Publication
Research Article
International Journal of MS Care
The Effect of Biofeedback as a Psychological Intervention in Multiple Sclerosis: A Randomized Controlled Study
Author(s):
Background: Relaxation, mindfulness, social support, and education (RMSSE) have been shown to improve emotional symptoms, coping, and fatigue in multiple sclerosis (MS). Biofeedback was trialed as a psychological intervention, designed to improve self-control, in two groups of patients with MS. Both groups received RMSSE, and one group additionally received biofeedback.
Methods: Forty people with relapsing-remitting MS were recruited from three sites in Sydney, Australia. The mean disability score (Expanded Disability Status Scale; EDSS) was 2.41 ± 1.46 (95% confidence interval [CI], 1.46–3.36); the mean age in years was 45.9 ± 12.42 (95% CI, 41.92–49.87). Participants were randomly assigned to two active treatment groups (n = 20 per group). All participants received one 1-hour session per week for 3 weeks of RMSSE, while biofeedback equipment measured breathing rate and muscle tension. Members of one group used biofeedback screens to regulate physiological response.
Results: Whole-group pre- and post-treatment scores demonstrated a reduction of 38% for anxiety and 39% for depression scores (P = .007 and P = .009, respectively). A post-treatment comparison failed to demonstrate any significant difference between the two active treatment groups in anxiety and depression scores. The biofeedback group showed significant pre- to post-treatment improvement or trends toward improvement in anxiety, fatigue, and stress (P = .05, .02, and .03, respectively). Comparison of pre-post treatment results between groups showed improvements for the biofeedback group in breathing rate and muscle tension (P = .06 and .09).
Conclusions: For relapsing-remitting MS patients receiving biofeedback in addition to RMSSE, there was a demonstrable reduction in anxiety, fatigue, and stress. There was also a trend toward significant reduction of breathing rate and muscle tension in favor of biofeedback.
A strong relationship has been observed between depression and fatigue in people with multiple sclerosis (MS) independent of age, length of illness, and Expanded Disability Status Scale (EDSS) scores.1 Control of stress has recently been implicated in reducing MS exacerbations.2 3 No standardized guidelines exist for effective management of the high levels of fatigue, depression, or stress found in association with MS, despite wide reporting of these symptoms.3–7
Recent controlled studies within MS populations have addressed psychosocial aspects of health management, including evaluation of the effectiveness of group treatment with cognitive-behavioral therapy (CBT)8; bibliotherapy, psychotherapy, and social discussion5 7; mindfulness meditation2; and individual stress-reduction therapy.3 Each study found improvements across outcome measures such as disease progression, fatigue, quality of life, and depression. In contrast, mood in patients not offered any psychosocial intervention may worsen over time.7
Beliefs about control or self-regulation have been found to mediate fatigue in MS,9 and negative bias in memory has been shown to predict depression.10 Fatigue and depression, in turn, may influence the perception by the individual of life stress and the use of effective coping strategies.2 7 While the adoption of positive, problem-solving coping strategies predicts long-term positive affect and well-being in MS,11–13 fatigue, anxiety, and stress are associated with depression and reduced use of task-focused coping skills.14 The literature reviewed for this article found that interventions addressing mental health in MS were typically high-cost in terms of staffing or required multiple visits by the patient.2 3 5 7 While offering hope that nonpharmacologic psychological or psychosocial interventions can improve health outcomes in MS, their effect may be due to the patient's placing an emphasis or developing dependence on the treatment (or therapist or group) rather than developing self-regulation or an internal locus of control.
In order to maintain the gains in mood and coping observed in prior psychosocial intervention, a gain in self-regulation may be required. Interventions that improve perceived self-control and coping strategies in MS are needed.15–18 Biofeedback, an intervention that promotes better self-regulation, has been found to improve coping strategies in headache and chronic pain19 20 and foster positive beliefs about self-control.
It was hypothesized that biofeedback would be incrementally more effective than relaxation, mindfulness, social support, and education (RMSSE) in improving self-regulation, measured by breathing rate and muscle tension. Improved self-regulation may increase internal control beliefs through the use of biofeedback, causing reductions in self-reported anxiety, stress, fatigue, and depression and changes in self-reported coping style. This article reports on a randomized controlled trial of biofeedback in a cohort of patients with clinically definite relapsing-remitting MS (RRMS).
Methods
Participants
Ethics approval was granted by the Human Research and Ethics Committees of South Western Sydney Local Health District and Charles Sturt University, Bathurst, NSW, Australia. Patient contact was approved by each patient's treating neurologist. Recruitment was by telephone interview and/or letter invitation. Forty people with RRMS were recruited across three sites in Sydney, Australia (Figure 1). There were no financial incentives, nor was there any cost to participate in the study. Participants were asked to remain on their usual medication(s) with biofeedback added for the 3-week study.
CONSORT 2010 Flow Diagram
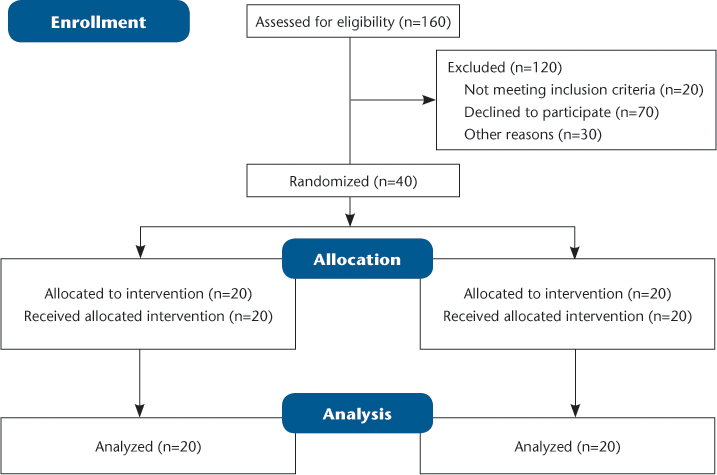
Participant numbers were estimated based on a large North American MS fatigue study (N = 6691) using the Fatigue Severity Scale.21 In that study, mean fatigue was 5.7 (SD 1.6). Power estimations of 80% were sought in order to determine whether the present intervention would reduce fatigue from severe (≥4) to mild (<4). A difference of at least one standard deviation was required. In a two-condition study, 5 participants per group gave 80% power (alpha level of .05); thus, with 20 participants in each group, greater than 99% power should be obtained.
Participants attended three 1-hour sessions at weekly intervals. At the first appointment, all participants signed consent forms; completed questionnaires including the Coping Inventory in Stressful Situations (CISS),22 Fatigue Severity Scale (FSS),23 and Depression Anxiety and Stress Scale (DASS)24; and were given information about the “flight/fight” response (Supplementary Appendix 1) and the procedure adopted for biofeedback25 (Supplementary Appendix 2). The session was continued with mindful breathing exercises and progressive muscle relaxation (PMR) demonstrated by the researcher (Supplementary Appendix 3). A full summary of session outlines appears in Supplementary Appendix 4.
Random allocation to groups was decided by collation of 20 computer-generated even numbers and 20 computer-generated odd numbers. The number list was used to allocate participants an even or an odd participant number at their first appointment. Because of the design of the study, the researcher was not blinded to the treatment arms. Only those randomized to the biofeedback arm were instructed on the use of equipment for self-regulation. At follow-up, 3 months after the last appointment, a packet of questionnaires was sent to each participant, including the CISS, FSS, and DASS. A stamped, self-addressed envelope was included for ease of return.
Intervention and Assessment of Measurement
Based on previous studies, an effective clinical intervention was recorded if breathing rate was reduced by 3 breaths per minute, below baseline, during relaxation and was maintained from week to week.20 26 27 During the first 30-second period of biofeedback recording, the breathing rate was recorded and labeled “base rate.” Breathing rate was recorded in the 30-second period after PMR, at the end of the first visit, and labeled “breathing rate t1.” “Breathing rate t1” was subtracted from base rate and the result called “breathing rate change.” Breathing rate was recorded in both ways at the subsequent two visits. The 30-second period at the end of PMR of each subsequent session was recorded as “breathing rate t2” or “breathing rate t3.” Breathing rate change for each participant was determined as follows: “Breathing rate t2” or “t3” was subtracted from the first session “base rate” and the result labeled “breathing rate change t2” or “breathing rate change t3.” Reduced trapezius electromyographic activity in the biofeedback group, compared with control, indicated whether the biofeedback group had learned to use the screens for self-regulation.25
Statistical software used was SPSS version 18.0 for students (IBM, Armonk, NY). Kolmogorov-Smirnov statistics were used with a Lilliefors significance level for testing normality, and the Shapiro Wilks statistic was included for sample sizes less than 100.
Whole-Group Analysis
The study investigated changes within groups over three visits and differences between groups. Effect size was calculated by subtracting outcome scores at t1 from outcome scores at t3. Significance was assessed using P values at 95% confidence interval (CI). Percentage improvements were calculated using the following formula:
Research28 was used to compare effect size for fatigue. One-way analysis of variance (ANOVA) was used to assess significance over three visits within groups.
Between-Groups Analysis
Initial analysis of difference between group outcomes adopted the independent-sample t test. Effect size was calculated by subtracting the control group's mean overall improvement (t3–t1) for each variable from the mean overall improvement (t3–t1) of the biofeedback group. Two-way ANOVA was used to assess main effects of biofeedback. In order to account for baseline differences between groups, baseline scores (t1) were entered as a covariate and group condition as a between-group factor.
Results
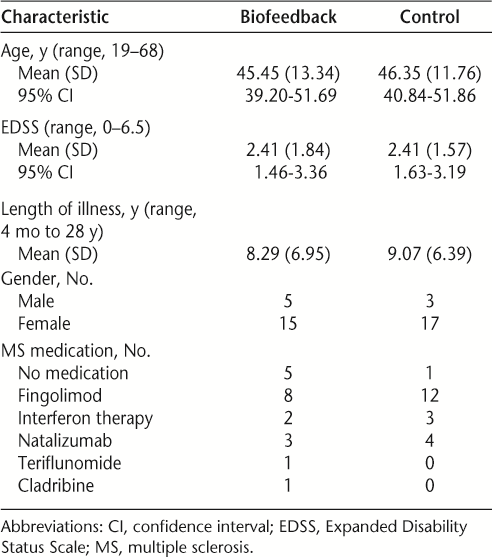
Demographics and baseline disease characteristics are described for each group in Table 1.
Demographics and baseline disease characteristics
Whole-Group Results
An effect of the intervention on the entire cohort was investigated from visit 1 (t1) to visit 3 (t3). Normality assumptions were met for age, EDSS, and all questionnaire measures. The two physiological independent variables, muscle tension and breathing rate, were distributed normally after logarithmic conversion.
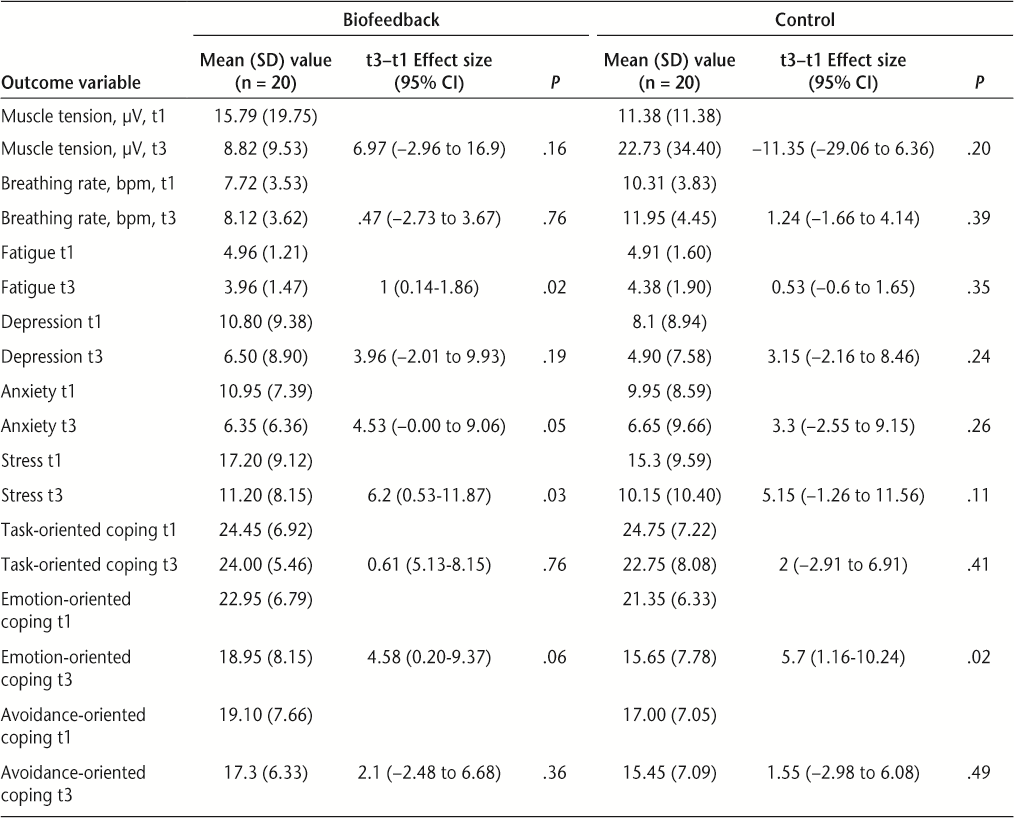
Measures of central tendency and the effect of the intervention (t3–t1) within groups are shown in Table 2. After three sessions, percentage improvements were calculated, with the overall cohort showing improvements in depression (t3–t1) of 39%, anxiety (t3–t1) of 38%, stress (t3–t1) of 34%, fatigue (t3–t1) of 13%, and emotional coping (t3–t1) of 22%.
Mean, standard deviation, effect size, and P value of variables before and after intervention
One-way ANOVA showed significant whole-group pre- to post-intervention improvements for the DASS components as follows: anxiety, F 18,21 = 3.02, P = .009, η2 = .721; depression, F 18,21 = 3.78, P = .007, η2 = .764. Fatigue, stress, and coping scores did not show whole-group improvement: fatigue, F 27,12 = 2.79, P = .32, η2 = .863; stress, F 25,14 = 1.324, P = .296, η2 = .703; coping (task), F 19,20 = 2.19, P = .045, η2 = .675; coping (emotion), F 19,20 = 1.49, P = .190, η2 = .587; coping (avoidance), F 20,19 = 1.76, P = .112, η2 = .649.
Home Practice
Thirty-seven of the 40 participants reported daily practice of the PMR and mindful breathing exercise, and 21 of these reported twice per day or more practice.
Between-Group Results
The biofeedback group anxiety improved significantly by 42% (effect size = 4.53 [95% CI, −0.00 to 9.06], P = .05), compared with nonsignificant improvement in controls (effect size = 3.3 [95% CI, −2.55 to 9.15], P = .26).
Using pre- to post-score differences only, at t3 the control group did not demonstrate significant improvements in questionnaire variables, while the biofeedback group demonstrated either significant improvement or a trend toward reduced anxiety (P = .05), fatigue (P = .02), and stress (P = .03), but not depression (P = .19).
Effect size for fatigue, from baseline to the end of treatment, showed improvement for the biofeedback group (effect size = 1 [95% CI, 0.14–1.86]; P = .02) compared with control (effect size = 0.53 [95% CI, −0.6 to 1.65]; P = .35). Biofeedback group clinical fatigue scores at t3 moved from “severe fatigue” to “mild fatigue.”
Despite random allocation to group, the biofeedback group had higher baseline scores on all measures of the DASS (anxiety, depression, stress) and higher fatigue than the control group. In order to statistically control for differences at baseline, questionnaire scores at t1 were entered as a covariate and “group condition” entered as a between factor in a series of separate ANOVAs computed using an alpha level of .05. There was no significant difference in effect of group conditions on questionnaire outcomes at t3: fatigue: F 1,39 = 0.59, P = .45, η2 = .016; depression: F 1,39 = 0.00, P = .97, η2 = .000; anxiety: F 1,39 = 0.184, P = .670, η2 = .005; stress: F 1,39 = 0.01, P = .93, η2 = .000; task coping: F 1,39 = 0.58, P = .45, η2 = .015; emotion coping: F 1,39 = 1.07, P = .31, η2 = .028; avoidance coping: F 1,39 = 0.32, P = .58, η2 = .009.
Within-group mean improvements, 95% CI, and P values were compared between groups by visit (Table 3). Outcomes on questionnaire variables did not show significant differences between groups.
Group condition difference, confidence interval, and P value at time 1 compared with time 3
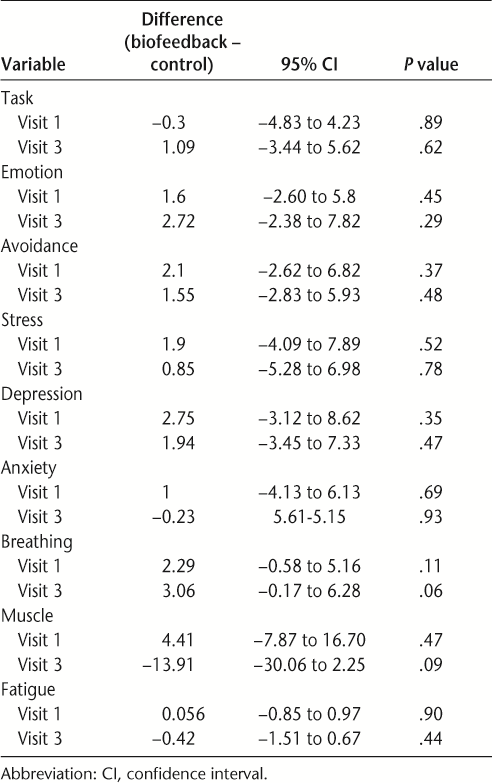
Physiological measures showed a trend toward significant differences at t3 (Table 2). The biofeedback group shoulder tension (mean t3 = 8.82 μV) was on average 13.91 μV lower than that of the control group (mean t3 = 22.73 μV), and the biofeedback group breathing rate (mean = 8.12 bpm) was lower than that of the control group (mean = 11.95 bpm). Table 3 shows the P values for difference between groups for breathing rate and muscle tension at t3 (P = .06 and .09, respectively).
After controlling for baseline and normality, ANOVA showed a trend toward breathing rate difference between groups for breathing rate at t3 as follows: F 1,39 = 3.347, P = .07, η2 = .083. The ANOVA showed a trend for significant change in muscle tension in the biofeedback condition: F 1,39 = 3.797, P = .06, η2 = .093.
At the final visit, all participants were asked verbally if the intervention had made a difference. The answer was recorded as a “yes” or “no” on the participants' files. One hundred percent of respondents in the biofeedback group indicated during semistructured interviews that the intervention had made a difference, compared with 84% of the control group.
Three-Month Follow-up
A follow-up questionnaire was sent to all 40 participants 3 months after the last visit. Nineteen participants returned surveys. Respondents from both groups (biofeedback [n = 13] and control [n = 6]) reported that they were practicing PMR with mindful breathing awareness 3 months later.
Discussion
This is the first randomized controlled study comparing the benefit in terms of mood, coping, and fatigue of RMSSE, with or without the addition of biofeedback, in patients with RRMS. Despite a large evidence base for effective outcomes in other conditions such as tension-type headache,19 chronic pain,20 and anxiety,29 biofeedback has been overlooked in interventions designed to improve mood, coping, and fatigue in MS. Promisingly, in this study, biofeedback was shown to be effective in reducing fatigue, stress, and anxiety. The biofeedback group also demonstrated improved control of breathing rate and muscle tension, indicating that they could be easily taught how to self-regulate these two physiological parameters.25 26 The study findings lend support to current research on the potential benefits of psychological interventions in improving the well-being of people with MS.2 3 8
Current models of care, which place emphasis on medication and reliance on specialists, minimize the role of the patient in managing symptoms. Since helplessness has been found to mediate both depressive mood and fatigue severity,30 MS patients troubled by fatigue may benefit from a psychological intervention targeting helplessness. Self-awareness and self-regulation may help MS patients develop internal coping strategies for life events rather than depending on external sources for help.26 30 31 Biofeedback with education not only increases self-regulation but may also generalize to a person's sense of perceived control or an internalization of their sense of self-control. Further, the intervention may be enhanced when combined with training in mindful breathing awareness (such as the simple breathing awareness activity in the present study), which can increase regulation of emotion, enabling prefrontal cortex activity and control of the flight/fight response.32 Mindful breathing has also been found to decrease depression, by increasing awareness of and control over internal processes such as thoughts,33 and to improve immune function.34
Anecdotally, patients often report stressful life events prior to relapse in MS, prompting research into the impact of stress on relapse, exacerbation, and remission.35 36 The observed reduction in breathing rate in both patient groups indicates that physiological stress reduction was achieved during the relaxation component at each visit. Biofeedback research indicates that reducing breathing rate by 3 or more breaths below base rate, as achieved in the present study, correlates with sympathetic reduction,26 which is associated with reduced anxiety and stress.27 At the third visit, both biofeedback and control groups improved in category from “mild” to “normal” stress on the DASS instrument,24 although the biofeedback group exhibited a larger effect size. The improvement in perceived stress in both groups may have been partly due to daily practice of PMR with mindful breathing. Thirty-seven of the 40 participants reported at least daily practice of PMR with mindful breathing between visits. The trend toward an improvement in stress in both groups warrants a larger study, as interventions that reduce stress may also reduce the rate of MS exacerbations3 34 and interact with coping mechanisms employed by the individual.17 35
The DASS anxiety scale measures physical manifestations of low mood, such as tight muscles and hyperarousal.24 It is hypothesized that the reductions in both muscle tension and anxiety observed in the biofeedback group were due to the learning gained through internalizing the feedback from access to the biofeedback equipment.
As a result of intervention, the biofeedback group achieved reduction of categorical anxiety24 by two levels. After adjusting for baseline, however, biofeedback was not found to have a statistical advantage.
The entire cohort improved in fatigue, as predicted by the literature.21 26 The biofeedback group improved in effect size for fatigue almost twice as much as controls. A practical significance21 in reduction from “severe fatigue” (mean = 4.9) to “mild fatigue” or “non-fatigue” (mean = 3.9) was observed only in the biofeedback group. Despite this, statistical support for an advantage of biofeedback over relaxation was not found.
The present study supports the existing literature showing that psychoeducational and psychosocial interventions can influence MS-related fatigue.2 37 38 Importantly, interventions that improve fatigue also interact with depression and other measures of well-being in MS.6 The interventions in the present study were novel in introducing self-regulation via biofeedback, and had the benefit of brevity (3 weeks) compared to other similar studies (for example, van Kessel et al.39 had an 8-week, and Mohr et al.3 a 24-week intervention). These factors contribute to reducing the time, staffing, and cost-related resource load of such an intervention.
Both anxiety and fatigue have been associated with negative cognitions about MS symptoms, such as worry and anticipation of embarrassment.9 38 The biofeedback group improved more in clinical categories of anxiety and fatigue than did the control group. Having learned to control physical manifestations of anxiety, breathing rate and muscle tension, the biofeedback group may have incorporated greater control beliefs about their symptoms.
It was hypothesized that if biofeedback provides an opportunity to develop an internal locus of control, biofeedback group participants would have reported that the intervention made a difference to coping39 or that they continued to practice relaxation after the study was completed. At follow-up, 19 participants returned surveys, including Coping Inventory for Stressful Situations,22 Fatigue Severity Scale,23 and DASS scales.24 Thirteen respondents were from the biofeedback group. These respondents reported ongoing improvements in coping, fatigue, and sleep, whereas the six control group respondents reported differences in relaxation and breathing awareness but not in fatigue, sleep, or coping.
Limitations of the present study include the relatively small sample and the potential benefits to the control group of RMSSE without biofeedback. Less than half of the cohort responded to the 3-month follow-up questionnaires, and hence inferences as to the long-term effects of biofeedback were inappropriate. A further possible confounder may be a bias favoring those who had found the intervention useful who responded to the 3-month follow-up survey.
Conclusion
The present study provides a novel use of biofeedback technology that may consolidate learning about, and control over, the physiological stress response leading to internalized control of the stress response and improvements in perceived stress, coping, anxiety, depression, and fatigue in RRMS. Although statistical support was not found in this study for incremental improvement of biofeedback over relaxation, possibly owing to too much similarity between group conditions, both groups improved in depression, anxiety, stress, and emotional coping. The biofeedback group members were able to demonstrate control of breathing rate and muscle tension using biofeedback equipment. Over three visits the biofeedback group had a reduction in clinical category of fatigue and anxiety.
The present study also provides a base for future research with more participants and more study arms, including no treatment, biofeedback, relaxation with mindfulness, and education. Home practice could be standardized with the use of a CD or portable biofeedback equipment. An extension of the present study may underscore whether improvements were maintained over time.
This study adds to the clinical evidence that people living with RRMS may benefit in terms of coping, fatigue, anxiety, stress, and depression from psychological interventions that increase the person's sense of control over their physiology. From the clinician's perspective, an RMSSE and biofeedback intervention may have significant cost-effectiveness and personnel resource implications.
PracticePoints
The present study examined a novel use of biofeedback technology that may consolidate learning about, and control over, the physiological stress response. Reliance on the therapist was minimized while internalized control of the stress response by the patient was emphasized.
While all participants benefited from participation, the study demonstrated effective reduction of anxiety, fatigue, and stress in the biofeedback group. Patients in the biofeedback group also demonstrated significant reductions compared to the control group in physiological measurements of breathing rate and muscle tension.
The study findings indicate the potential benefits of psychological interventions in improving the well-being of people with MS.
Acknowledgments
Suzanne Baker, Clinical Nurse, MS Clinic, Neurology Department, Liverpool Hospital, who provided introduction to patients suitable for the study.
References
Kroencke DC, Lynch SG, Denney DR. Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler. 2000; 6: 131–136.
Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010:75:1141–1149.
Mohr DC, Lovera J, Brown T, et al. A randomised trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012; 79: 412–419.
Lode K, Bru E, Klevan G, Myhr KM, Nyland H, Larsen JP. Depressive symptoms and coping in newly diagnosed patients with multiple sclerosis. Mult Scler. 2009; 15: 638–643.
Malcomson KS, Dunwoody L, Lowe-Strong AS. Psychosocial interventions in people with multiple sclerosis: a review. J Neurol. 2007; 254: 1–13.
Mohr DC, Hart SL, Goldberg A. Effects of treatment for depression on fatigue in multiple sclerosis. Psychosom Med. 2003; 65: 542–547.
Rigby SA, Thornton EW, Young CA. A randomized group intervention trial to enhance mood and self-efficacy in people with multiple sclerosis. Br J Health Psychol. 2008; 13: 619–631.
Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler J. 2011; 17: 1250–1257.
Knoop H, van Kessel K, Moss-Morris R. Which cognitions and behaviours mediate the positive effect of cognitive behavioural therapy on fatigue in patients with multiple sclerosis? Psychol Med. 2012; 42: 205–213.
Beeney J, Arnett PA. Stress and memory bias interact to predict depression in multiple sclerosis. Neuropsychology. 2008; 22: 118–126.
Lode K, Larsen JP, Bru E, Klevan G, Myhr KM, Nyland H. Patient information and coping styles in multiple sclerosis. Mult Scler. 2007; 13: 792–799.
Pakenham KI. Investigation of the coping antecedents to positive outcomes and distress in multiple sclerosis (MS). Psychol Health. 2006; 21: 633–649.
Schwartz C. Teaching coping skills enhances quality of life more than peer support: results of a randomized trial with multiple sclerosis patients. Health Psychol. 1999; 18: 211–220.
Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005; 76: 469–475.
Aldwin CM, Revenson TA. Does coping help? a reexamination of the relation between coping and mental health. J Pers Soc Psychol. 1987; 53: 337–348.
Goretti B, Portaccio E, Zipoli V, et al. Coping strategies, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2009; 30: 15–20.
Goretti B, Portaccio E, Zipoli V, Razzolini L, Amato M. Coping strategies, cognitive impairment, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2010; 31: 227–230.
Pakenham KI. Adjustment to multiple sclerosis: application of a stress and coping model. Health Psychol. 1999; 18: 383–392.
Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007; 128: 111–127.
Kapitza KP, Passie T, Bernateck M, Karst M. First non-contingent respiratory biofeedback placebo versus contingent biofeedback in patients with chronic low back pain: a randomized, controlled, double-blind trial. Appl Psychophysiol Biofeedback. 2010; 35: 207–217.
Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008; 6:100.
Endler NS, Parker JD. A Coping Inventory for Stressful Situations. Toronto, Canada: Multi-Health Systems; 1999.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46: 1121–1123.
Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney, Australia: Psychological Foundation of Australia; 1995.
Schwartz M, Andrasik F. Biofeedback: A Practitioner's Guide. 3rd ed. New York, NY: The Guilford Press; 2003.
Balaña FJM, Pastor ME. Latest contributions about the influence of breathing to learning with biofeedback electrical conductance of the skin. Span J Motiv Emotion. 2000;3(4).
Pastor MC, Menendez FJ, Sanz MT, Abad EV. The influence of respiration on biofeedback techniques. Appl Psychophysiol Biofeedback. 2008; 33: 49–54.
Bakshi R, Shaikh ZA, Miletich RS, et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler. 2000; 6: 181–185.
Rice KM, Blanchard EB. Biofeedback in the treatment of anxiety disorders. Clin Psychol Rev. 1982; 2: 557–577.
van der Werf SP, Evers A, Jongen PJH, Bleijenberg G. The role of helplessness as mediator between neurological disability, emotional instability, experienced fatigue and depression in patients with multiple sclerosis. Mult Scler. 2003; 9: 89–94.
Boekaerts M, Penelope P, Eva B, Barry M. Coping with stressful situations: an important aspect of self-regulation. In: International Encyclopedia of Education. Oxford, UK: Elsevier; 2010:570–575.
Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res. 2010; 68: 539–544.
Carmody J, Baer RA, Lykins E, Olendzki N. An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. J Clin Psychol. 2009; 65: 613–626.
Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003; 65: 564–570.
Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: part II. direct and indirect relationships. Mult Scler. 2006; 12: 465–475.
Kroecke DC, Denney DR. Stress and coping in multiple sclerosis: exacerbation, remission and chronic subgroups. Mult Scler. 1999; 5: 89–93.
Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011; 17: 604–612.
Skerrett TN, Moss-Morris R. Fatigue and social impairment in multiple sclerosis: the role of patients' cognitive and behavioral responses to their symptoms. J Psychosom Res. 2006; 61: 587–593.
van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson M. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008; 70: 205–213.
Financial Disclosures: The authors have no conflicts of interest to disclose.







