Publication
Research Article
International Journal of MS Care
A Survey of Cannabis Use in a Large US-Based Cohort of People with Multiple Sclerosis
Abstract
Background:
As cannabis products become increasingly accessible across the United States, it is important to understand the contemporary use of cannabis for managing multiple sclerosis (MS) symptoms.
Methods:
We invited participants with MS from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry (aged 18 years or older) to complete a supplemental survey on cannabis use between March and April 2020. Participants reported cannabis use, treated symptoms, patterns, preferences, methods of use, and the factors limiting use. Findings are reported using descriptive statistics.
Results:
Of the 6934 participants invited, 3249 responded. Of the respondents, 31% reported having ever used cannabis to treat MS symptoms, with 20% currently using cannabis. The remaining 69% had never used cannabis for MS symptoms, for reasons including not enough data about efficacy (40%) and safety (27%), and concerns about legality (25%) and cost (18%). The most common symptoms current users were attempting to treat were spasticity (80%), pain (69%), and sleep problems (61%). Ever users (vs never users) were more likely to be younger, be non-White, have lower education, reside in the Northeast and West, be unemployed, be younger at symptom onset, be currently smoking, and have higher levels of disability and MS-related symptoms (all P < .001).
Conclusions:
Despite concerns about insufficient safety and efficacy data, legality, and cost, almost one-third of NARCOMS Registry respondents report having tried nonprescription cannabis products in an attempt to alleviate their symptoms. Given the lack of efficacy and safety data on such products, future research in this area is warranted.
In 2010, more than 700,000 adults with multiple sclerosis (MS) were estimated to reside in the United States.1 Approximately 95% of people with MS experience multiple symptoms, such as dysarthria, pain, fatigue, sleep disturbances, depression and anxiety, spasticity, mobility impairment, sexual dysfunction, and bladder and bowel dysfunction.2 These symptoms adversely affect health-related quality of life.2
Of people with MS, more than 80% experience spasticity,3–5 which is generally treated with oral antispasticity medications, rehabilitation, and botulinum toxin; intrathecal baclofen is typically a second-line therapy.6–8 However, medications are often incompletely effective and can be associated with dose-limiting adverse effects.6,7 Also, although there is a broad range of rehabilitative treatments available to treat people with MS, there is a critical lack of high-quality evidence showing the effectiveness of various modalities, and more research is needed.8 A survey of those with MS-related spasticity found that almost 50% were unsatisfied with their current treatment.5
Interest in the use of cannabis for managing MS symptoms continues to increase as more data (both anecdotal and from clinical studies) become available and access becomes easier from a legal perspective. Although there are currently no US Food and Drug Administration (FDA)–approved cannabis products for the treatment of symptoms associated with MS, recent surveys conducted in Oregon and Colorado indicate that up to 74% of people with MS have considered using cannabis products, and 25% to 54% have tried cannabis products for their symptoms, particularly spasticity, sleep disturbances, and pain.9,10 A recent nationwide survey to characterize pain in people with MS that also included questions about cannabis use reported similar interest and use, with a focus on the treatment of pain and sleep disturbances but not on spasticity.11
Given the rapidly evolving landscape of cannabis products and regulations, an assessment of cannabis use by people with MS in a large, nationwide sample not limited to those selected on the basis of a specific symptom is warranted. We conducted a supplemental survey on cannabis use among North American Research Committee on Multiple Sclerosis (NARCOMS) Registry participants to better understand the factors associated with contemporary use of cannabis products for MS symptoms, with a focus on common MS symptoms, including spasticity. Specifically, we evaluated the prevalence of cannabis use in people with MS; demographic factors associated with its use; treated symptoms; patterns, preferences, and methods of use; and factors limiting its use.
Methods
Study and Survey Design
The NARCOMS Registry is a voluntary self-report registry for adults (18 years or older) with MS (https://www.narcoms.org/).12 After enrollment, participants are asked to update their information every 6 months either online or by paper questionnaire, according to their preference. In addition to the semiannual updates, NARCOMS Registry participants are occasionally invited to participate in supplemental surveys.
Those who were active US-based NARCOMS Registry participants as of February 28, 2020, were invited to participate in this online supplemental survey (N = 6934); no other eligibility criteria were applied. The survey was conducted using the REDCap system hosted at Washington University in St Louis.13 Participants had the option to link their previous NARCOMS Registry semiannual update survey data (which included demographic and clinical characteristics) to this supplemental cannabis survey to reduce survey burden. Those who wanted to remain anonymous and chose not to link their surveys were presented with questions to ascertain demographic and clinical information.
Ethical Considerations
The cannabis survey was approved by the Washington University institutional review board. By responding to the cannabis survey, participants were giving their informed consent to participate in the research. Because the use of cannabis is not legal throughout the United States, NARCOMS obtained a certificate of confidentiality to protect the privacy of research participants by prohibiting disclosure of identifiable, sensitive research information to anyone not connected to the research, except when the participant consented, or in limited other specific situations.
Demographic and Clinical Data Collected
For participants who gave permission to link their semiannual update information with the cannabis survey, their semiannual update responses from 2019 were used to ascertain annual household income, employment status, alcohol use, smoking status, state of residence, disability status, MS clinical course (ie, MS history), and disease-modifying therapy use (yes/no). Annual household income was reported as less than $15,000, $15,000 to $30,000, $30,001 to $50,000, $50,001 to $100,000, more than $100,000, or “I do not wish to answer.” Current employment status was categorized as employed full time, employed part time, or not employed. Alcohol use was categorized as never, monthly or less, 2 to 4 times per month, 2 to 3 times per week, or 4 or more times per week. Smoking status was categorized as not at all, some days, or every day. State of residence was categorized into census regions (Northeast, South, Midwest, and West). Clinical course was categorized as clinically isolated syndrome, relapsing-remitting, secondary progressive, primary progressive, don’t know or unsure, MS diagnosis not confirmed by a physician, or other.
The NARCOMS Registry enrollment questionnaire provided information regarding birth date, gender, race, educational level, and age at MS symptom onset and diagnosis. Race was categorized as White, African American, and various other choices including other. The highest levels of education reached were categorized as less than bachelor’s degree (less than high school, high school/GED, associate’s degree, and technical degree) and postsecondary (bachelor’s degree, postgraduate education).
Participants reported disability status using the Patient-Determined Disease Steps scale, a single-item measure with response options ranging from 0 (normal) to 8 (bedridden), which correlates well with a physician-scored Expanded Disability Status Scale score. Patient-Determined Disease Steps scale scores were categorized as mild (0–1), moderate (2–4), or severe (5–8).3
A numerical rating scale of 0 to 10 was used to rate spasticity, pain, and sleep in the past week, with 0 being no spasticity/pain/sleep problems and 10 being worst possible spasticity/pain/sleep problems. Numerical rating scale scores were categorized as normal (0), mild (1–3), moderate (4–6), or severe (7–10).
Cannabis Use Data Collected
Participants were instructed that for the purposes of this survey, cannabis/marijuana referred to Δ9-tetrahydrocannabinol (THC)–containing products derived from the cannabis/marijuana plant and did not include products marketed as only cannabidiol (CBD) or hemp CBD. This is henceforth referred to as simply cannabis. Where possible, questions regarding cannabis were sourced from national surveys to ensure that the questions were validated (see Appendix S1, which is published in the online version of this article at ijmsc.org).
Participants were asked whether they had ever used cannabis to treat their MS symptoms (ever users). If so, it was determined whether they were current users (use within 30 days of taking the survey) or past users (previous use had been >30 days before taking the survey). Current users were asked about their cannabis use during the 30 days preceding the survey, including frequency, timing, and administration route preference. Those who had never used cannabis to treat their MS symptoms were classified as never users.
Participants were asked about MS symptoms that they used cannabis to treat and when they had started using cannabis for their MS symptoms in relation to rehabilitation therapy. Symptom options included muscle spasms, cramps, or spasticity; tremors; overactive bladder or bladder symptoms; pain; migraine or headaches; anxiety; depression; insomnia or sleep problems; nausea or gastrointestinal problems; and other. We considered respondents who chose the muscle spasms, cramps, or spasticity option to have spasticity in our analysis.
Spasticity was further assessed by asking which aspects of spasticity cannabis was used to treat, with options including muscle stiffness, muscle spasms or cramps, pain associated with spasticity, problems sleeping associated with spasticity, or walking problems associated with spasticity. In addition, participants were asked about other concomitant spasticity treatments they were using.
Past users and current users were also asked why they used less cannabis for their MS than they might otherwise. Never users were asked why they did not use cannabis for MS.
Statistical Analysis
Descriptive statistics were used to summarize responses, with mean ± SD or median (25th, 75th percentiles) for continuous variables and frequency (percentage) for categorical variables. Differences between groups were determined by t test or Wilcoxon test for continuous variables and by χ2 or Fisher exact test, as appropriate, for categorical variables.
Results
Participants
Of the 6934 invited NARCOMS Registry participants, 3249 (46.9%) responded to the survey, of whom 3240 responded to the item that asked about cannabis use for MS. Only 225 respondents (7%) opted not to link the cannabis survey with their semiannual update survey.
Respondents were distributed over the four census regions of the United States (Table S1). Most respondents were female and White. Approximately two-thirds had a bachelor’s degree or higher and were not working. The mean ± SD respondent age at survey completion was 61.3 ± 10.0 years. Respondents had a mean ± SD age at symptom onset of 31.2 ± 10.3 years and a median (25th, 75th percentile) disability level of 3 (gait disability) (1 [mild disability], 6 [bilateral support]). More than half of the respondents had a relapsing-remitting MS clinical course, and approximately one-quarter had secondary progressive MS. Almost two-thirds of the respondents were taking a disease-modifying therapy.
Compared with nonrespondents, respondents were slightly older (61 vs 60 years, P < .001), were more likely to be White (89% vs 85%, P < .001) and to have a bachelor’s degree or higher (61% vs 57%, P = .003), and reported drinking alcohol slightly more frequently (P = .043). None of these differences were clinically meaningful.
Cannabis Use and Nonuse
Of the 3240 respondents, 1012 (31%) reported ever using cannabis to treat MS symptoms (ever users). Overall, 636 respondents (20%) reported current use (current users [within 30 days of taking the survey]), 376 (12%) reported using it in the past (past users), and 2228 (69%) reported having never used it (never users).
Of the 1012 ever users, 636 (63%) were currently using cannabis for their MS and 376 (37%) were past users. Compared with never users, ever users were more likely to be younger, be non-White, have a lower educational status, reside in the Northeast and West, and be not working (all P < .001) (Table S1). In addition, ever users were more likely to report younger age at MS symptom onset, current smoking, higher disability level, and higher levels of spasticity, pain, and sleep problems (all P < .001). Compared with past users, current users were more likely to be male (P = .001) and slightly younger (P = .009). No other statistically significant differences were identified.
Patterns, Preferences, and Methods of Use for Current Users
Of the 604 current users who responded to the cannabis use question, 244 (40%) reported cannabis use on all of the past 30 days and 120 (20%) reported use on 20 to 29 of the past 30 days. On average, current users reported cannabis use on a mean ± SD of 19.6 ± 11.2 days of the past 30 days. Most current users (80%) used cannabis in the evenings, with additional reported use in the morning (26%), in the afternoon (28%), or overnight (25%). The primary mode of use was smoking it (33%) or eating it (20%) (Figure 1).
Primary mode of cannabis use
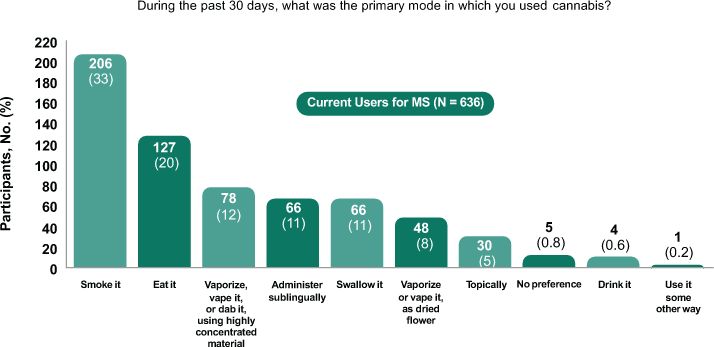
Reasons for Not Using Cannabis or Using Less Than Otherwise Might Use
Among the 2228 never users, top reasons for not using cannabis included not enough data about how well it works (n = 885), not enough data about safety (n = 607), concerns about legality (n = 549), lack of insurance coverage for cannabis/marijuana (n = 499), and concerns about cost (n = 402).
Overall, 325 (51%) of the 636 current users reported using less cannabis than they otherwise might. Among current users, the top three reasons for using less cannabis than otherwise might were cost (n = 177), lack of insurance coverage for cannabis (n = 154), and concerns about legality (n = 79). Overall, 333 of 376 past users (89%) reported using less cannabis than they otherwise might. For past users, the top three reasons were cost (n = 110), lack of insurance coverage for cannabis (n = 98), feeling high/not liking the way it feels (n = 79).
Symptoms Current Users Are Intending to Treat with Cannabis
Current users reported using cannabis in an attempt to treat a variety of MS symptoms (Figure 2), the most common of which were muscle spasms/cramps/spasticity (80%), pain (69%), and insomnia/sleep problems (61%). Most often, current users were using cannabis to treat a combination of spasticity, pain, and sleep problems (Figure S1). Compared with never users, ever users tended to have more severe spasticity, pain, and sleep problems, as reflected by their median numerical rating scale scores: spasticity (5.0 vs 3.0), pain (5.0 vs 2.5), and sleep problems (5.0 vs 3.0). Similar proportions of current users had mild, moderate, and severe spasticity, pain, and sleep problems (Table S1).
In current users, proportion of people with multiple sclerosis (MS) attempting to treat MS symptoms with cannabis
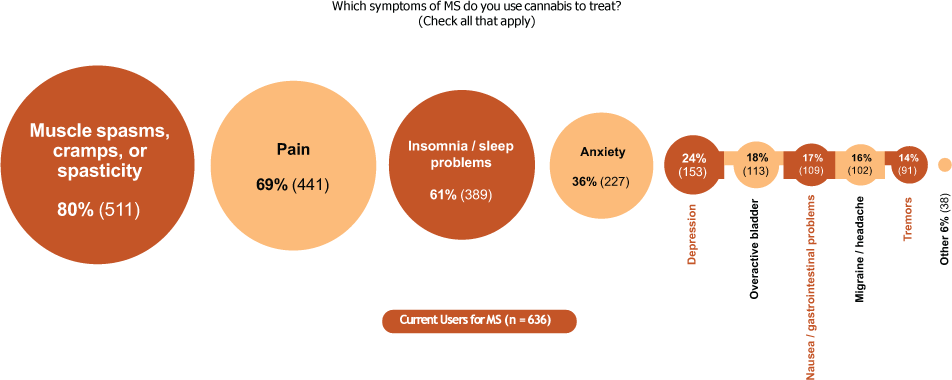
Among people with MS who were currently trying to treat spasticity with cannabis (n = 511), the most common aspects intended to address were muscle spasms or cramps (61%), muscle stiffness (53%), problems sleeping at night associated with spasticity (52%), and pain associated with spasticity (51%). Less frequently, cannabis use was intended to treat walking problems associated with spasticity (26%).
Concomitant Therapies and Cannabis Use in the Treatment Algorithm
Most current users started using cannabis after trying one or more MS symptom management drugs (77%) and after trying rehabilitation therapy (55%).
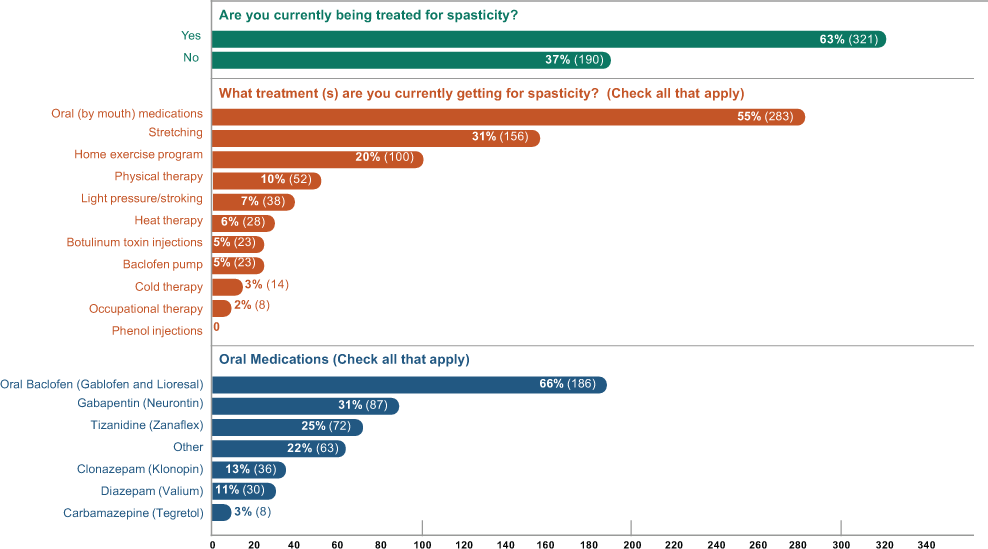
Of the 511 current users treating their spasticity with cannabis, 37% reported no other concomitant spasticity treatments; the remaining 63% reported using concomitant therapies, with the most common being prescription oral antispasticity medications (55%), stretching (31%), a home exercise program (20%), and physical therapy (10%) (Figure 3).
Spasticity treatment modalities in current cannabis users who specifically treat their spasticity with cannabis
Discussion
In this large population of people with MS, almost one-third of participants have tried cannabis products for their MS symptoms and one-fifth were currently using cannabis for their symptoms, most commonly spasticity, pain, and sleep problems.
Compared with past users, current users were more likely to be male and younger. Compared with never users, ever users were more likely to be younger and non-White, have a lower educational status, reside in the Northeast and West, be unemployed, be younger at MS symptom onset, have a higher disability level, and have higher levels of spasticity, pain, and sleep problems. The primary modes of cannabis use were smoking and eating. The most common reasons for having never used cannabis products—not enough data on their efficacy or safety and concerns about legality—highlight the need for reliable clinical efficacy and safety data to guide therapeutic decisions. Although 18% of never users also reported concerns about the cost of cannabis to treat their MS, there were no significant differences in total household incomes between ever users and never users.
Authors of a previous anonymized NARCOMS Registry survey conducted in 2014 reported that almost half of the 5481 respondents had considered using cannabis for their MS symptoms, but only 26% had used it for their MS symptoms, with only 16% using cannabis at the time of the survey.14 Thus, ever use and current use of cannabis were higher in the present survey, which may reflect increasing acceptance of cannabis use and/or fewer legal barriers to use. However, that survey was anonymous, so we are unable to understand changes in individual participants over time. In the previous survey, male gender, higher disability level, and younger age were associated with a higher likelihood of current use, which is in line with the findings of the present survey. The most common symptoms that participants used cannabis to attempt to treat in the previous survey were spasticity, pain, and bladder symptoms.14 The present survey results were largely in line with this observation; however, problems with sleep rather than bladder symptoms were a top symptom cited in the current survey.
In general, there was some variability in cannabis use in recent surveys of people with MS, with the proportion of participants who had ever used cannabis ranging from 49% to 54%, current use from 30% to 50%, use within the past year from 21% to 42%, and daily use from 19% to 58%.9–11,15–17 The variability in results in other recent surveys may be partly explained by geographic location and differences in participant characteristics. In contrast with other recent surveys, the proportions of current users (20%) and ever users (31%) seemed to be lower in this survey. One explanation might be that because randomized controlled study data on cannabinoid treatment for MS symptoms are available only for THC-containing cannabis products, this survey was limited to THC-containing cannabis (ie, excluded CBD-only cannabis use). The prevalence of cannabis use might have been higher if CBD-only products were included. This survey also specified using cannabis to treat MS symptoms. In contrast, the other surveys reported values for participants who had previously used cannabis for any reason.9,16,17 In addition, different patient populations (age, gender, disability level, duration of disease) may have contributed different estimates of ever and current use.
Among current users, daily use was within the range reported in the literature, with 40% of participants reporting cannabis use on all of the past 30 days. Interestingly, in a 2016 to 2017 US survey that included 169,036 adults who reported either no medical condition (46%) or any medical condition (54%) (eg, cardiovascular, pulmonary, metabolic, inflammatory, or renal disorder; cancer; or mental illness), 11.2% of those with a medical condition used cannabis daily to treatment their symptoms.18 This finding suggests that the prevalence of daily cannabis use to treat symptoms of MS is higher (at 19%–58%) than for other medical conditions in general.9–11,15–18
In other recent surveys of cannabis use in people with MS (2019–2020), the most common symptoms that cannabis was used to treat were also pain, spasticity, and sleep problems,9–11,16,17 with mood, depression, anxiety, and stress also cited.9,16,17 The same is true for a 2016 survey of cannabis use among Canadians with MS, with the addition of anxiety as a common symptom.19
The most common symptoms reported in the present survey are generally in line with these previous reports, with a slightly higher proportion of respondents reporting spasticity in this survey. Definitions of spasticity have varied in previous surveys, with some mentioning only spasticity and others mentioning other descriptors such as muscle tightness, muscle stiffness, or spasms. In the present survey, when responding to the item regarding which symptoms of MS participants were using cannabis to treat, the definition of spasticity was more descriptive than in others, including muscle spasms, cramps, or spasticity. This may have led to a higher proportion of respondents indicating that they were using cannabis to treat spasticity. Also, the severity of spasticity, which was not necessarily assessed in previous surveys, may have been different in the present survey than in others.
The results of this survey and previous surveys indicate that despite there being no FDA-approved products for physicians to prescribe, cannabis use for MS symptoms is quite common. People with MS are using a variety of cannabis products, with differing cannabinoid ratios and contents and differing modes of use (smoking, eating, etc), presumably leading to differing safety and efficacy effects. Although some people with MS are choosing to self-medicate with the various cannabis products available to them, many cite issues limiting their use, most commonly a lack of data on efficacy or safety, or concerns about legality. Inaccurate labeling or presence of contaminants in non–FDA-regulated products has also been reported.20–22
Limitations
The NARCOMS Registry is voluntary and may not represent the general population; however, it is comparable with other populations, with an age distribution close to the peak age in the prevalence reported by Wallin et al.1 Despite efforts to ensure confidentiality, response bias may have affected some responses. In addition, because this was a single survey, it represents a single snapshot in time, with patterns of cannabis use likely to be fluid. Because this survey excluded participants using CBD-only products, these results relate only to people with MS using THC-containing cannabis products. The analyses were also limited by incomplete responses for some elements. Participants were not explicitly asked about past recreational use of cannabis. This may have influenced how open they were to trying cannabis to treat MS symptoms. Participants were not expressly asked whether the symptom being treated was diagnosed by their treating physician. Because spasticity in particular has the potential to be misidentified by patients, this limits our understanding of what participants might have been treating. However, previous work in the NARCOMS Registry population has shown that individuals who self-report spasticity also report treatment with commonly used therapies for spasticity, such as stretching, physical therapy, and oral medications and that more severe spasticity is associated with more severe impairments in mobility, bladder function, and fatigue as expected.5 Participants were not asked why they stopped or changed previous interventions for their symptoms; so, it is not known whether this was due to lack of effectiveness or adverse effects. Understanding those issues might further inform our understanding of the limitations of current therapeutic options for symptom management in MS and the potential utility of cannabis as an alternative option. Finally, women were somewhat overrepresented, even after accounting for the greater prevalence of MS in women than in men.1
Conclusions
Taken together, the data collected in this NARCOMS Registry survey highlight the prevalent use of cannabis among people with MS and identify patient-centric concerns associated with cannabis options that currently exist in the United States. Given the gaps in efficacy and safety data, insurance coverage, and legality, the survey results suggest an enduring unmet need for appropriately researched and regulated cannabinoid treatments for MS symptoms such as spasticity, pain, and sleep problems.
PRACTICE POINTS
Approximately one-third of adults with MS from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry used cannabis to treat MS symptoms at some point, and 20% were currently using it. A total of 636 current users most commonly reported using cannabis to treat MS-related spasticity symptoms (80%), pain (69%), or sleep problems (61%).
Respondents with MS used cannabis for spasticity to treat muscle spasms/cramps (61%), muscle stiffness (53%), problems sleeping at night associated with spasticity (52%), and pain associated with spasticity (51%); 37% reported using no concomitant spasticity treatments.
Issues limiting cannabis use among NARCOMS Registry respondents included insufficient safety and efficacy data, legality, cost, and feeling high/not liking the way it feels.
Acknowledgments
The authors thank Dr Lesley Taylor of Alchemy Medical Writing Ltd for medical writing and editorial support, which was funded by Greenwich Biosciences Inc. The NARCOMS Registry is a project of the Consortium of Multiple Sclerosis Centers (CMSC) and the Foundation of the CMSC.
References
Wallin MT, Culpepper WJ, Campbell JD, The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019; 92: e1029– e1040.
Henze T, Flachenecker P, Zettl UK. Importance and treatment of spasticity in multiple sclerosis: results of the MOVE 1 study. Nervenarzt. 2013; 84: 214– 222.
Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004; 10: 589– 595.
Milinis K, Tennant A, Young CA; TONiC Study Group. Spasticity in multiple sclerosis: associations with impairments and overall quality of life. Mult Scler Relat Disord. 2016; 5: 34– 39.
Bethoux F, Marrie RA. A cross-sectional study of the impact of spasticity on daily activities in multiple sclerosis. Patient. 2016; 9: 537– 546.
Tintoré M. Advances in the management of multiple sclerosis symptoms: pathophysiology and assessment of spasticity in multiple sclerosis. Neurodegener Dis Manag. 2015; 5( suppl): 15– 17.
Urits I, Adamian L, Fiocchi J, Advances in the understanding and management of chronic pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep. 2019; 23: 59.
Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019; 1: CD012732.
Rice J, Hugos C, Hildebrand A, Cameron M. Cannabis use in people with multiple sclerosis and spasticity: a cross-sectional analysis. Mult Scler Relat Disord. 2020; 41: 102009.
Weinkle L, Domen CH, Shelton I, Sillau S, Nair K, Alvarez E. Exploring cannabis use by patients with multiple sclerosis in a state where cannabis is legal. Mult Scler Relat Disord. 2019; 27: 383– 390.
Braley TJ, Whibley D, Alschuler KN, Cannabinoid use among Americans with MS: current trends and gaps in knowledge. Mult Scler J Exp Transl Clin. 2020; 6: 2055217320959816.
Vollmer TL, Ni W, Stanton S, The NARCOMS patient registry: a resource for investigators. Int J MS Care. 1999; 1: 28– 34.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377– 381.
Cofield SS, Salter A, Tyry T, Perspectives on marijuana use and effectiveness. Neurol Clin Pract. 2017; 7: 333– 343.
Hildebrand A, Minnier J, Cameron MH. Cannabis use for symptom relief in multiple sclerosis: a cross-sectional survey of webinar attendees in the US and Canada. Mult Scler Relat Disord. 2020; 38: 101516.
Schabas AJ, Vukojevic V, Taylor C, Cannabis-based product use in a multiple sclerosis cohort. Mult Scler J Exp Transl Clin. 2019; 5: 2055217319869360.
Gustavsen S, Søndergaard HB, Andresen SR, Illegal cannabis use is common among Danes with multiple sclerosis. Mult Scler Relat Disord. 2019; 33: 5– 12.
Dai H, Richter KP. A national survey of marijuana use among US adults with medical conditions, 2016–2017. JAMA Netw Open. 2019; 2: e1911936.
Banwell E, Pavisian B, Lee L, Feinstein A. Attitudes to cannabis and patterns of use among Canadians with multiple sclerosis. Mult Scler Relat Disord. 2016; 10: 123– 126.
Oldfield K, Ryan J, Doopen M, Kung S, Braithwaite I, Newton-Howes G. A systematic review of the label accuracy of cannabinoid-based products in regulated markets: is what’s on the label what’s in the product? Australas Psychiatry . 2021; 29: 88– 96.
Bon-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017; 318: 1708– 1709.
Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015; 313: 2491– 2493.







