Publication
Research Article
International Journal of MS Care
Critical Appraisal of Evidence for Improving Gait Speed in People with Multiple Sclerosis
Background: Research has not yet compared the treatment effects of dalfampridine with traditional rehabilitation of gait impairments in multiple sclerosis (MS). The purpose of this review was to critically appraise the evidence for dalfampridine and gait training for increasing gait speed in people with MS.
Methods: A systematic search of the research literature was conducted. Consideration was given to only randomized controlled trials (RCTs), systematic reviews, and meta-analyses. For selection of gait training studies, only studies involving task-specific gait training interventions and measuring treatment effects on gait speed were considered.
Results: Treatment effects on gait speed were extracted from four studies examining the efficacy of dalfampridine and six gait training RCTs. Overall mean increase in gait speed with dalfampridine was 0.07 m/s (95% confidence interval [CI], 0.04–0.09 m/s) compared to 0.06 m/s (95% CI, 0.02–0.10 m/s) for gait training. Among dalfampridine responders (38% of participants in RCTs), the mean increase in gait speed was 0.16 m/s (95% CI, 0.13–0.18 m/s). Mean increases for individual gait training interventions ranged from 0.01 to 0.39 m/s; however, CIs were wide due to small sample sizes.
Conclusions: Current evidence is insufficient to conclude whether dalfampridine or gait training is superior for improving gait speed in people with MS. These findings should be viewed cautiously due to differences in study populations and small sample sizes in gait training studies. Both treatment approaches provide only short-lived improvements. Head-to-head comparison trials and studies combining both treatment modalities are needed.
Mobility limitations affect more than 90% of people with multiple sclerosis (MS).1 Impairments in the ability to walk can lead to reduced community participation and low levels of physical activity, depression, and poor quality of life.2–4 Considering that the onset of MS typically occurs in people 20 to 40 years old,5 which is much earlier in life than the typical onset of other common neurologic disorders (eg, stroke and Parkinson's disease), the potential impact of walking limitations on quality of life in people with MS is substantial. Thus, it is not surprising that people with MS report walking as one of their priorities.6 Dalfampridine extended-release (dalfampridine-ER), although a relatively new treatment option, is rapidly growing as a pharmacologic approach for the management of gait-related impairment in MS.7
The efficacy of treatment with dalfampridine-ER (10-mg tablets taken twice daily) for improving gait speed in people with MS has been established in two phase 3 clinical trials.8 9 The pooled analysis of these two trials10 showed that individuals treated with dalfampridine-ER had a greater improvement in gait speed from baseline (13.8%) than those treated with the placebo (6.5%, P < .001). However, there has been no research examining how the efficacy of this new treatment approach compares to that of physical therapy, the traditional clinical management approach for treating mobility limitations in MS. The purpose of this review was to critically appraise the evidence for dalfampridine-ER and physical therapy approaches to increase gait speed in people with MS, with an emphasis on evaluating and comparing the magnitude of treatment effects. This review focuses primarily on the effects of dalfampridine-ER and physical therapy for increasing gait speed because gait speed is the primary outcome by which the efficacy of dalfampridine-ER has been evaluated and is the indicated use of the drug as approved by the US Food and Drug Administration.
To locate sources for this review, a systematic search of the research literature was conducted using PubMed, the Cochrane Database of Systematic Reviews, and the Physiotherapy Evidence Database. A health sciences librarian was consulted and gave input regarding the search strategy. The search strategy used in PubMed is shown in Supplementary Table 1 (published in the online version of this article at ijmsc.org), and was last conducted on November 25, 2014. Consideration was given to randomized controlled trials (RCTs), systematic reviews, and meta-analyses only. For physical therapy/rehabilitation studies, only those that involved task-specific gait training and measured treatment effects on gait speed were selected. Task-specific training is defined as “the repetitive practice of a task that is specific to the intended outcome.”11(p1581) Studies that trained gait through repetitive practice of stepping using any modality (eg, treadmill, overground, and robotic assistance) were considered. This review was not intended to be a formal systematic review but rather an appraisal of the current results and effect sizes from the published clinical trials of dalfampridine compared with gait training.
Effects of Dalfampridine on Gait Speed in MS
The pharmacokinetics of dalfampridine-ER have been examined in several clinical studies12–16 and summarized in recent review articles7 17 and will not be detailed herein. To briefly summarize the mechanism of action, dalfampridine (chemical name: 4-aminopyridine) is a potassium-channel blocker that is believed to improve motor function in people with MS by increasing nerve conduction through demyelinated axons.18
One phase 2 trial,19 two phase 3 trials,8 9 and a pooled analysis of the phase 3 data10 have examined the efficacy of dalfampridine-ER in MS. The results are summarized in Table 1. The phase 2 trial19 was a multicenter, randomized, double-blind, placebo-controlled, parallel-group design involving 206 participants. Participants were individuals with any form of MS who had not had a recent relapse or change in medication, were aged 18 to 70 years, and could complete the Timed 25-Foot Walk test (T25FW) in 8 to 60 seconds, which equates to gait speed ranging from 0.13 to 0.95 m/s. Participants were randomly assigned to one of four treatment groups: twice-daily dalfampridine-ER in 10-, 15-, or 20-mg doses or placebo. After baseline assessment, there was a 2-week single-blind dose-escalation phase, followed by a 12-week double-blind stable-dose phase and a 1-week dose-reduction phase. Although the dalfampridine-ER groups showed larger percentage increases in gait speed from baseline (approximately 7%–11% increases [estimated from a graphical presentation of data; actual values were not reported]) than the placebo group (an approximately 2.5% increase [estimated from a graphical presentation of data; actual values were not reported]), there were no significant differences between any of the groups.
Effects of dalfampridine extended-release on gait speed
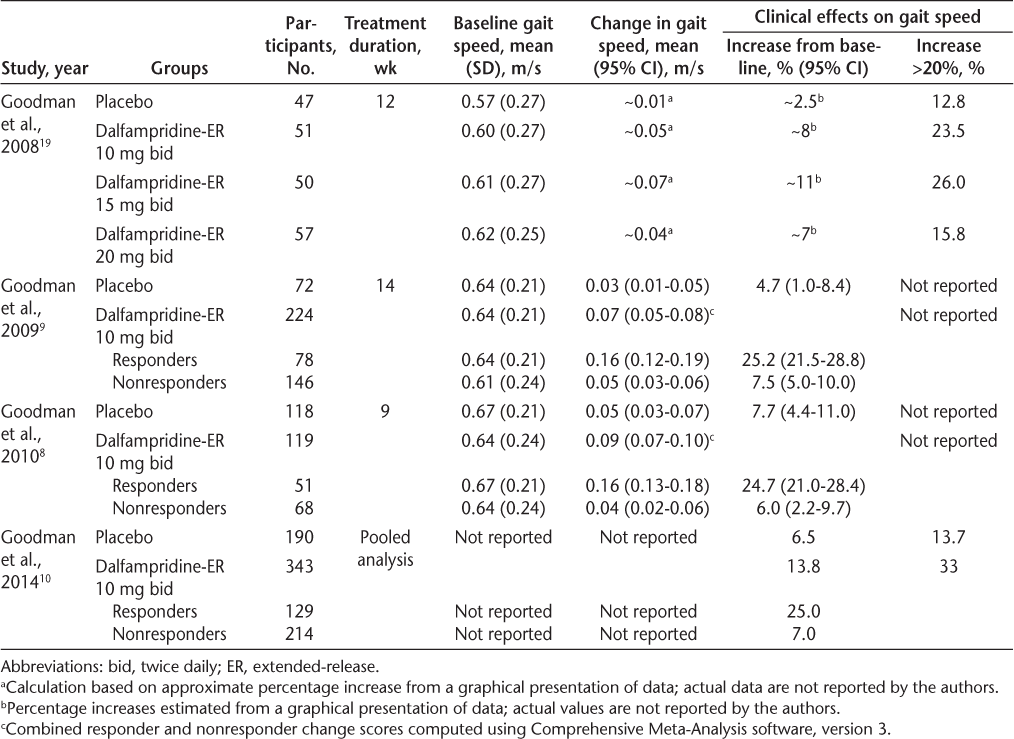
To evaluate the clinical significance of the phase 2 efficacy results, Goodman et al.19 compared the proportion of participants in each group who had an average improvement in gait speed greater than 20% from baseline, a value they specified a priori to be clinically meaningful. Although the dalfampridine-ER groups had larger percentages of participants with average increases in gait speed exceeding 20% (23.5% taking 10 mg; 26.0%, 15 mg; and 15.8%, 20 mg) than the placebo group (12.8%), none of the differences were statistically significant. The authors conducted an additional post hoc responder analysis, which revealed a significantly higher proportion of responders in the dalfampridine-ER 10-, 15-, and 20-mg groups (35.3%, 36.0%, and 38.6%, respectively) than in the placebo group (8.5%). For this analysis, a responder was defined as a participant who experienced a consistent improvement during the treatment phase, specifically, a person whose gait speed for at least three assessments during the stable-dose treatment phase was faster than his or her maximum speed in the five nontreatment visits (ie, before or after the intervention period). The post hoc responder analysis prompted criticism by Kryscio20 that Goodman et al. had excessively “massaged” the data after the planned analyses yielded nonsignificant results and that by doing so had increased the type I error rate. Subsequently, the phase 3 efficacy studies were powered for this responder analysis approach.
The two phase 3 clinical trials8 9 used a similar multisite, randomized, double-blind, placebo-controlled, parallel-group design. The minimum gait speed for inclusion was increased from 0.13 m/s in the phase 2 study to 0.17 m/s (maximum of 45 seconds to complete the T25FW). In the first phase 3 trial,9 participants were randomized at a ratio of 3:1 to receive 10 mg twice daily of dalfampridine-ER or placebo. There was a 2-week placebo run-in phase, followed by a 14-week treatment period and then a 4-week no-treatment follow-up. The primary efficacy variable was responder status, defined as consistency of gait speed improvement (ie, gait speed for at least three of the four assessments during the treatment period greater than the maximum speed in any of the off-drug assessments). The results showed that the proportion of participants meeting the responder criterion was significantly greater in the dalfampridine-ER group (35%) than in the placebo group (8%, P < .0001). The average increase in gait speed from baseline during the treatment period was 25.2% for dalfampridine-ER responders (0.16 m/s, 95% confidence interval [CI], 0.12–0.19 m/s), 7.5% for dalfampridine-ER nonresponders (0.05 m/s, 95% CI, 0.03–0.06 m/s), and 4.7% for the placebo group (0.03 m/s, 95% CI, 0.01–0.05 m/s) (Table 1). An analysis of all the dalfampridine-ER participants (ie, responders and nonresponders combined) compared with the placebo group was not reported by the authors because the primary efficacy variable nominated for the phase 3 trial was responder status. Thus, for this review, to provide a more accurate estimate of the effect size of dalfampridine-ER 10 mg twice daily on gait speed, an analysis of the reported data combining responders and nonresponders was performed using Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ). This analysis showed that in the first phase 3 clinical trial,9 the mean change in gait speed from baseline for the dalfampridine-ER group was 0.07 m/s (95% CI, 0.05–0.08 m/s), compared with the reported 0.03 m/s (95% CI, 0.01–0.05 m/s) for the placebo group.
In the second phase 3 trial,8 the participants were randomly assigned in a 1:1 ratio to receive 10 mg of dalfampridine-ER twice daily or placebo. The study design was the same as in the previous phase 3 trial9 except that the treatment period was 9 weeks instead of 14 weeks and the nontreatment follow-up period was 2 weeks instead of 4 weeks. Again, the proportion of participants who were responders was significantly higher in the dalfampridine-ER group (42.9%) than in the placebo group (9.3%, P < .0001). The average increase in gait speed from baseline during the treatment period was 24.7% for dalfampridine-ER responders (0.16 m/s; 95% CI, 0.13–0.18 m/s), 6.0% for dalfampridine-ER nonresponders (0.04 m/s; 95% CI, 0.02–0.06 m/s), and 7.7% for the placebo group (0.05 m/s; 95% CI, 0.03–0.07 m/s) (Table 1). The overall effect of dalfampridine-ER combining responders and nonresponders (calculated using Comprehensive Meta-Analysis software, version 3) was 0.09 m/s (95% CI, 0.07–0.10 m/s). Importantly, the phase 3 trials showed that although the treatment effects were retained throughout the treatment period, the effects were reversed when treatment was discontinued.
The data from the two phase 3 trials were recently pooled for further analysis and examination of participant subsets.10 The efficacy results were consistent with those of the individual trials, demonstrating a larger number of responders in the dalfampridine-ER group (37.6%) than in the placebo group (8.9%, P < .0001). Further results are presented in Table 1. The pooled analysis also found that the dalfampridine-ER responder rate was independent of demographic characteristics, disease duration, level of disability, baseline walking speed, type of MS, and use of immunomodulatory therapies.
Effects of Gait Training on Gait Speed in MS
The physical rehabilitation RCTs identified by the current search comprised interventions that can be classified as gait training interventions, including conventional gait training and treadmill training with or without body-weight support or robot assistance,21–26 aerobic exercise,25 27 progressive resistance exercise,27–34 functional electrical stimulation with or without exercise,28 29 35 conventional physical therapy,31–34 36–38 and other interventions, including whole-body vibration,39–41 torso weighting,42 Wii activities,43 44 massage,45 and rhythmic auditory stimulation.46 Although many of these studies measured treatment effects on walking ability,21–28 30 31 34 36–38 42 46 47 this review focuses on only the task-specific gait training RCTs.21–26
The individual study findings from the gait training RCTs (k = 6) are summarized in Table 2. The results of five of these six RCTs,21 22 24–26 as well as one uncontrolled trial48 and two case series reports,49 50 have been previously synthesized in a systematic review on the effectiveness of treadmill training, body-weight–supported treadmill training, and robot-assisted treadmill training (using the Lokomat [Hocoma Inc USA, Norwell, MA]) in people with MS.51 The purpose of the previous systematic review was to determine whether one gait training approach is superior to the others. Overall, Swinnen et al.51 found positive effects of treadmill training on gait speed in people with MS, with effect sizes ranging from small to large. There was no clear evidence from the review that one form of gait training was more effective than another.
Effects of gait training on gait speed
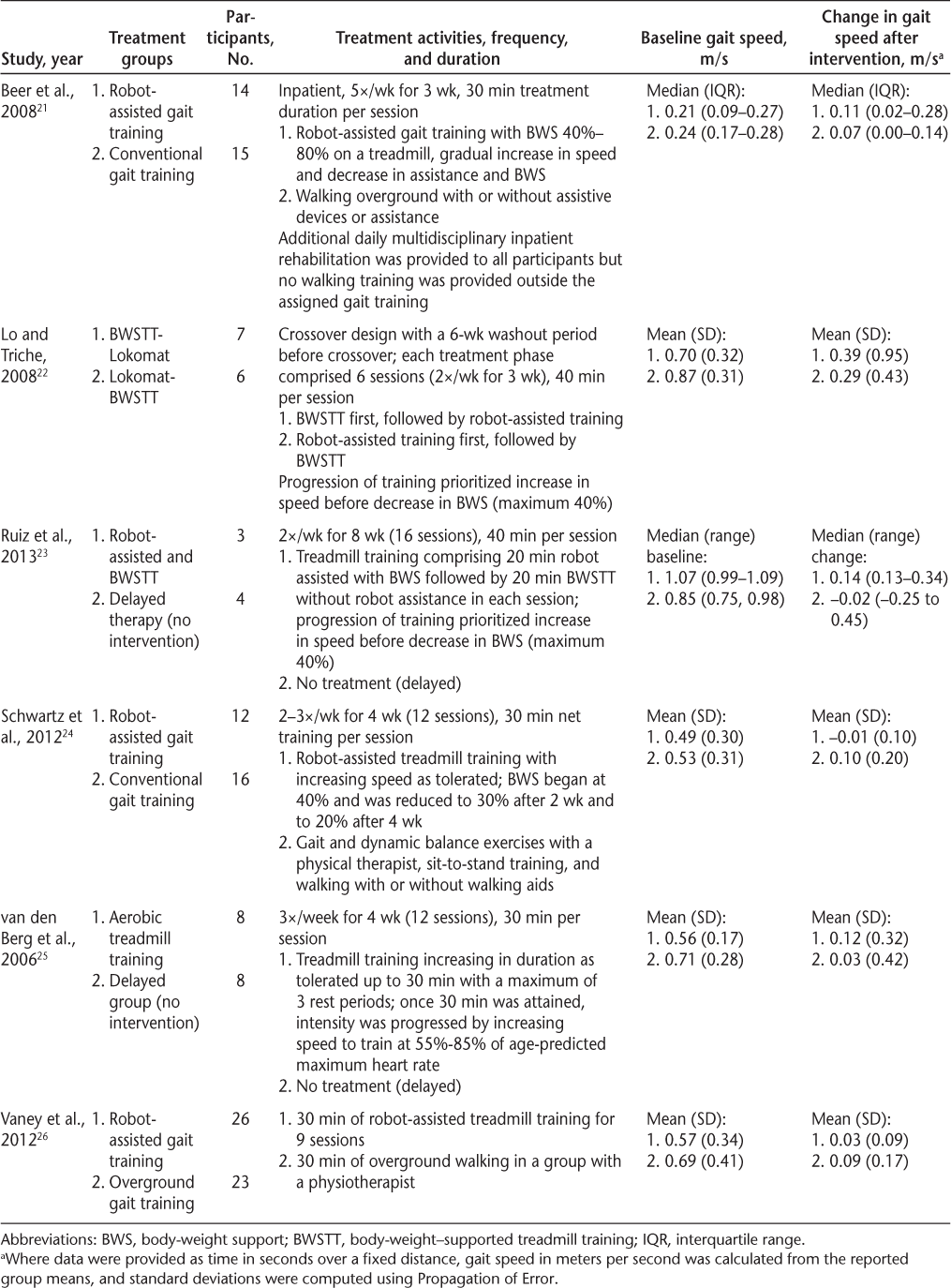
Most of the six gait training RCTs located for this review used robot-assisted or treadmill modalities for gait training, with or without body-weight support (Table 2), including one crossover design of both robot-assisted and treadmill training.22 Ruiz et al.23 included both robot-assisted and treadmill training independently in each session. Conventional (overground) gait training was examined in three of the studies as a comparison group,21 24 26 and two studies had a no-treatment (delayed) comparison.23 25 Treatment durations ranged from 9 to 16 sessions (30–40 minutes per session). Frequency varied from two to three times per week to five times per week in the two inpatient studies.21 26 Conventional gait training was not well described but included the assistance of a physical therapist to train walking “in the gym room or sometimes outside on uneven surfaces with walking aids of (the patient's) choice,”26 “walking overground (with or without walking aids),”21 or “gait and dynamic balance exercises, standing from sitting training, and walking with or without walking aids.”24
To summarize the findings: Beer et al.21 found significant within-group differences for conventional (overground) gait training (median increase, 0.11 m/s) and robot-assisted treadmill training (median increase, 0.07 m/s), with a large effect size that was not statistically significant for the between-group difference, in favor of the robot-assisted group. In a randomized crossover design of body-weight–supported treadmill training and robot-assisted treadmill training, Lo and Triche22 found that both groups significantly improved their speed on the T25FW after the crossed-over intervention (mean increases, 0.39 and 0.29 m/s, respectively), but there was wide variation. At the end of the first phase (six sessions over 3 weeks), which provided a direct comparison of the treadmill and robot-assisted interventions, there were no statistically significant differences in changes in the T25FW, although the absolute change scores favored the treadmill group.22 Schwartz et al.,24 who compared robot-assisted gait training on a treadmill with conventional gait training provided by a physical therapist, found that at the end of the 4 weeks of treatment, only conventional gait training showed significant improvements in gait speed (0.10 m/s). Similarly, Vaney et al.26 also found a between-group difference in favor of the overground walking group compared with robot-assisted gait training (adjusted mean difference, 0.05 m/s, 95% CI, −0.03 to 0.13). Van den Berg et al.25 found that aerobic treadmill training for 30 minutes three times per week for 4 weeks significantly improved gait speed (0.12 m/s increase) compared with a nonintervention control group (0.03 m/s increase), although the intervention group walked significantly more slowly (mean ± SD, 0.56 ± 0.17 m/s) than the control group (mean ± SD, 0.71 ± 0.28 m/s) at baseline.
The most recent RCT,23 which was not included in the systematic review by Swinnen et al.,51 compared a combination of robot-assisted gait training and body-weight–supported treadmill training (without robot assistance) for 16 sessions over 2 months with a no-treatment control. The sample size was very small (N = 7), and although the median change in gait speed in the gait training group (0.14 m/s) was greater than that in the no-intervention control group (−0.02 m/s), the difference was not statistically significant.
Discussion: Dalfampridine Versus Gait Training
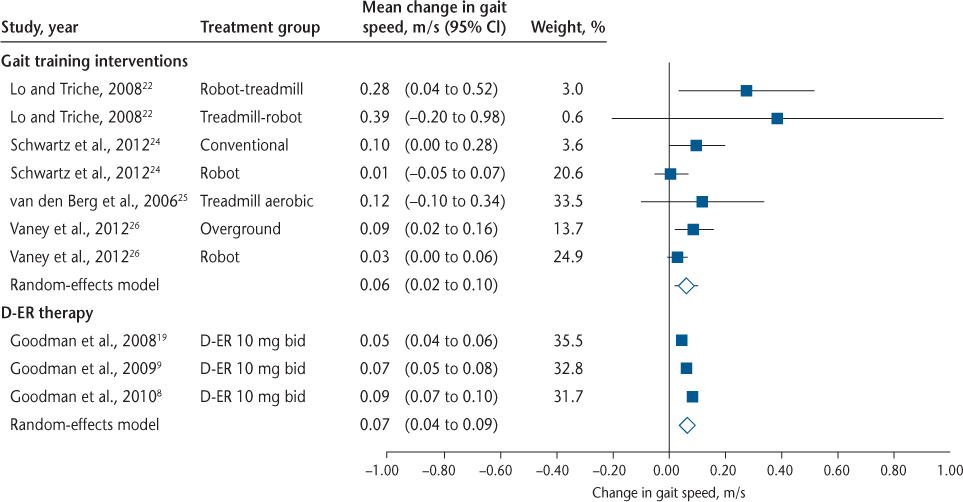
Figure 1 summarizes the mean change in gait speed and 95% CIs for individual studies and overall effect sizes for the gait training RCTs22 24–26 and the dalfampridine-ER clinical trials.8 9 19 Mean changes in gait speed are for individual treatment groups and reflect within-group change, not between-group differences. For Goodman et al., 20099 and 2010,8 data were combined for responders and nonresponders to more accurately reflect the effect size of dalfampridine-ER on gait speed. The wider CIs for the gait training interventions are due to considerably smaller sample sizes relative to the dalfampridine-ER studies. Two gait training studies21 23 were excluded from the quantitative synthesis because data were reported as medians and interquartile ranges; the change in gait speed in these studies is shown in Table 2. The synthesis also excludes the no-treatment control groups because the focus of this review was on comparing dalfampridine-ER with gait training. Figure 1 clearly illustrates that across task-specific gait training RCTs in MS, the overall effect size (0.06 m/s; 95% CI, 0.02–0.10) is similar to that observed for dalfampridine-ER when accounting for both responders and nonresponders (0.07 m/s; 95% CI, 0.04–0.09). Although the effect size is considerably larger if only the responders are considered (0.16 m/s; 95% CI, 0.13–0.18) (Table 1),8 9 it is not possible to compare this effect size with that of the gait training studies because the gait training studies do not include responder analyses. It is not presently known what proportion of patients respond favorably to gait training in the context of a clinical trial.
Forest plot showing mean changes in gait speed and 95% confidence intervals (CIs) for individual studies and overall effect sizes for gait training randomized controlled trials22 24–26 and dalfampridine extended-release (D-ER) clinical trials.8 9 19 Mean changes in gait speed are for individual treatment groups and reflect within-group change, not between-group differences. For Goodman et al. 20099 and 2010,8 the data represent changes for all participants (combined responders and nonresponders). Analyses were performed using Comprehensive Meta-Analysis software, version 3. bid, twice daily.
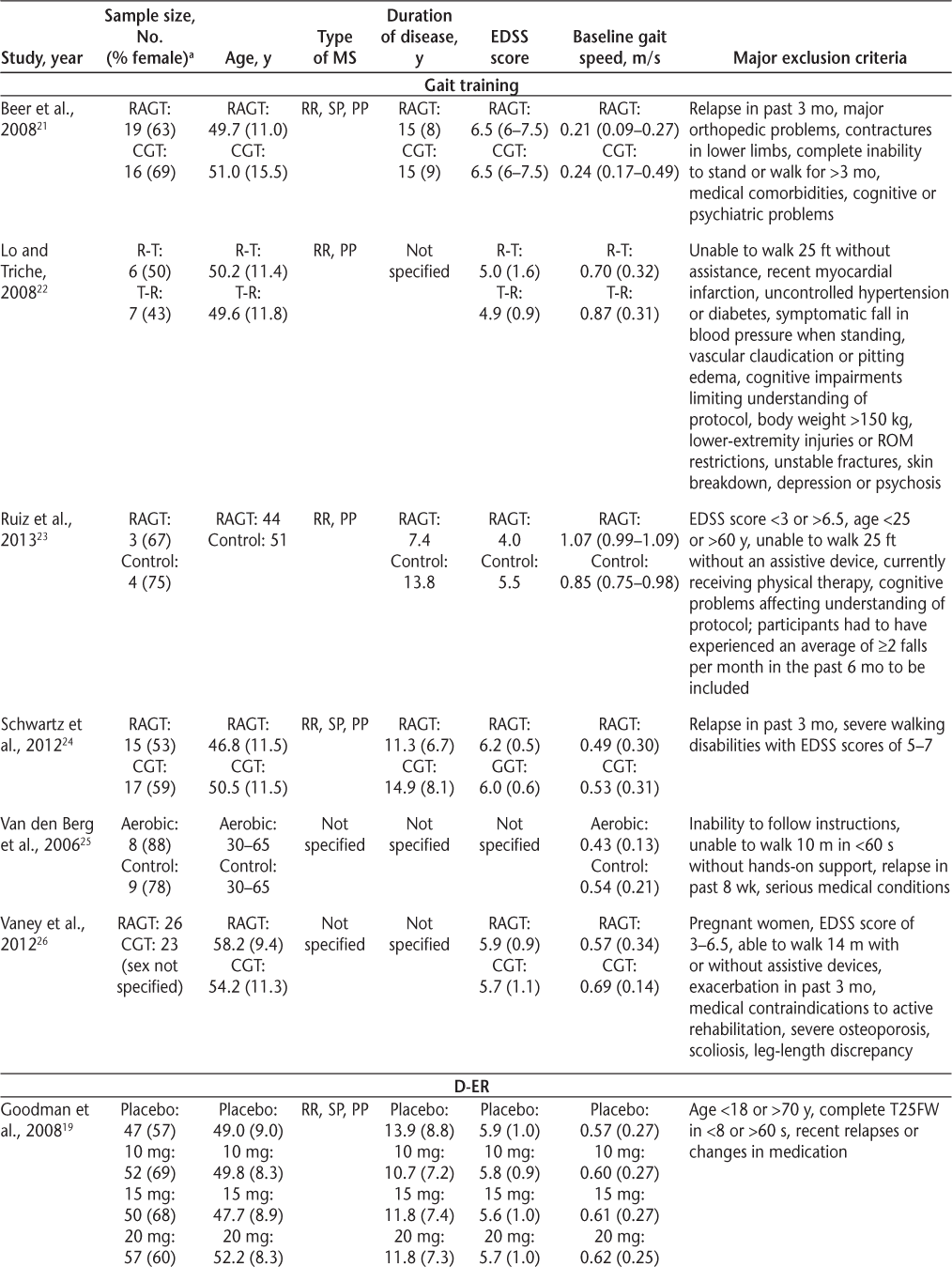
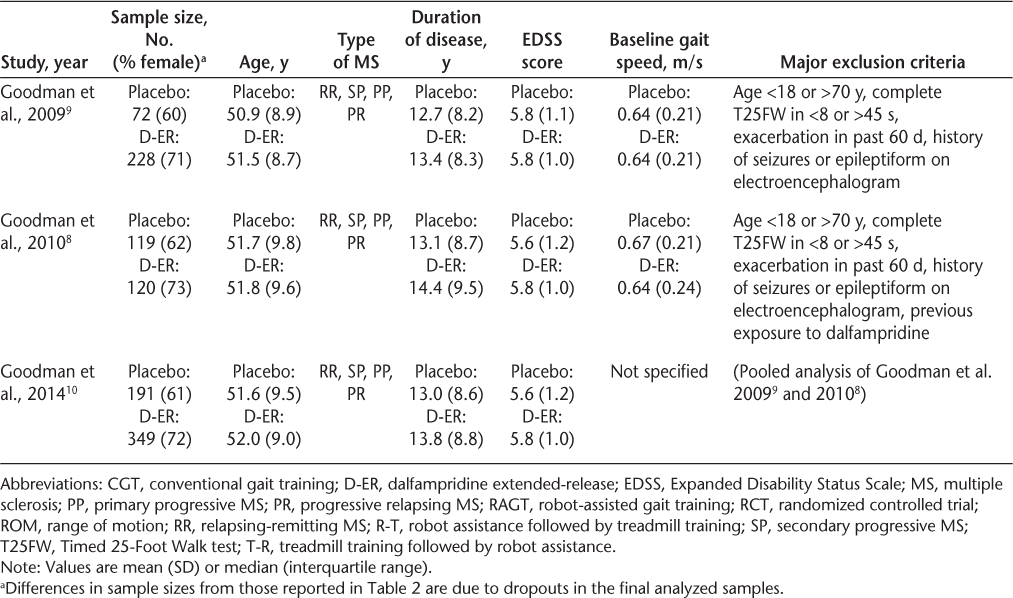
Participant characteristics and major exclusion criteria for each of the studies are summarized in Table 3. Across all of the studies, the participants were predominantly women, with a mean or median age in the late 40s or early 50s. All of the studies excluded participants with recent relapses. The cognitive function of the participants was largely unreported. Mean or median disease duration was relatively consistent (typically, 11–15 years) across studies but was not always specified in the gait training studies. Despite similar demographic characteristics, direct comparisons of treatment effects between the gait training studies and the dalfampridine-ER trials should be made with caution because the studies differed in terms of average baseline gait speed and level of disability (Table 3). The dalfampridine trials had considerably larger sample sizes and longer treatment periods than any of the gait training trials. Indeed, several of the gait training trials were reported as pilot studies, with very small sample sizes and insufficient statistical power. Thus, the effect sizes observed for the gait training studies should be viewed cautiously. Of the six gait training RCTs, only Lo and Triche22 reported an effect size greater than the average 0.16 m/s increase in gait speed observed for dalfampridine responders.8 9 However, most of the gait training interventions, regardless of modality, produced larger mean increases in gait speed than did dalfampridine-ER in the dalfampridine nonresponders. Given that 62% of people who receive dalfampridine-ER are nonresponders,10 there is a tremendous need for development of other interventions for gait rehabilitation for people with MS.
Sample characteristics of the gait training and D-ER RCTs
Sample characteristics of the gait training and D-ER RCTs
The therapeutic effects of dalfampridine-ER last only as long as the medication is being taken, and they are reversed when the treatment is discontinued.8 9 Post-intervention follow-up was lacking in most of the gait training studies. However, Beer et al.21 and Schwartz et al.24 reported that at 6-month follow-up assessments, gait parameters had returned to baseline values. Therefore, based on current evidence, both dalfampridine-ER and gait training treatment options seem to produce only short-lived improvements.
This review examined only task-specific gait training rehabilitation interventions, which were considered to provide the optimal comparison with dalfampridine-ER given the large amount of evidence that task-specific training is optimal for improving functional activities in people with neurologic disorders.52 Nonetheless, a more comprehensive review of other physical rehabilitation clinical trials on gait performance in people with MS may be warranted to fully appreciate the effect size of physical rehabilitation on gait speed. Indeed, there is strong evidence that exercise therapy improves mobility-related activities, although specific effects on gait speed are not clear.53 54 This review focused exclusively on gait speed because this was the primary efficacy measure for the pivotal phase 3 dalfampridine-ER trials. Although gait speed is considered the “functional vital sign,”55 treatment effects on other mobility parameters and self-reported measures of perceived disability should also be taken into account to evaluate clinical significance. A review of these outcomes was beyond the scope of the present article.
There have been questions raised about whether the treatment benefit of dalfampridine outweighs the costs56; however, the cost-effectiveness of dalfampridine and gait training have not been assessed. The cost of dalfampridine in the United States is high: the wholesale price of a bottle of sixty 10-mg dalfampridine-ER tablets (a 30 days' supply) was $1267.39 in 2010.17 In the United Kingdom, the cost of dalfampridine is £4700 per year (2014), approximately $7200 USD.57 Gait training costs could not be estimated because of variations in the content, duration, and number of treatment sessions.
This review highlights some important priorities for future research. First, there is a need to directly compare dalfampridine-ER with gait training and other physical therapy interventions. Moreover, combining gait training/physical therapy (such as exercise) with dalfampridine-ER may increase the effect size on gait speed and increase the proportion of patients who respond (ie, experience a clinically meaningful treatment effect). To date, gait training studies have not included a responder analysis; much larger gait training trials are needed to afford such an analysis. Cost-effectiveness should also be considered in future research trials of dalfampridine and rehabilitation to enable comparison of the costs and benefits of each treatment approach. As Hayes7 argues, another important priority for future research is to examine whether dalfampridine-ER can produce clinically meaningful improvements in instrumental activities of daily living and employment. Indeed, walking in the real world is far more complex than walking in the clinic or laboratory setting, and real-world ambulation typically involves the concurrent performance of cognitive tasks, such as talking (ie, dual tasking). Therefore, future research should also examine the effects of rehabilitation (drug and/or physical) on more realistic locomotor tasks, such as dual-task walking and obstacle negotiation.
Conclusion
There are presently no studies that have directly compared oral dalfampridine-ER 10 mg given twice daily with other treatment options for gait impairment, such as gait training. Thus, comparisons of treatment efficacy for these two options can be made only from relative treatment effects in individual studies. The quantitative summary of existing evidence in this review demonstrates that the overall effect size of dalfampridine-ER is similar to that observed in a pooled analysis of gait training RCTs, although important differences in the sample populations mean that these comparisons should be viewed cautiously. In addition, a wide variation in the response to both forms of therapy is expected. Although 38% of participants in RCTs demonstrated a consistent therapeutic response to dalfampridine-ER, the proportion of responders in gait training studies has not been assessed, and the correlation between study responders and real-life responders has not been established. Both treatment approaches provide only short-term improvements; however, retention was infrequently assessed in the gait training studies. Furthermore, the clinical significance of the changes in gait speed observed with dalfampridine or gait training has not yet been fully determined. In summary, the current evidence is insufficient to conclude whether dalfampridine-ER or gait training is superior for improving gait speed in people with MS. Trials comparing the two approaches directly and examining the effects of a combined intervention are warranted.
PracticePoints
The mean increase in gait speed with dalfampridine in people with MS is 0.07 m/s (95% confidence interval [CI], 0.04–0.09 m/s). A proportion of patients (referred to as “responders,” 37.6%) experience larger treatment effects: 0.16 m/s (95% CI, 0.13–0.18 m/s).
Mean increases in gait speed for individual gait training interventions range from 0.01 m/s (95% CI, −0.05 to 0.07 m/s) to 0.39 m/s (95% CI, −0.20 to 0.98 m/s). The CIs are wide due to very small sample sizes.
The current evidence is insufficient to conclude whether dalfampridine or gait training is superior for improving gait speed in people with MS. Direct comparisons of dalfampridine and gait training are needed, as well as examination of dalfampridine combined with physical therapy.
References
Hemmett L, Holmes J, Barnes M, et al. What drives quality of life in multiple sclerosis? QJM. 2004;97:671–676.
Motl RW, McAuley E. Physical activity and health-related quality of life over time in adults with multiple sclerosis. Rehabil Psychol. 2014;59:415–421.
Motl RW, McAuley E, Wynn D, et al. Physical activity, self-efficacy, and health-related quality of life in persons with multiple sclerosis: analysis of associations between individual-level changes over one year. Qual Life Res. 2013;22:253–261.
Cavanaugh JT, Gappmaier VO, Dibble LE, et al. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011;35:26–33.
Poser S, Raun N, Poser W. Age at onset, initial symptomatology and the course of multiple sclerosis. Acta Neurol Scand. 1982;66:355–362.
Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991.
Hayes KC. Impact of extended-release dalfampridine on walking ability in patients with multiple sclerosis. Neuropsychiatr Dis Treat. 2011;7:229–239.
Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68:494–502.
Goodman AD, Brown TR, Krupp LB, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738.
Goodman AD, Brown TR, Schapiro RT, et al. A pooled analysis of two phase 3 clinical trials of dalfampridine in patients with multiple sclerosis. Int J MS Care. 2014;16:153–160.
Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87:1580–1602.
Henney HR III, Faust B, Blight AR. Effect of food on the single-dose pharmacokinetics and tolerability of dalfampridine extended-release tablets in healthy volunteers. Am J Health Syst Pharm. 2011;68:2148–2154.
Samara E, Winkle P, Pardo P, et al. Pharmacokinetics of dalfampridine extended release 7.5-mg tablets in healthy subjects and individuals with mild and moderate renal impairment: an open-label study. J Clin Pharmacol. 2014;54:53–60.
Smith W, Swan S, Marbury T, et al. Single-dose pharmacokinetics of sustained-release fampridine (Fampridine-SR) in healthy volunteers and adults with renal impairment. J Clin Pharmacol. 2010;50:151–159.
Vollmer T, Henney HR III. Pharmacokinetics and tolerability of single escalating doses of fampridine sustained-release tablets in patients with multiple sclerosis: a phase I–II, open-label trial. Clin Ther. 2009;31:2206–2214.
Bever CT Jr, Anderson PA, Leslie J, et al. Treatment with oral 3,4 diaminopyridine improves leg strength in multiple sclerosis patients: results of a randomized, double-blind, placebo-controlled, crossover trial. Neurology. 1996;47:1457–1462.
Lamore R III, Jacob E, Jacob SC, et al. Dalfampridine (Ampyra). Drug Forecast. 2010;35:665–669.
Judge S, Bever CJ. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther. 2006;111:224–259.
Goodman AD, Brown TR, Cohen JA, et al. Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology. 2008;71:1134–1141.
Kryscio RJ. Fampridine for MS responders: clinically relevant or hypothesis generating? Neurology. 2008;71:1130–1131.
Beer S, Aschbacher B, Manoglou D, et al. Robot-assisted gait training in multiple sclerosis: a pilot randomized trial. Mult Scler. 2008;14:231–236.
Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008;22:661–671.
Ruiz J, Labas MP, Triche EW, et al. Combination of robot-assisted and conventional body-weight-supported treadmill training improves gait in persons with multiple sclerosis: a pilot study. J Neurol Phys Ther. 2013;37:187–193.
Schwartz I, Sajin A, Moreh E, et al. Robot-assisted gait training in multiple sclerosis patients: a randomized trial. Mult Scler. 2012;18:881–890.
van den Berg M, Dawes H, Wade DT, et al. Treadmill training for individuals with multiple sclerosis: a pilot randomised trial. J Neurol Neurosurg Psychiatry. 2006;77:531–533.
Vaney C, Gattlen B, Lugon-Moulin V, et al. Robotic-assisted step training (Lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil Neural Repair. 2012;26:212–221.
Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis: a randomized study. Neurology. 2004;63:2034–2038.
Barrett CL, Mann GE, Taylor PN, et al. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler. 2009;15:493–504.
Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler. 2011;17:468–477.
Cakt BD, Nacir B, Genc H, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil. 2010;89:446–457.
Dodd KJ, Taylor NF, Shields N, et al. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult Scler. 2011;17:1362–1374.
Harvey L, Smith AD, Jones R. The effect of weighted leg raises on quadriceps strength, EMG parameters and functional activities in people with multiple sclerosis. Physiotherapy. 1999;85:154–161.
Hayes HA, Gappmaier E, LaStayo PC. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: a randomized controlled trial. J Neurol Phys Ther. 2011;35:2–10.
Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. 2012;26:579–593.
Taylor P, Barrett C, Mann G, et al. A feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis. Neuromodulation. 2014;17:75–84; discussion 84.
Storr LK, Sorensen PS, Ravnborg M. The efficacy of multidisciplinary rehabilitation in stable multiple sclerosis patients. Mult Scler. 2006;12:235–242.
Wiles CM, Newcombe RG, Fuller KJ, et al. Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70:174–179.
Lord SE, Wade DT, Halligan PW. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: a pilot randomized controlled study. Clin Rehabil. 1998;12:477–486.
Broekmans T, Roelants M, Alders G, et al. Exploring the effects of a 20-week whole-body vibration training programme on leg muscle performance and function in persons with multiple sclerosis. J Rehabil Med. 2010;42:866–872.
Hilgers C, Mundermann A, Riehle H, et al. Effects of whole-body vibration training on physical function in patients with multiple sclerosis. NeuroRehabilitation. 2013;32:655–663.
Schyns F, Paul L, Finlay K, et al. Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil. 2009;23:771–781.
Widener GL, Allen DD, Gibson-Horn C. Randomized clinical trial of balance-based torso weighting for improving upright mobility in people with multiple sclerosis. Neurorehabil Neural Repair. 2009;23:784–791.
Nilsagard YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomised, controlled multi-centre study. Mult Scler. 2013;19:209–216.
Prosperini L, Fortuna D, Gianni C, et al. Home-based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair. 2013;27:516–525.
Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. 2013;27:1126–1136.
Conklyn D, Stough D, Novak E, et al. A home-based walking program using rhythmic auditory stimulation improves gait performance in patients with multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2010;24:835–842.
Ratchford JN, Shore W, Hammond ER, et al. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010;27:121–128.
Newman MA, Dawes H, van den Berg M, et al. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: a pilot study. Mult Scler. 2007;13:113–119.
Giesser B, Beres-Jones J, Budovitch A, et al. Locomotor training using body weight support on a treadmill improves mobility in persons with multiple sclerosis: a pilot study. Mult Scler. 2007;13:224–231.
Pilutti LA, Lelli DA, Paulseth JE, et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011;92:31–36.
Swinnen E, Beckwee D, Pinte D, et al. Treadmill training in multiple sclerosis: can body weight support or robot assistance provide added value? a systematic review. Mult Scler Int. 2012;2012:240274.
Carr J, Shepherd R. Neurological Rehabilitation: Optimizing Motor Performance. 2nd ed. Edinburgh, UK: Churchill Livingstone Elsevier; 2010.
Rietberg MB, Brooks D, Uitdehaag BM, et al. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005;1:CD003980.
Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. 2009;23:108–116.
Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322.
Hauser SL, Johnston SC. 4-aminopyridine: new life for an old drug. Ann Neurol. 2010;68:A8–A9.
Multiple Sclerosis Trust. Information, Education, Research and Support. Herts, UK: Multiple Sclerosis Trust; 2014.
Financial Disclosures: Dr. Plummer is a member of the Physical Therapy in Post Ischemic Stroke Advisory Board for Acorda Therapeutics Inc.







