Publication
Research Article
International Journal of MS Care
Associations Between Treatment Satisfaction, Medication Beliefs, and Adherence to Disease-Modifying Therapies in Patients with Multiple Sclerosis
Author(s):
Abstract
Background:
Adherence to disease-modifying therapy (DMT) remains problematic for many patients with multiple sclerosis (MS). An improved understanding of factors affecting DMT adherence may inform effective interventions. This study examined associations between treatment satisfaction, medication beliefs, and DMT adherence.
Methods:
A survey was mailed in 2016 to 600 adult patients with relapsing-remitting MS taking an injectable or oral DMT. Patients were sampled from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry. The survey measured self-reported DMT adherence (doses taken divided by doses prescribed during previous 2-week period—adherence ≥0.80), DMT satisfaction using the Treatment Satisfaction Questionnaire for Medication version II, medication beliefs using the Beliefs About Medicines Questionnaire, and demographic and clinical covariates. Relationships between variables were examined using multivariate logistic regression.
Results:
Final analyses included 489 usable surveys. Mean ± SD participant age was 60.5 ± 8.3 years. Most respondents were white (93.8%), female (86.6%), taking an injectable DMT (66.9%), and adherent to DMT (92.8%). Significant predictors of DMT adherence were age (odds ratio [OR], 1.086; 95% CI, 1.020–1.158; P = .011), type of DMT (oral vs. injectable; OR, 23.350; 95% CI, 2.254–241.892; P = .008), and DMT experience (naive vs. experienced; OR, 2.831; 95% CI, 1.018–7.878; P = .046).
Conclusions:
In patients with MS sampled from a patient registry, treatment satisfaction and medication beliefs were not significantly associated with DMT adherence. Based on significant predictors, younger patients, patients taking injectable DMTs, and patients with previous experience with another DMT(s) are at higher risk for nonadherence. Future research is warranted to assess relationships between variables in more diverse MS populations.
Multiple sclerosis (MS) is an unpredictable demyelinating disease of the central nervous system that is associated with significant burden to patients and their families. Although there is no cure for MS, several disease-modifying therapies (DMTs) are available for use in patients with relapsing forms of MS who manifest current disease activity as demonstrated by clinical symptoms and/or recent magnetic resonance imaging lesions. The DMTs modify or change the course of MS by reducing the number of relapses and/or by slowing disease progression. However, to achieve optimal clinical benefit, patients must be adherent to their DMT.
Poor adherence to DMTs is a major issue for many patients, with rates ranging from 41% to 88%.1 Given the potential negative effect on economic, clinical, and humanistic outcomes, there is a major need to address poor DMT adherence in patients with MS. Tan and colleagues2 found that, on average, DMT-adherent patients with MS incur lower medical costs than nonadherent patients. In a retrospective cohort study using a US administrative claims database, the rate of severe relapse during the 2-year study period was lower for patients with MS adherent to DMT than for patients nonadherent to DMT.3 An observational, multicenter, multinational study by Devonshire and colleagues4 found that DMT-adherent patients with MS had better quality-of-life scores on all nine dimensions of the Multiple Sclerosis International Quality of Life Questionnaire.
Understanding the factors associated with DMT adherence may provide opportunities for the development of interventions and strategies aimed at helping patients with MS adhere to their DMT. The World Health Organization Medication Adherence Model categorizes the barriers to adherence into five broad domains: social and economic factors (eg, age, race, employment, literacy), health care team and system-related factors (eg, quality of health care services), condition-related factors (eg, severity of symptoms, comorbid conditions), therapy-related factors (eg, convenience, side effects), and patient-related factors (eg, forgetfulness, beliefs about disease and medication).5 Given that patient-related factors (eg, beliefs) are often the most amenable to change through interventions and educational programs,6 this study further explored patient-related factors potentially associated with DMT adherence, specifically, treatment satisfaction and medication beliefs.
Treatment satisfaction is a construct not captured in established theoretical models of individual decision making. When evaluating the quality of pharmaceutical products and services, treatment satisfaction is a useful patient-reported outcome and quality metric.7 Furthermore, it can be used as a tool to screen for patients who may become nonadherent due to unsatisfactory components of their medication.7 In a review of studies assessing the link between medication adherence, persistence, and treatment satisfaction, Barbosa and colleagues8 found that 16 studies demonstrated a statistically significant link between satisfaction and adherence or persistence.
The association between patients' beliefs and medication adherence is well established in the literature, with some studies suggesting that beliefs are the strongest predictor of adherence.9,10 An important reason for focusing on patients' beliefs when studying adherence is that beliefs tend to be fixed over time unless an intervention is made.11 This suggests that patients' beliefs may be modified, if needed. Interventions (eg, educational programs) that target specific negative beliefs may have a positive impact on adherence.12
Regarding patients' medication beliefs, this study focused on perceived necessity and perceived concern. The Necessity-Concerns Framework, developed by Horne and colleagues,13 assumes that adherence decisions are influenced by a cost-benefit assessment. With respect to this study, patients' beliefs about the necessity to take their DMT for managing their MS are balanced against concerns about the potential adverse effects of their DMT. The utility of the Necessity-Concerns Framework in predicting adherence has been supported by several studies.13 In a meta-analysis of 94 studies, greater medication adherence was associated with strong perceptions of necessity of treatment and lower concerns about treatment.13
In summary, DMT adherence may be affected by several factors (eg, social and economic, health care system, condition, therapy, and patient). Studies of patients with other diseases suggest that satisfaction and medication beliefs are associated with medication adherence; however, few studies have provided support for these relationships in patients with MS taking DMTs. Thus, the objectives of this study were 1) to describe patients' treatment satisfaction (in terms of effectiveness, side effects, convenience, and global satisfaction), perceived necessity, perceived concern, and adherence with their current DMT and 2) to explore the factors that influence the likelihood of DMT adherence.
Methods
Study Design and Sample
This study used a nonexperimental, cross-sectional design. Study participants were patients with MS identified from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, which is a registry for MS research, treatment, and patient education. The Registry comprises more than 37,000 persons with MS who have volunteered to provide information about their experience with the disease through biannual surveys. The survey for this study was sent separately from the NARCOMS biannual surveys.
Eligible participants were Registry members who were aged 18 years or older, diagnosed as having relapsing-remitting MS, prescribed a US Food and Drug Administration (FDA)–approved injectable or oral DMT, and able to complete the self-administered survey instrument. Registry members currently prescribed an intravenous infusion DMT or a non–FDA-approved DMT or who are not on any DMT were excluded. Patients taking an intravenous infusion DMT were excluded because adherence tends to not be an issue, as infusions are administered by a health care professional in an infusion clinic. Eligibility was based on Registry members' self-reported information from the NARCOMS Fall 2015 biannual survey. This study was approved by The University of Texas at Austin institutional review board.
Survey Development
The survey included previously developed and validated scales, including the Treatment Satisfaction Questionnaire for Medication version II (TSQM vII) and the Beliefs About Medicines Questionnaire (BMQ). Several demographic and clinical characteristics were also measured. These scales and demographic and clinical characteristics are discussed in further detail later herein.
Before distribution, the survey was pilot tested with ten patients with MS from local MS support groups who met the study inclusion criteria. The purpose of the pilot test was to identify issues with format, instructions, and clarity/completeness of question-and-answer choices. Based on participants' comments, no modifications to the survey were necessary.
Study Measures
Figure 1 shows the conceptual model that provides the proposed relationships between study variables.
Study model

Dependent Variable
DMT Adherence. Adherence to DMT was measured using self-reported questions adapted from an approach outlined by Siegel et al.14 Participants were asked the following: 1) “How many times during the last 2 weeks were you supposed to take your DMT (twice day, once a day, every other day, 3 times a week, once a week, once every 2 weeks)?” and 2) “How many doses did you miss or forget?” Adherence to DMT was defined as the percentage of adherence during the past 2 weeks (ie, the quotient of total doses taken divided by total doses prescribed). For the univariate and multivariate logistic regression analyses, participants were categorized as adherent (DMT adherence ≥0.80) and nonadherent (DMT adherence <0.80).
Independent Variables
The TSQM vII. Satisfaction with DMT was measured using the TSQM vII, an 11-item questionnaire that assesses patients' satisfaction with their medication.7 It includes four subscales: satisfaction with medication effectiveness, dissatisfaction with medication side effects, satisfaction with medication convenience, and global satisfaction.
The TSQM subscales have been found to explain 9% to 20% of the variance in adherence.7 The original TSQM, vI, has been used with patients with MS15,16 and has been found to be reliable, with Cronbach α values for subscales ranging from 0.79 to 0.93.16 The TSQM vII was used in this study because it has equivalent measurement characteristics as the TSQM vI but uses four fewer items and more consistent wording.7
Patients responded to items on the TSQM vII using 5-point (1 = extremely dissatisfied to 5 = not at all dissatisfied) and 7-point (1 = extremely dissatisfied to 7 = extremely satisfied) Likert scales. After applying the scale scoring algorithm, possible scores for each of the TSQM vII subscales ranged from 0 to 100, with higher scores indicating greater satisfaction.
The BMQ. Beliefs about DMT were measured using the BMQ, a ten-item questionnaire that includes two subscales: perceived necessity and perceived concern.9 Although the BMQ has not been used in the MS population, there is evidence of reliability for both subscales in other populations (eg, perceived necessity subscale: asthmatic [α = 0.80], diabetic [α = 0.74], cardiac [α = 0.76], psychiatric [α = 0.74], and general medical [α = 0.86]; perceived concerns subscale: asthmatic [α = 0.75], diabetic [α = 0.80], cardiac [α = 0.76], psychiatric [α = 0.63], and general medical [α = 0.65]).9
Patients responded to each item on the BMQ using a 5-point (1 = strongly disagree to 5 = strongly agree) Likert scale. Possible scores for the perceived necessity and perceived concern subscales ranged from 5 to 25. Higher scores on respective subscales indicate greater perceived necessity of DMTs and greater perceived concern about DMTs.
Covariates
Demographic Characteristics. Demographic characteristics included age (year born), race (white, Hispanic or Latino, black or African American, American Indian or Alaska Native, Asian or Pacific Islander, multiple races, other), sex (female, male), education (some high school, high school degree or equivalent [eg, GED], some college but no degree, associate degree, bachelor degree, graduate or profession degree), and health insurance (private/commercial/prepaid health plans, Medicare, Medicaid, Tricare, Department of Veterans Affairs, other, no health insurance).
Clinical Characteristics. Clinical characteristics included time with MS (number of years diagnosed as having MS), type of DMT (injectable, oral), time on DMT (number of months on current DMT), DMT experience (DMT experienced, DMT naive), and frequency of DMT administration (once every 2 weeks, once a week, 3 times a week, every other day, every day, twice a day).
Survey Distribution
After identifying 600 eligible NARCOMS Registry members, a study packet was mailed to each potential participant in March 2016. The study packet included cover letter; a paper-and-pencil survey with a self-addressed, prepaid business reply envelope to return to The University of Texas at Austin researchers on completion; and a $10 Amazon gift card in appreciation for their participation. The surveys were anonymous, and the incentive gift card was sent with the invitation for the Registry members to keep regardless of whether they chose to participate. To maintain confidentiality, all the materials were sent to the Registry members by NARCOMS staff.
The cover letter highlighted the purpose of the study, the importance of patients' participation, the voluntary nature of the study, the anonymity of the study, the approximate time to complete the survey, and the researchers' contact information. The cover letter indicated that patients may contact research personnel if they have any questions about the study. The survey was available only in English.
Data Analysis
The data were analyzed using IBM SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). Descriptive statistics (eg, means, SDs, frequencies, and percentages) were calculated for all the study variables. Mean imputation was used to handle observations with missing data such that each missing value was imputed with the mean of the observed values for that variable. Results for all statistical tests were considered significant at P < .05.
Objective 1 was analyzed using descriptive statistics (means, SDs, and ranges). Internal consistency reliability as measured by the Cronbach α was assessed for all the multi-item scales (TSQM vII and BMQ subscales).
Objective 2 was analyzed using univariate and multivariate logistic regression analyses to determine the associations between treatment satisfaction (in terms of satisfaction with medication effectiveness, dissatisfaction with medication side effects, satisfaction with medication convenience, and global satisfaction), perceived necessity, and perceived concern and DMT adherence while controlling for demographic (ie, age, race, sex, marital status, education, health insurance) and clinical (ie, time with MS, type of DMT, time on DMT, DMT experience) covariates.
First, in the univariate logistic regression analyses, each primary independent variable was assessed individually as a predictor of DMT adherence. Second, all predictor variables were simultaneously entered into the logistic regression model. The dependent variable for this analysis was binomial: DMT adherence of 0.80 or greater (“1”) or DMT adherence less than 0.80 (“0”).
Results
Study Participants
A total of 505 surveys were returned (initial response rate: 84.2%). After preliminary analysis (ie, verifying eligibility and cleaning data), it was determined that 12 respondents were not currently taking an injectable or oral DMT and four respondents returned incomplete surveys. A total of 489 usable surveys (usable response rate: 81.5%) were included in the final analysis.
Table 1 provides an overview of participants' demographic and clinical characteristics. The mean ± SD age of participants was 60.5 ± 8.3 (median, 61) years. Most were white (93.8%), were female (86.6%), had an Associate's degree or higher (55.9%), and were married or partnered (63.8%). Approximately 60.0% of participants were disabled or retired, and 36.0% had private health insurance.
Demographic and clinical characteristics of respondents
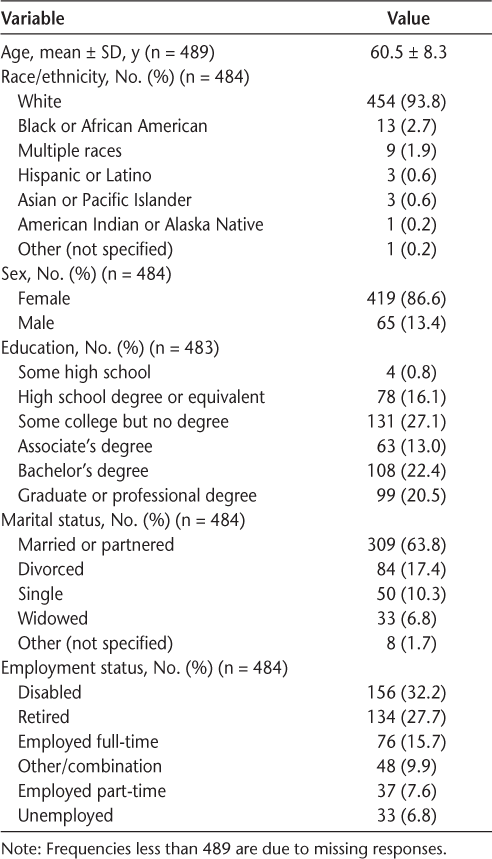
The mean ± SD time with MS was 21.1 ± 8.1 years, and the mean ± SD time on their current DMT was 8.7 ± 6.9 years. Most patients were currently taking an injectable DMT (66.9%) and were DMT experienced (previously took another DMT; 64.8%).
DMT Adherence
The mean ± SD adherence rate was 0.95 ± 0.14, with actual reported rates ranging from 0.00 (complete nonadherence) to 100.00 (complete adherence). When dichotomized, 92.8% were considered adherent (≥ 0.80).
Satisfaction with DMT
Overall, respondents reported high mean ± SD levels of satisfaction with DMT regarding effectiveness (75.4 ± 19.2; n = 489), convenience (81.0 ± 17.5; n = 489), and overall global satisfaction (81.2 ± 18.4; n = 489). For the side effects subscale, mean ± SD dissatisfaction levels (higher scores indicate lower levels of dissatisfaction) were generally low (88.3 ± 19.9). All the TSQM vII subscales had high internal consistency: effectiveness (α = 0.84), side effects (α = 0.90), convenience (α = 0.88), and global satisfaction (α = 0.91).
Beliefs About DMT
Overall, respondents reported moderate levels of perceived necessity (mean ± SD, 18.3 ± 3.8; n = 489) and lower levels of perceived concern (mean ± SD, 10.6 ± 3.7; n = 489). The BMQ subscales had high internal consistency: perceived necessity (α = 0.84) and perceived concern (α = 0.71).
Univariate Logistic Regression Analyses
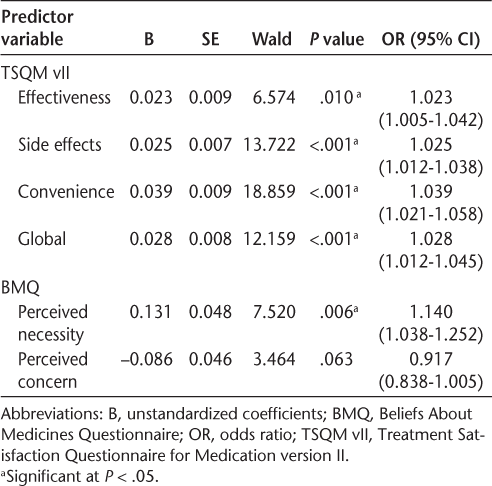
Table 2 provides an overview of the results of the univariate logistic regression analyses with each independent variable and DMT adherence. Individually, each of the satisfaction measures and perceived necessity were found to be significantly associated with adherence. Perceived concern was not significantly associated with adherence.
Univariate analyses between treatment satisfaction, medication beliefs, and disease-modifying therapy adherence
Multivariate Logistic Regression Analysis
Table 3 provides an overview of the results of the multivariate analysis. None of the treatment satisfaction measures or medication beliefs (perceived necessity and perceived concern) were found to be significantly associated with DMT adherence.
Multivariate analysis between treatment satisfaction, medication beliefs, and DMT adherence (n = 442)a
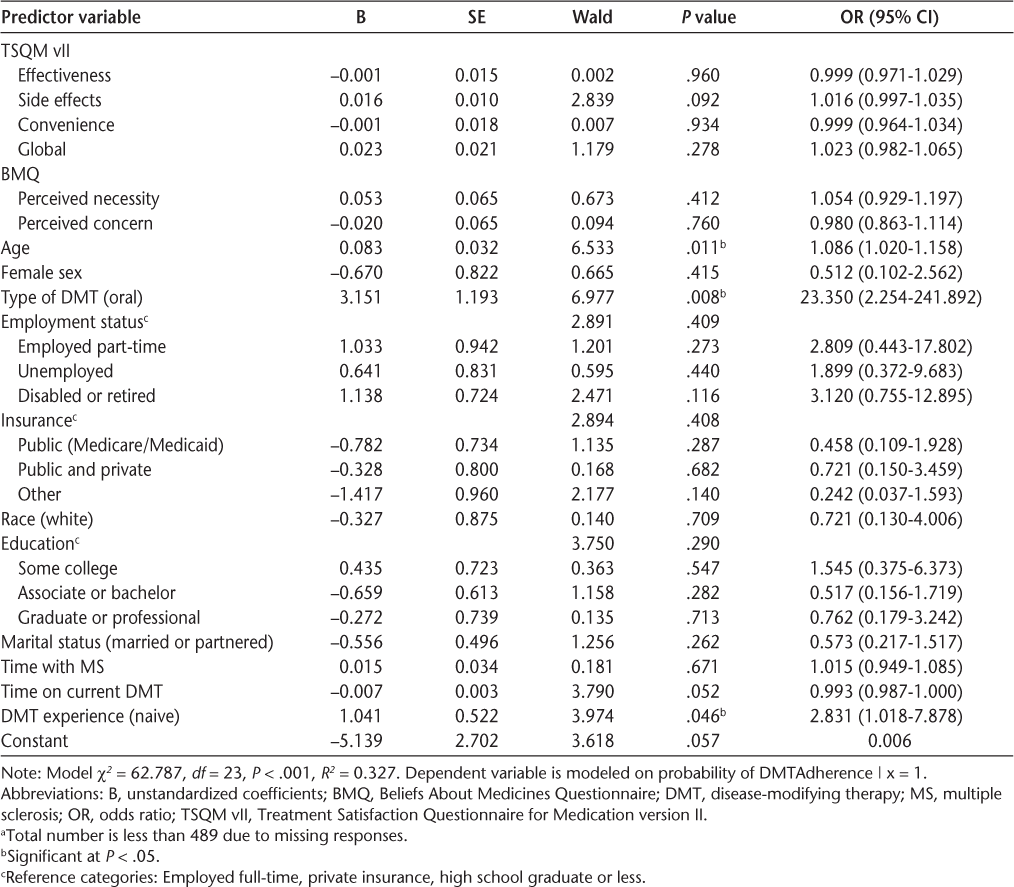
Among the covariates, age (odds ratio [OR], 1.086; 95% CI, 1.020–1.158; P = .011), type of DMT (oral vs. injectable [reference]: OR, 23.350; 95% CI, 2.254–241.892; P = .008), and DMT experience (naive vs. experienced [reference]: OR, 2.831; 95% CI, 1.018–7.878; P = .046) were found to be significant predictors of adherence.
Discussion
This study found DMT adherence rates to be higher than those previously reported in patients with MS. Across all participants, the mean DMT adherence rate was 0.95, and more than 90% of participants were categorized as adherent when the measure was dichotomized. In comparison, a systematic review by Menzin and colleagues1 found that DMT adherence rates ranged from 41% to 88%; however, none of the adherence rates reported in this review were for oral DMTs, which may be associated with greater adherence, as evidenced by findings from the present multivariate analyses.
Another explanation for the higher DMT adherence rates in the present study is that the sample—NARCOMS Registry members—may be more proactive and cognizant about their health care and medication-taking behavior compared with other, non-Registry patients with MS, given that they volunteer to complete biannual surveys that track disease activity and health outcomes over time. Another possible explanation for the high DMT adherence rate is that self-reported measures are often affected by response bias. Adams and colleagues17 found that in 87% of 37 comparisons between self-reported adherence rates and more objective rates, the median overestimation of adherence using self-reported rates was 27% (absolute difference). Finally, the mean age of participants in the present sample was high, and age has been found to be positively associated with adherence in patients with MS18–21; thus, this could have driven the higher adherence rates in the present study.
Participants reported high levels of treatment satisfaction across all TSQM vII measures (ie, satisfaction with effectiveness, convenience, and global satisfaction). Furthermore, scores on the TSQM vII side effects subscale were high, indicating low levels of dissatisfaction with their current DMT. These findings are consistent with those from a study by Hanson et al.22 that assessed DMT satisfaction using the TSQM vI. The scores were 70.1, 79.4, 71.7, and 68.9 for the effectiveness, side effects, convenience, and global satisfaction subscales, respectively. In terms of beliefs about DMT, respondents reported moderate levels of perceived necessity and lower levels of perceived concern. To our knowledge, this is the first study to use the BMQ in patients with MS; thus, there is no benchmark with which to compare these scores for patients with MS. More research is warranted to validate these findings.
In the multivariate logistic regression analysis, none of the primary independent variables (treatment satisfaction, perceived necessity, and perceived concern) were significant predictors of DMT adherence. This is likely due to the homogeneity of the sample in terms of demographic variables and DMT adherence. For example, although MS primarily affects people of white race and females, the present sample was over-represented with these individuals, accounting for 94% and 76% of the sample, respectively. Furthermore, more than 90% of the sample was considered adherent, which provides little variability to be explained by the predictor variables. Thus, it is possible that treatment satisfaction and medication beliefs may be important determinants of DMT adherence in other patients with MS.
To our knowledge, this is the first study to quantitatively assess the associations between perceived necessity and perceived concern and DMT adherence in patients with MS. Given that few patients were considered nonadherent in the present study and that other medication beliefs (eg, perceived severity,20 perceived benefit,20 perceived barriers,23 and perceived convenience23) have been found to be associated with DMT adherence, future research is warranted to confirm or refute these findings (ie, no significant relationships between perceived necessity, perceived concern, and DMT adherence) in other diverse MS populations with varied adherence levels.
In the present study, only covariates (age, DMT experience, and type of DMT) were significantly related to adherence in the multivariate model. Age was a significant and positive predictor of DMT adherence such that the odds of adherence increased with age. This finding is consistent with several other studies that report a positive association between age and DMT adherence in patients with MS18–21. Given this finding, health care providers should monitor DMT adherence in younger patients because they may be at greater risk for nonadherence.
Regarding DMT experience, the odds of DMT adherence were higher for patients who were treatment naive or patients who were taking their first DMT. One potential explanation is that treatment-naive patients were responding better (eg, in terms of effectiveness and satisfaction) to their current DMT than patients who have switched from another DMT; and thus, downstream DMT adherence was greater for treatment-naive patients. In a post hoc analysis (not shown in the Results section), mean time on the current DMT was significantly higher for treatment-naive patients (14.2 years) than for treatment-experienced patients (5.6 years), which may suggest that these patients were responding better to treatment. Accordingly, patients who have switched from other DMTs may need to be monitored for potential issues regarding DMT nonadherence.
The other significant predictor in this study was type of DMT, such that the odds of DMT adherence were higher for patients taking an oral DMT versus an injectable DMT. The FDA approved the first oral DMT for the treatment of patients with relapsing forms of MS in 2010. Since then, two additional oral DMTs have been approved. Given the relatively recent approvals, few studies have compared adherence between oral and injectable DMTs. Among these few studies, adherence rates to DMTs tended to be higher for the oral DMTs compared with the injectable DMTs,24,25 which is consistent with the present study findings. It is possible that the higher adherence rates for oral DMTs are due to the easier administration process associated with oral DMTs. Also, patients taking oral DMTs do not experience potential injection-specific issues such as injection anxiety or needle phobia and injection site pain or reactions, which have been found to be associated with nonadherence.21,26 As such, health care providers should monitor patients taking injectable DMTs for potentially modifiable issues that may lead to nonadherence.
Findings from this study should be considered in light of several limitations. First, this is a cross-sectional study; thus, relationships between variables are representative of only a single point in time. Past and future relationships between variables cannot be assumed. Second, due to the self-report nature of the study, social desirability and recall bias may confound results; however, previous studies with self-reported data from patients with MS have shown a high level of validity.23 Furthermore, DMT adherence was measured over a 2-week period rather than a 4-week or 1-month period to overcome potential issues of recall bias. Third, participants were sampled from an MS registry with patients who respond to biannual surveys about their MS and MS-related outcomes, and these patients may be more cognizant about and proactive in their health and medication-taking behavior. Also, the participants were quite homogenous in terms of demographic and clinical characteristics, including age, sex, and education; thus, the findings may not be generalizable to other patients with MS, particularly those outside of this registry. Also, this may have resulted in the low variability in DMT adherence, which partly explains the lack of statistical significance found between the predictor variables. Thus, future research is warranted to confirm or refute these findings in a more general MS population.
In summary, participants in the present study reported high treatment satisfaction (ie, effectiveness, side effects, convenience, and global satisfaction), positive medication beliefs (ie, high levels of perceived necessity and low levels of perceived concern), and high levels of DMT adherence. The treatment satisfaction measures and perceived necessity were associated with DMT adherence in the univariate logistic regression analyses. However, in the multivariate analysis, only age and type of DMT were found to be significant predictors. These findings suggest that younger patients and those taking injectable DMTs are at higher risk for nonadherence. As such, attention should be placed on these types of patients to facilitate optimal DMT adherence.
PRACTICE POINTS
Participants in this study were patients with MS from the North American Research Committee on Multiple Sclerosis Registry. Of the 489 patients included in the final analyses, 92.8% were considered disease-modifying therapy (DMT) adherent.
Treatment satisfaction (effectiveness, side effects, convenience, and global satisfaction) and medication beliefs (perceived necessity and perceived concern) were not significantly associated with DMT adherence in a multivariate model; still, these variables may be important determinants in other patients with MS who are having issues with DMT adherence, which has been evidenced in previous studies.
Health care providers should monitor younger patients, patients taking injectable DMTs, and patients who have taken another DMT(s) in the past because age, type of DMT, and DMT experience were found to be significant predictors of DMT adherence.
Acknowledgments
The authors acknowledge NARCOMS and particularly thank Tuula Tyry for identifying Registry participants and mailing the survey to them. NARCOMS is supported, in part, by the Consortium of Multiple Sclerosis Centers (CMSC) and the Foundation of the CMSC.
References
Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19:24S–40S.
Tan X, Patel I, Chang J. Review of the four item Morisky Medication Adherence Scale (MMAS-4) and eight item Morisky Medication Adherence Scale (MMAS-8). Inov Pharm. 2014;5:165.
Ivanova JI, Bergman RE, Birnbaum HG, et al. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ. 2012;15:601–609.
Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77.
Sabate E. Adherence to Long-term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
Gittelsohn J, Lee K. Integrating educational, environmental, and behavioral economic strategies may improve the effectiveness of obesity interventions. Appl Econ Perspect Pol. 2013;35:52–68.
Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8:9S–24S.
Barbosa CD, Balp MM, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48.
Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567.
Berglund E, Lytsy P, Westerling R. Adherence to and beliefs in lipid-lowering medical treatments: a structural equation modeling approach including the necessity-concern framework. Patient Educ Couns. 2013;92:105–112.
Porteous T, Francis J, Bond C, Hannaford P. Temporal stability of beliefs about medicines: implications for optimising adherence. Patient Educ Couns. 2010;79:225–230.
Sharaf F. Impact of health education on compliance among patients of chronic diseases in Al Qassim. Int J Health Sci. 2010;4:139–148.
Horne R, Chapman SCE, Parham R, et al. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8:e80633.
Siegel SD, Turner AP, Haselkorn JK. Adherence to disease-modifying therapies in multiple sclerosis: does caregiver social support matter? Rehabil Psychol. 2009;53:73–79.
Boeru G, Milanov I, De Robertis F, et al. ExtaviJect® 30G device for subcutaneous self-injection of interferon beta-1b for multiple sclerosis: a prospective European study. Med Devices (Auckl). 2013;6:175–184.
Glanz BI, Musallam A, Rintell DJ, et al. Treatment satisfaction in multiple sclerosis. Int J MS Care. 2014;16:68–75.
Adams AS, Soumerai SB, Lomas J, et al. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999;11:187–192.
Halpern R, Agarwal S, Dembek C, et al. Comparison of adherence and persistence among multiple sclerosis patients treated with disease modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence. 2011;5:73–84.
Halpern R, Agarwal S, Borton L, et al. Adherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysis. Adv Ther. 2011;28:761–775.
Turner AP, Kivlahan DR, Sloan AP, et al. Predicting ongoing adherence to disease modifying therapies in multiple sclerosis: utility of the health beliefs model. Mult Scler. 2007;13:1146–1152.
Turner AP, Williams RM, Sloan AP, et al. Injection anxiety remains a long-term barrier to medication adherence in multiple sclerosis. Rehabil Psychol. 2009;54:116–121.
Hanson KA, Agashivala N, Stringer SM, et al. A cross-sectional survey of patient satisfaction and subjective experiences of treatment with fingolimod. Patient Prefer Adherence. 2013;7:309–318.
Wicks P, Massagli M, Kulkarni A, Dastani H. Use of an online community to develop patient-reported outcome instruments: the Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). J Med Internet Res. 2011;13:e12.
Agashivala N, Wu N, Abouzaid S. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
Bergvall N, Petrilla A, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims data-base analysis. J Med Econ. 2014;17:696–707.
Fernandez O, Aguera J, Izquierdo G, et al. Adherence to interferon β-1b treatment in patients with multiple sclerosis in Spain. PLoS One. 2012;7:e35600.







