity by specific types and doses of disease-modifying agents or classes of symptomatic medications. Nevertheless, these data suggest that the behavioral intervention is efficacious for increasing physical activity behavior regardless of the use of disease-modifying agents and symptomatic pharmacotherapies. One unresolved question is the possible combinatory efficacy of the behavioral intervention with other rehabilitation therapies for changing outcomes.
One additional observation regarding interindividual variability was that 15 people in the waitlist control (ie, 39% of the group) demonstrated a positive change score for the composite measure of physical activity (ie, increased physical activity). This is interesting because the behavioral intervention still resulted in a significantly larger increase in physical activity, even when a substantial proportion of participants in the control group demonstrated an increase in physical activity. This further demonstrates that some of the interindividual variability in change over time is unrelated to an individual's receipt of a behavioral intervention. Such naturally occurring change in physical activity should be the focus of future research, but the planned and exploratory analyses suggested that this was not associated with disability status, clinical course of MS, sex, age, or disease duration.
The main limitation of the present analysis was the unplanned secondary analysis of data regarding clinical variables that might explain the interindividual variability in physical activity change. Indeed, there are known limitations of secondary data analyses, including the lack of foresight and preplanning regarding possible moderator variables. There are certainly other clinical characteristics of people with MS that might moderate the effect of the behavioral intervention. Another possible moderator is experience with the Internet, and this might have influenced the effect of the behavioral intervention. Note that people with MS represent one of the largest Internet user groups,21 particularly for health information,22 but there is still substantial variability in experience that might have influenced the effect of the behavioral intervention for changing physical activity.
Overall, we demonstrated that the behavioral intervention based on SCT and delivered through the Internet was efficacious for changing physical activity in people with MS, but there was variability in its efficacy. The intervention was most effective for those with RRMS, mild disability, or normal weight status. Such results should be confirmed in future studies, and researchers might consider the importance of qualitative and mixed-methods research across disease types, disability levels, and weight status for identifying the possible need for more targeted behavioral interventions. The additional refinement will go a long way in promoting physical activity behavior change with the possibility of meaningful outcomes for all people with MS.
PracticePoints
Behavioral interventions have increased physical activity in people with MS, but there is interindividual variability in the efficacy of this approach for behavior change.
Three variables—clinical course of MS, disability status, and body-mass index—moderated the efficacy of the behavioral intervention for increasing physical activity.
References
Garrett M, Coote S. Multiple sclerosis and exercise in people with minimal gait impairment: a review. Phys Ther Rev. 2009; 14: 169–180.
Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol. 2012; 8: 487–497.
Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013; 94: 2342–2348.
Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004; 31: 143–164.
Suh Y, Weikert M, Dlugonski D, Balantrapu S, Motl RW. Social cognitive variables as correlates of physical activity in persons with multiple sclerosis: findings from a longitudinal, observational study. Behav Med. 2011; 37: 87–94.
Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychol Health Med. 2012; 17: 636–651.
Motl RW, Dlugonski D, Wójcicki TR, McAuley E, Mohr D. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler. 2011; 17: 116–128.
Pilutti LA, Dlugonski D, Sandroff BM, Klaren R, Motl RW. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler. 2014; 20: 594–601.
Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996; 39: 432–441.
Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985; 10: 141–146.
Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003; 35: 1381–1395.
Sandroff BM, Motl RW, Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev. 2012; 49: 467–475.
Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012; 126: 256–262.
Motl RW, Zhu W, Park Y, McAuley E, Scott JA, Snook EM. Reliability of scores from physical activity monitors in adults with multiple sclerosis. Adapt Physical Act Q. 2007; 24: 245–253.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996; 46: 907–911.
Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013; 13:37.
Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis-associated disability differ between races? Neurology. 2006; 66: 1235–1240.
Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Prof Psychol Res Pract. 2009; 40: 532–538.
Dlugonski D, Motl RW, McAuley E. Increasing physical activity in multiple sclerosis: replicating Internet intervention effects using objective and self-report outcomes. J Rehabil Res Dev. 2011; 48: 1129–1136.
Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta analysis. Mult Scler. 2005; 11: 459–463.
National Multiple Sclerosis Society. New survey finds technology plays a critical role in the lives of people with multiple sclerosis yet many are not using it to overcome disease-related challenges [news release]. http://www.microsoft.com/presspass/features/2007/oct07/10-26ms.mspx. Published October 26, 2007. Accessed July 25, 2013.
Wardell L, Hum S, Laizner AM, Lapierre Y. Multiple sclerosis patients' interest in and likelihood of using online health-care services. Int J MS Care. 2009; 11: 79–89.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This project was funded, in part, by pilot grant PP 1695 from the National Multiple Sclerosis Society.







